Abstract
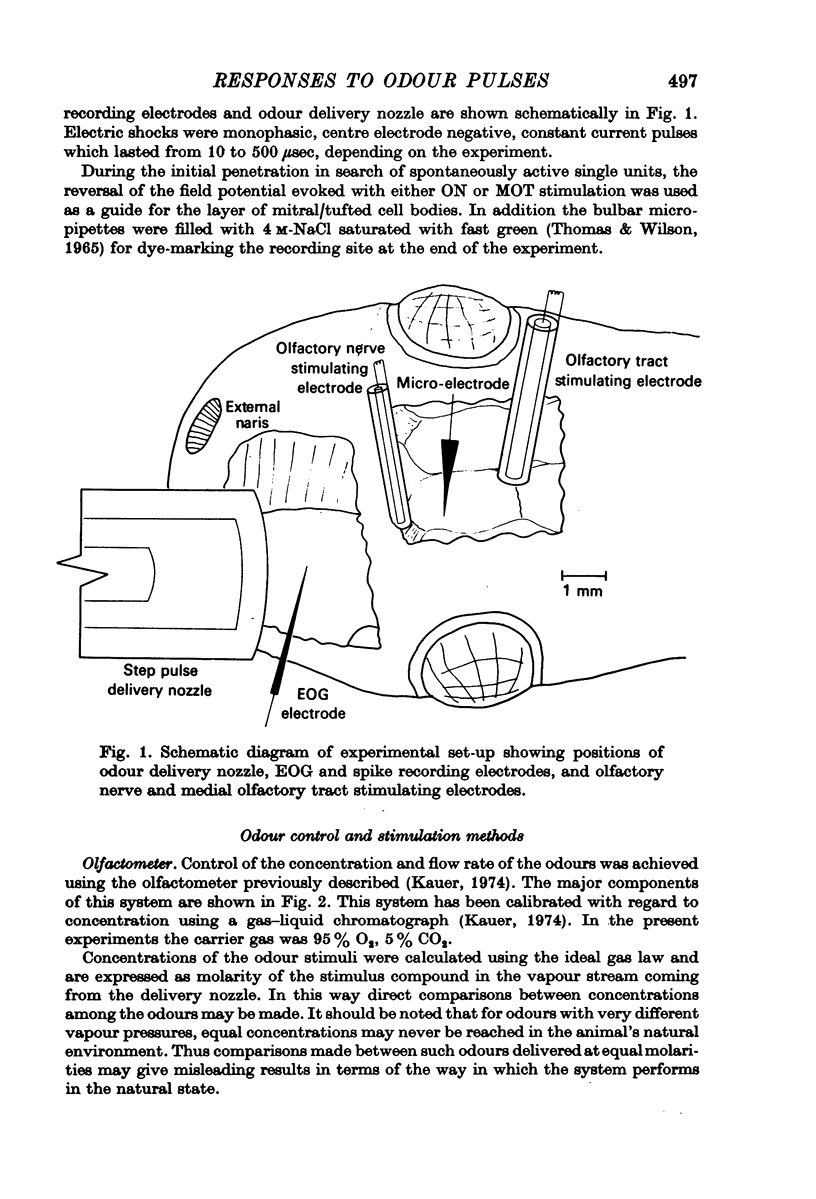
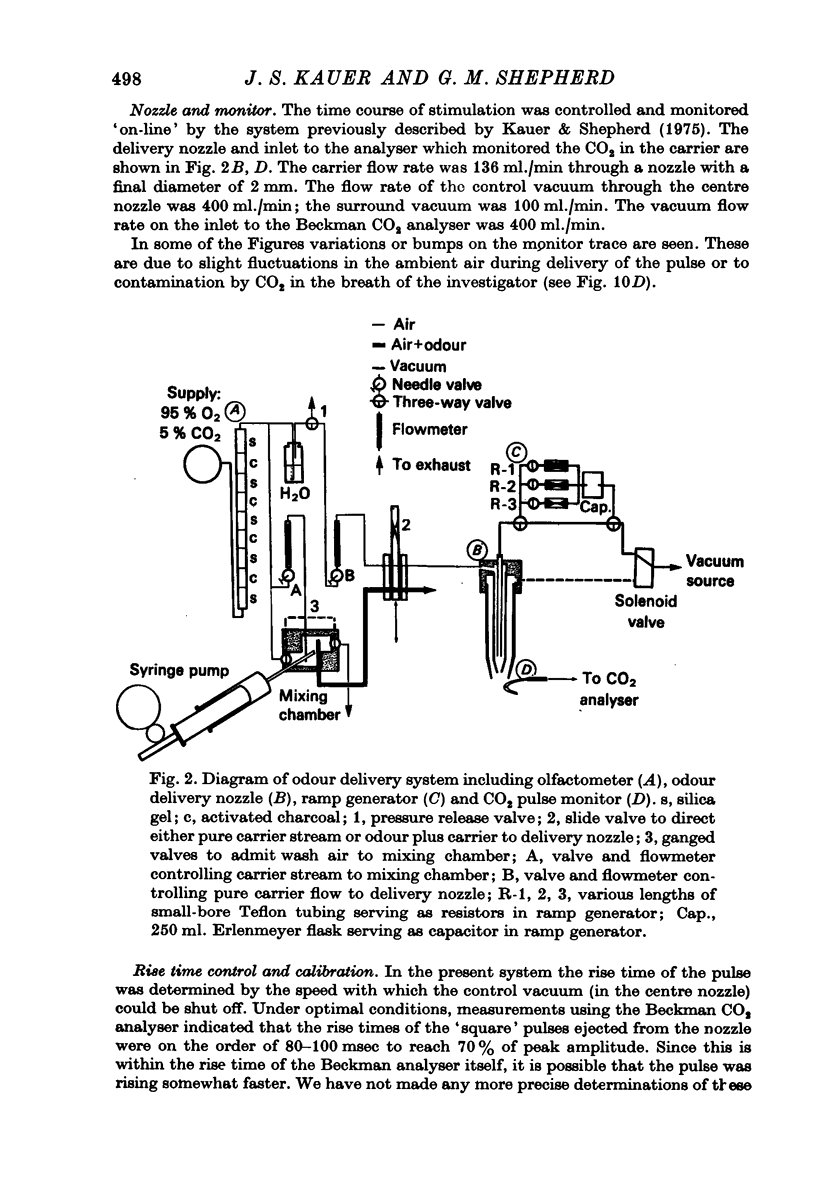
1. A method for delivering odour pulses of controlled onset, steady plateau and abrupt termination, has been developed and applied to a single unit study of mitral cell responses in the olfactory bulb of the salamander. The pulses have been monitored during the experiments near the site of stimulation on the olfactory mucosa.
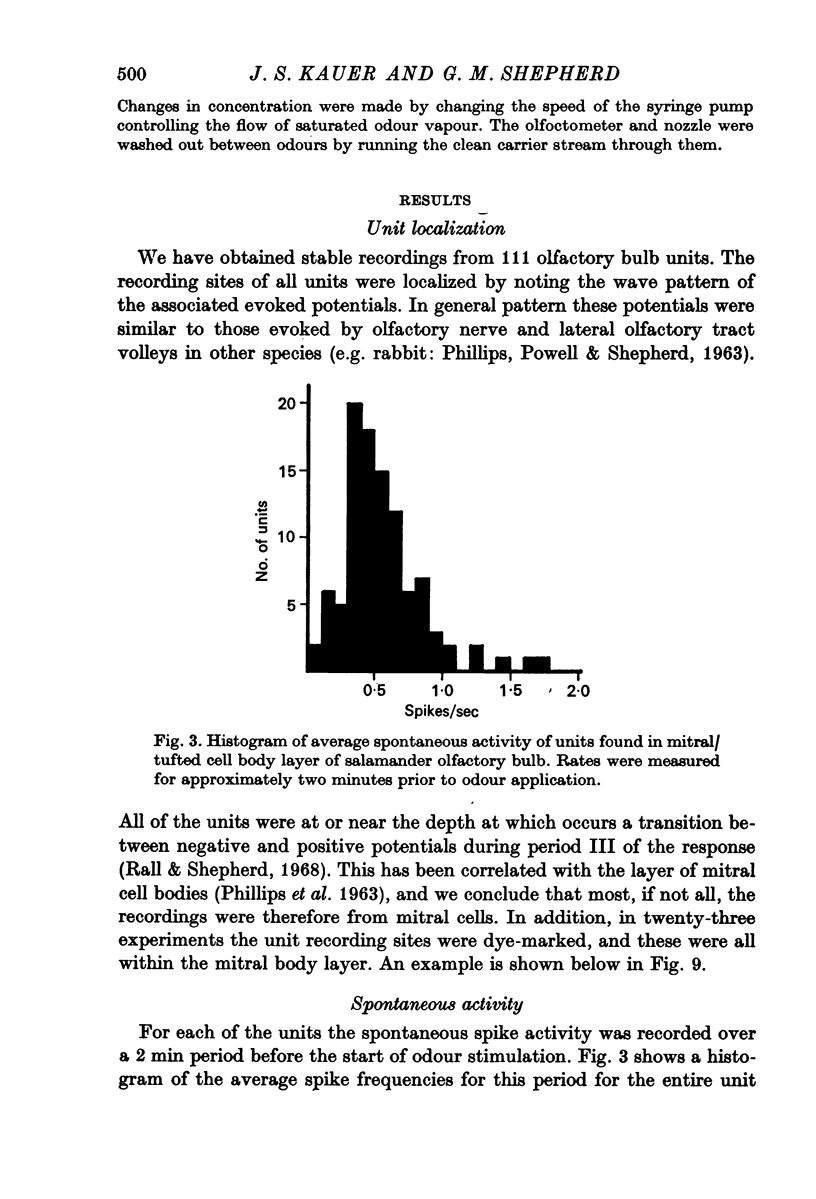
2. Responses have been categorized as excitatory or suppressive based on the initial response to the odour pulse.
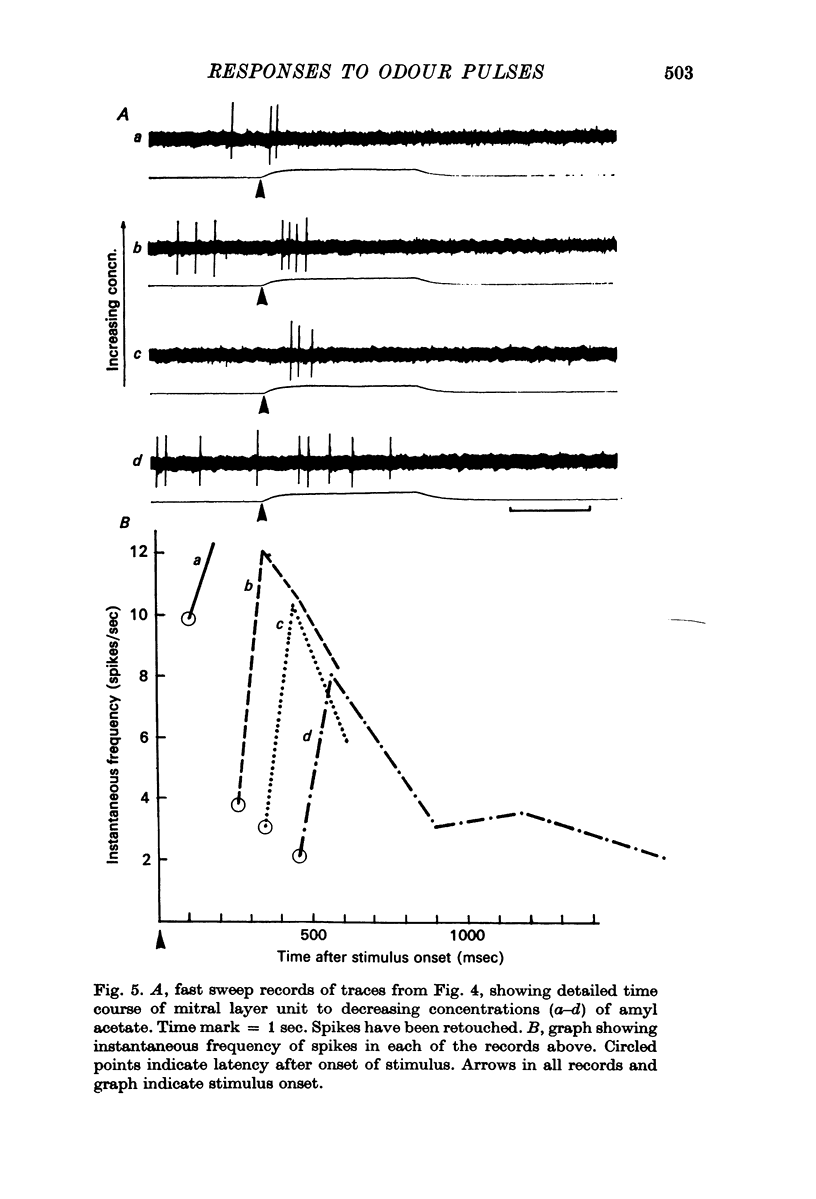
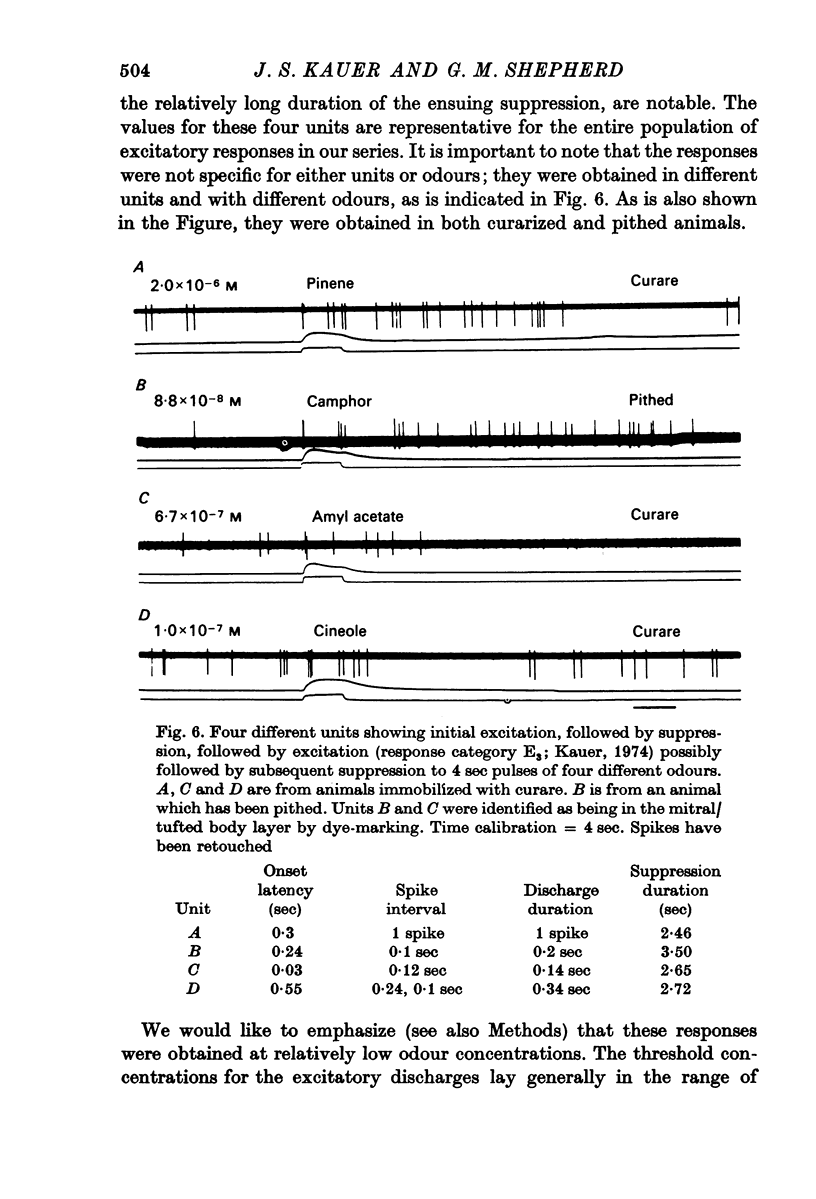
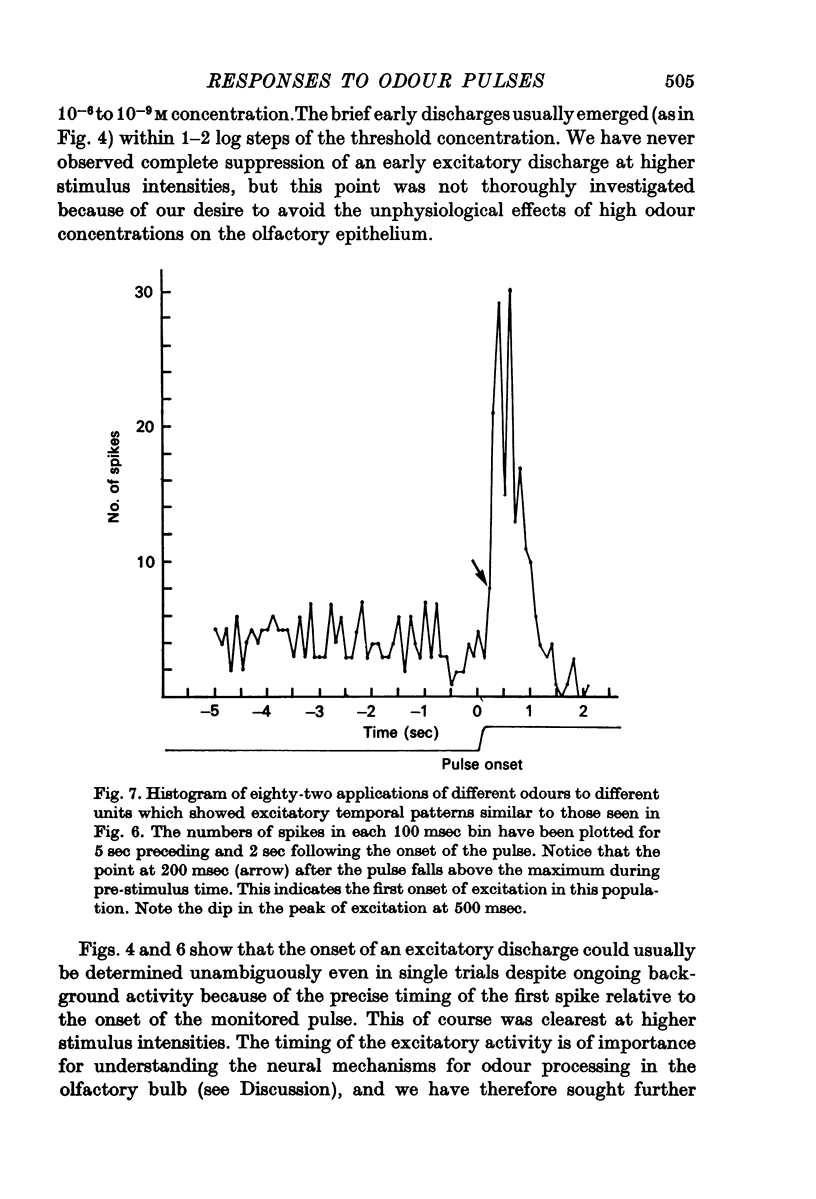
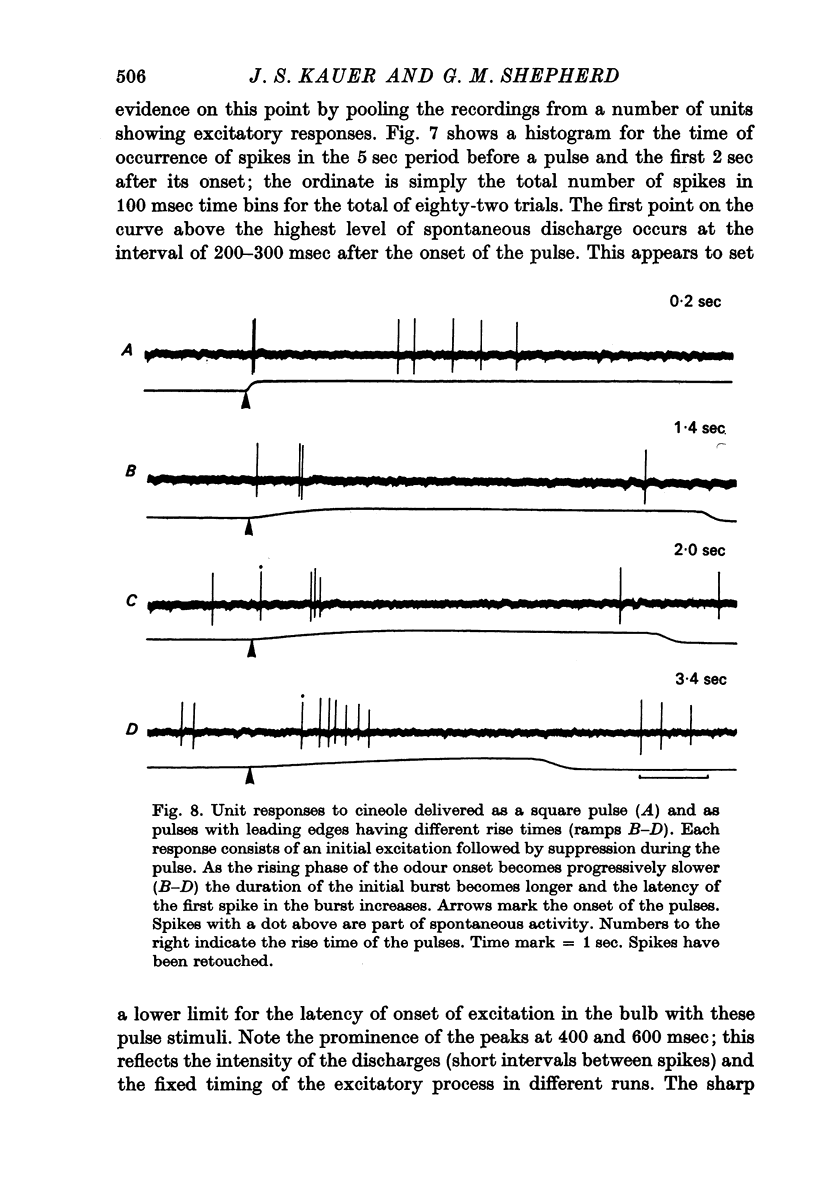
3. Initially excitatory responses had sustained discharges near threshold. With increasing concentration, the discharge changed to a brief burst followed by suppression. The briefest latency of a unit response was 120 msec, using stimulation of medium concentration, after the start of the pulse; the majority of units appeared to be excited within 200-300 msec. Ramp stimuli gave increasing periods of excitation as the rise time of the odour front became less abrupt.
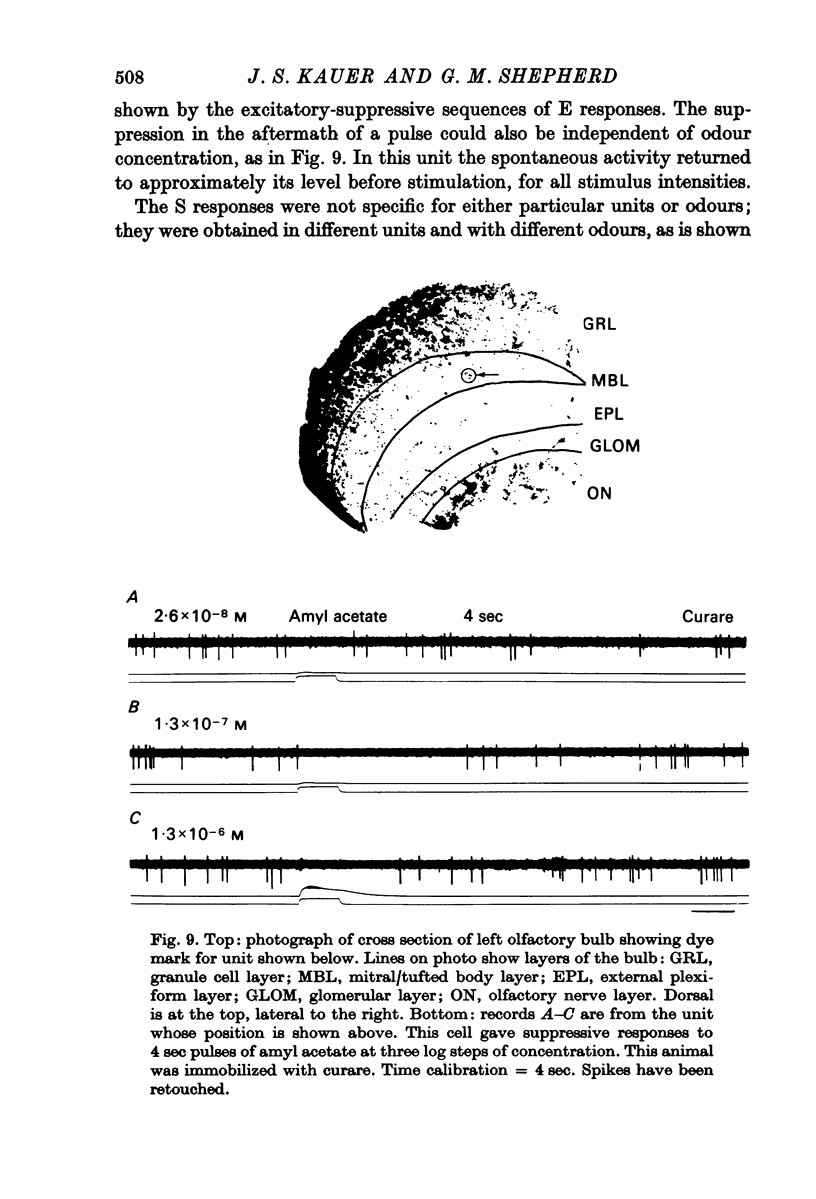
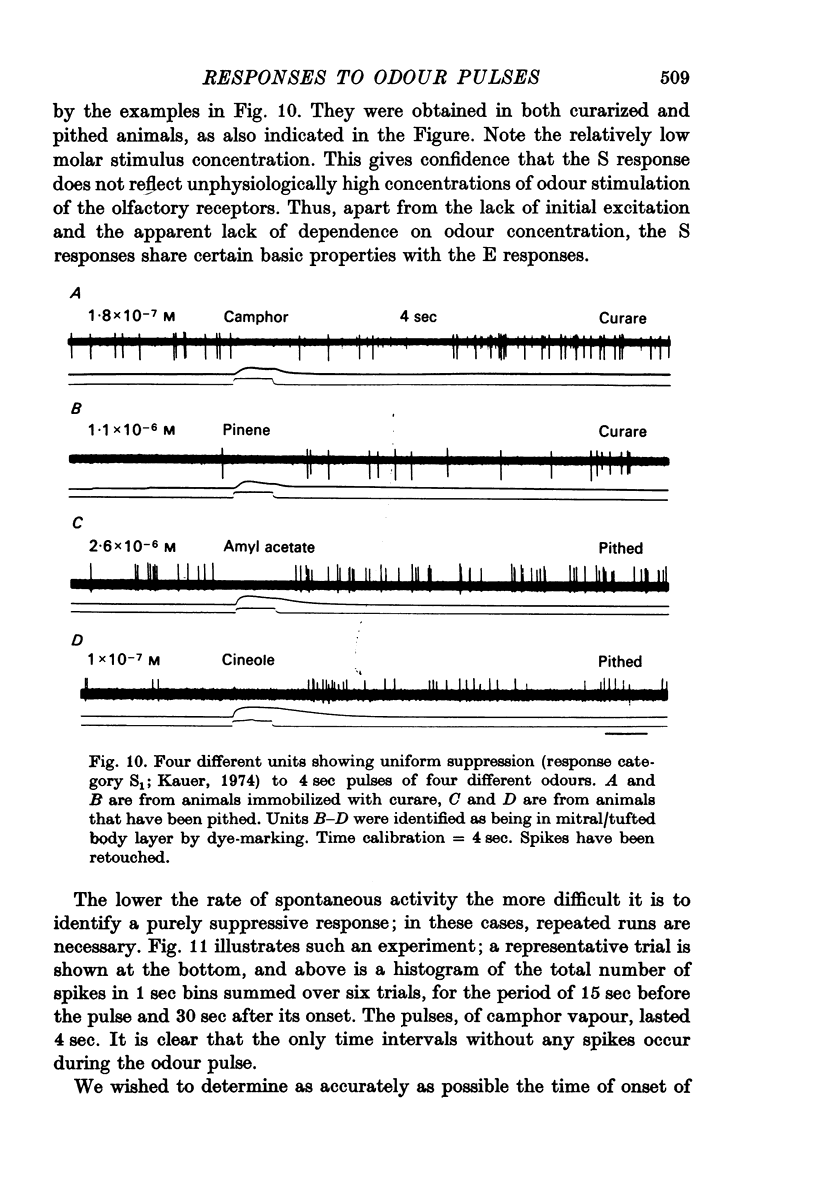
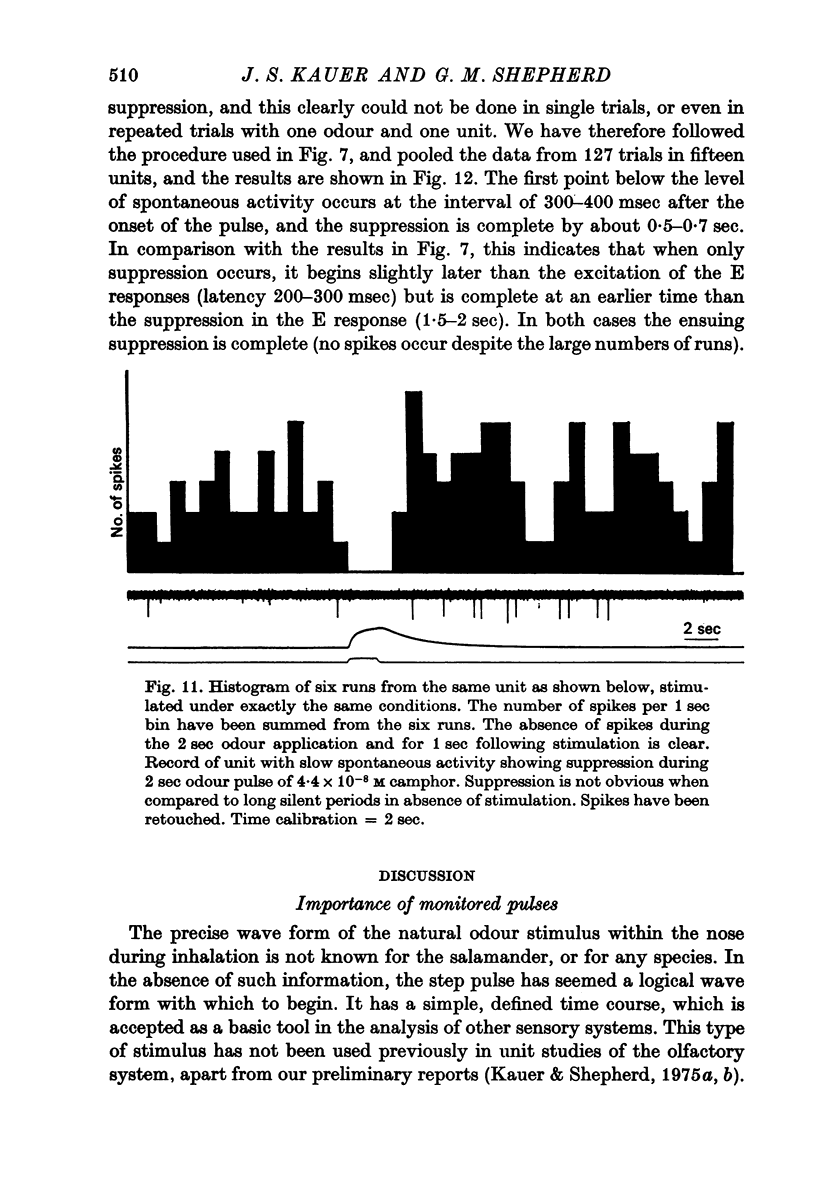
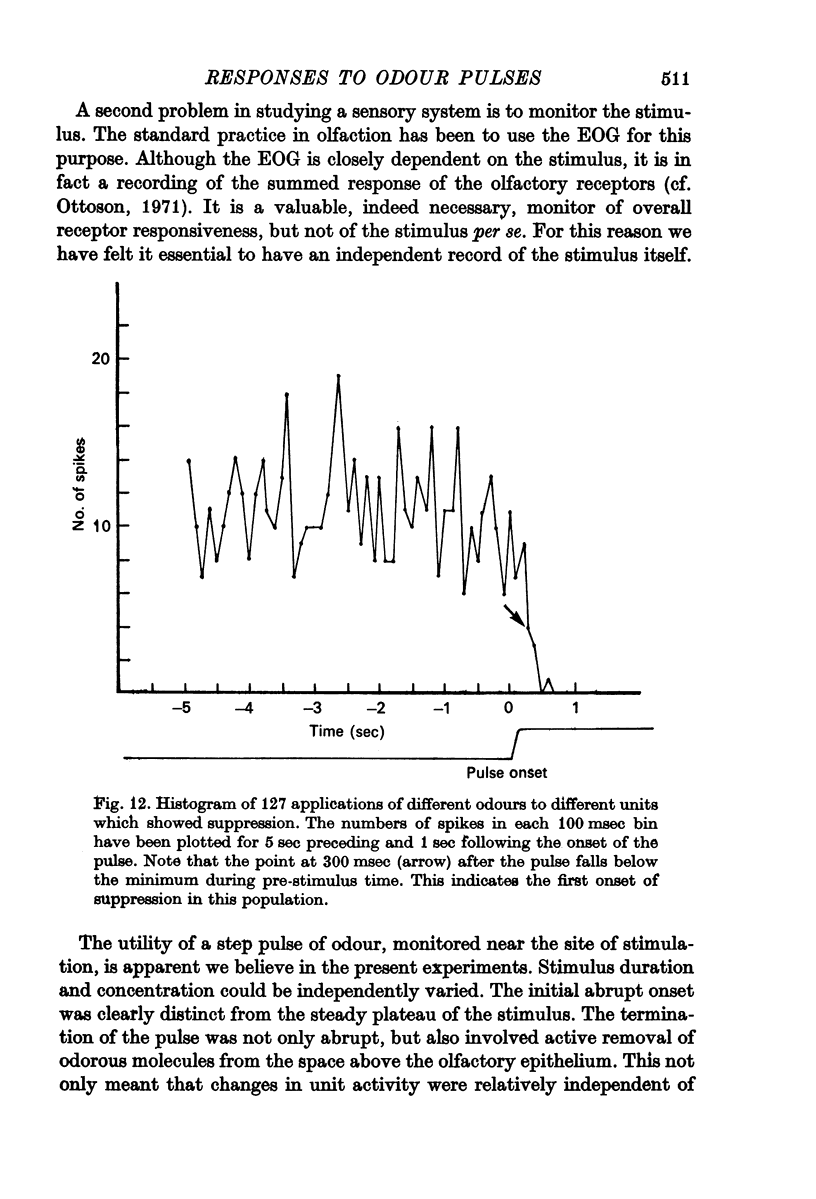
4. Initially suppressive responses showed suppression at all levels of concentration. The majority of units appeared to have an onset of suppression about 300-400 msec after the start of the pulse.
5. These basic responses, involving suppression or excitatory—suppressive sequences, can be correlated with some basic properties of the synaptic circuits in the olfactory bulb. The time courses of the initial responses appear to be within the time periods of the inhalation cycle of the salamander, and therefore may reflect mechanisms of processing of natural olfactory stimuli.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broadwell R. D., Jacobowitz D. M. Olfactory relationships of the telencephalon and diencephalon in the rabbit. III. The ipsilateral centrifugal fibers to the olfactory bulbar and retrobulbar formations. J Comp Neurol. 1976 Dec 1;170(3):321–345. doi: 10.1002/cne.901700305. [DOI] [PubMed] [Google Scholar]
- CALLENS M., BOISACQ-SCHEPENS N. TONIC DEPRESSIVE INFLUENCES ON THE OLFACTORY BULB BY THE CONTRALATERAL OLFACTORY BULB AND BY CENTRAL NERVOUS SYSTEM STRUCTURES. Arch Int Physiol Biochim. 1963 Aug;71:621–623. [PubMed] [Google Scholar]
- Gesteland R. C., Lettvin J. Y., Pitts W. H. Chemical transmission in the nose of the frog. J Physiol. 1965 Dec;181(3):525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Analysis of unitary spikes recorded extracellularly from frog olfactory receptor cells and axons. J Physiol. 1973 Nov;234(3):533–551. doi: 10.1113/jphysiol.1973.sp010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Short-axon cells in the olfactory bulb: dendrodendritic synaptic interactions. J Physiol. 1975 Oct;251(2):523–548. doi: 10.1113/jphysiol.1975.sp011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Unitary responses in frog olfactory epithelium to sterically related molecules at low concentrations. J Gen Physiol. 1974 Aug;64(2):241–261. [PMC free article] [PubMed] [Google Scholar]
- Holley A., Duchamp A., Revial M. F., Juge A. Qualitative and quantitative discrimination in the frog olfactory receptors: analysis from electrophysiological data. Ann N Y Acad Sci. 1974 Sep 27;237(0):102–114. doi: 10.1111/j.1749-6632.1974.tb49847.x. [DOI] [PubMed] [Google Scholar]
- KERR D. I., HAGBARTH K. E. An investigation of olfactory centrifugal fiber system. J Neurophysiol. 1955 Jul;18(4):362–374. doi: 10.1152/jn.1955.18.4.362. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kauer J. S., Moulton D. G. Responses of olfactory bulb neurones to odour stimulation of small nasal areas in the salamander. J Physiol. 1974 Dec;243(3):717–737. doi: 10.1113/jphysiol.1974.sp010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. S., Shepherd G. M. Olfactory stimulation with controlled and monitored step pulses of odor. Brain Res. 1975 Feb 21;85(1):108–113. doi: 10.1016/0006-8993(75)91014-8. [DOI] [PubMed] [Google Scholar]
- Macrides F., Chorover S. L. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972 Jan 7;175(4017):84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- O'Connell R. J., Mozell M. M. Quantitative stimulation of frog olfactory receptors. J Neurophysiol. 1969 Jan;32(1):51–63. doi: 10.1152/jn.1969.32.1.51. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Price J. L. The origin of the centrifugal fibres to the olfactory bulb. Brain Res. 1969 Jul;14(2):542–545. doi: 10.1016/0006-8993(69)90135-8. [DOI] [PubMed] [Google Scholar]
- Price J. L. The termination of centrifugal fibres in the olfactory bulb. Brain Res. 1968 Mar;7(3):483–486. doi: 10.1016/0006-8993(68)90019-x. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO OLFACTORY NERVE VOLLEYS IN THE RABBIT. J Physiol. 1963 Aug;168:89–100. doi: 10.1113/jphysiol.1963.sp007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M. Physiological evidence for dendrodendritic synaptic interactions in the rabbit's olfactory glomerulus. Brain Res. 1971 Sep 10;32(1):212–217. doi: 10.1016/0006-8993(71)90168-5. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972 Oct;52(4):864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Precise localization of Renshaw cells with a new marking technique. Nature. 1965 Apr 10;206(980):211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization in the olfactory glomerulus of the mouse. Brain Res. 1972 Feb 11;37(1):69–80. doi: 10.1016/0006-8993(72)90346-0. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization of the mammalian olfactory glomerulus: new findings including an intraspecific variation. Brain Res. 1973 Oct 12;60(2):299–313. doi: 10.1016/0006-8993(73)90792-0. [DOI] [PubMed] [Google Scholar]


