Abstract
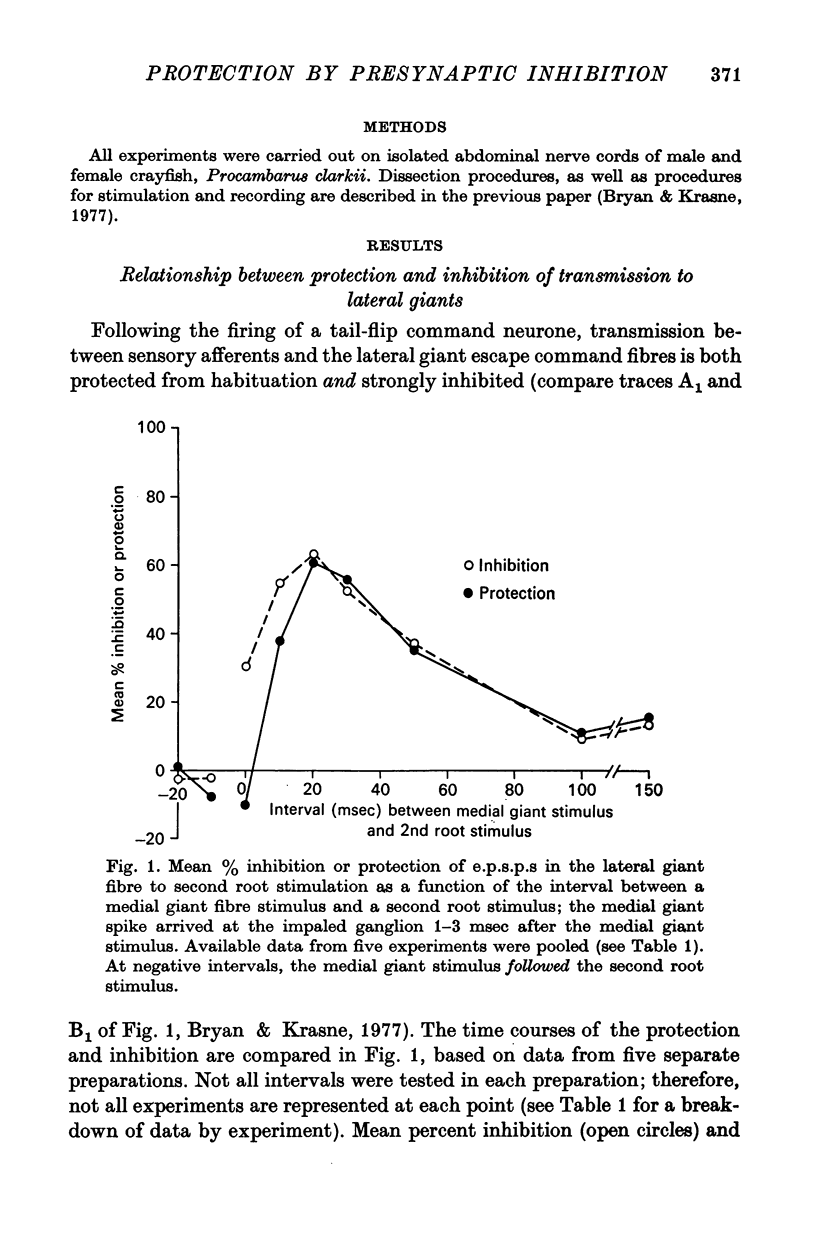
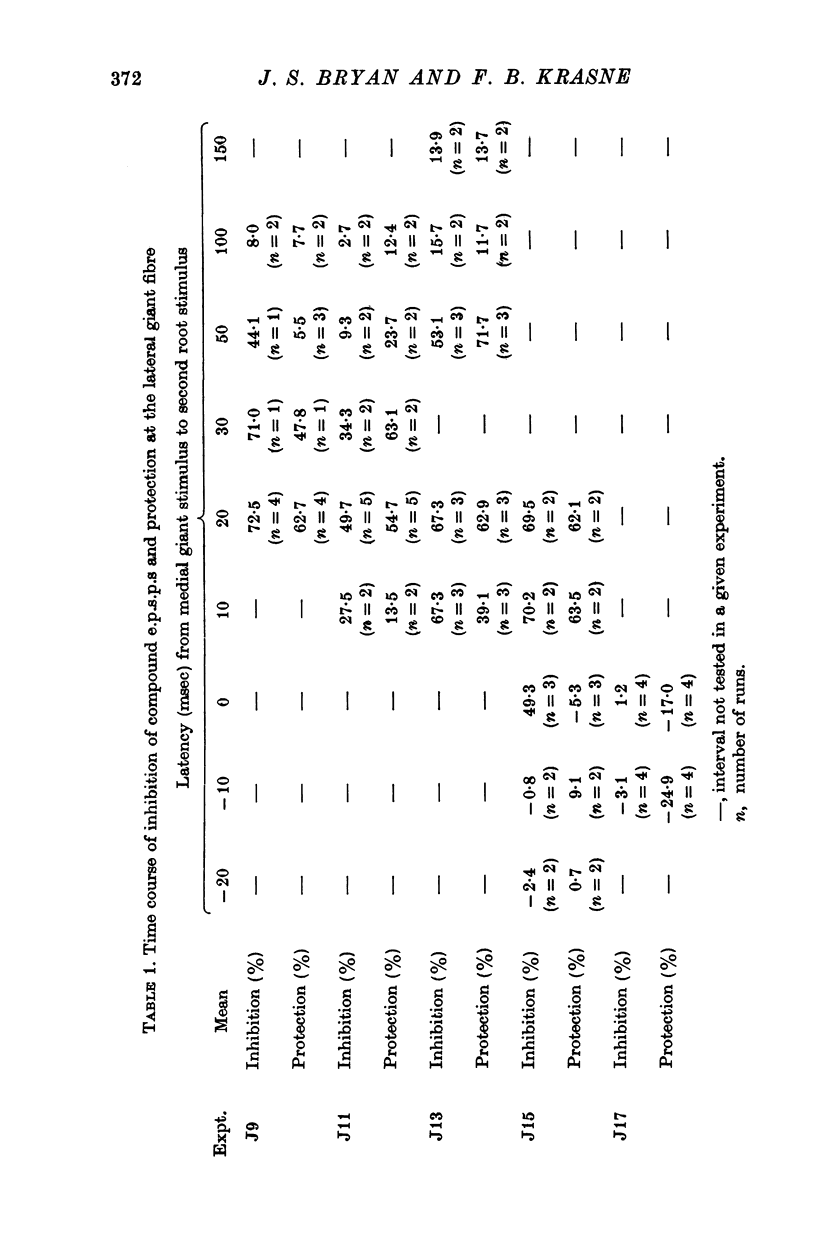
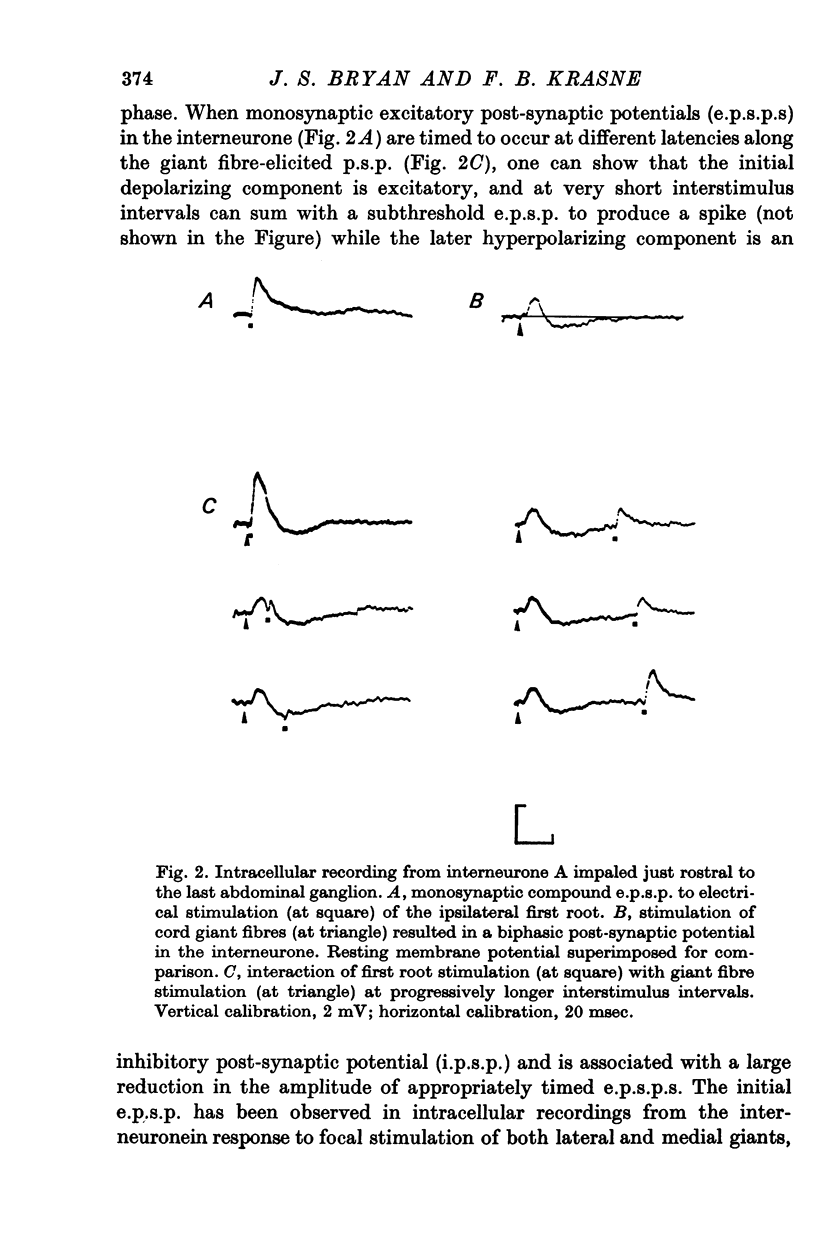
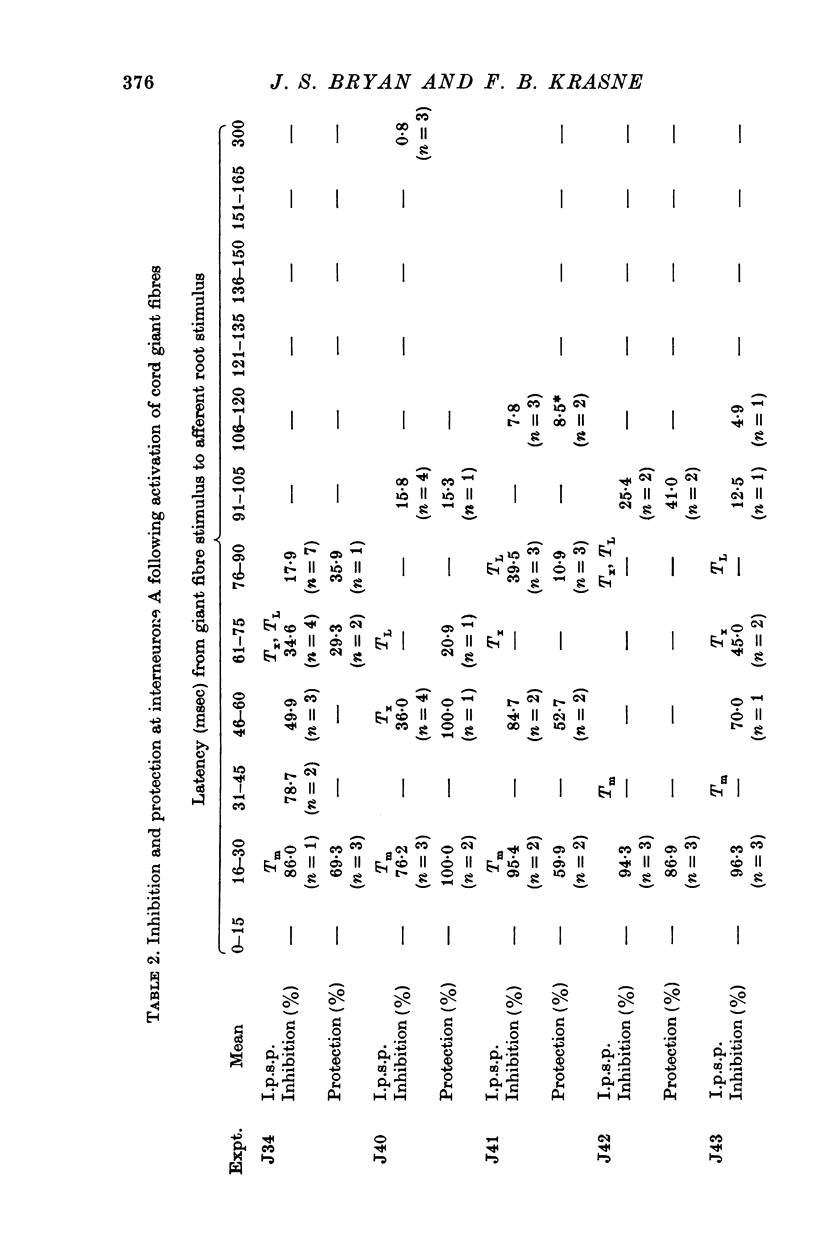
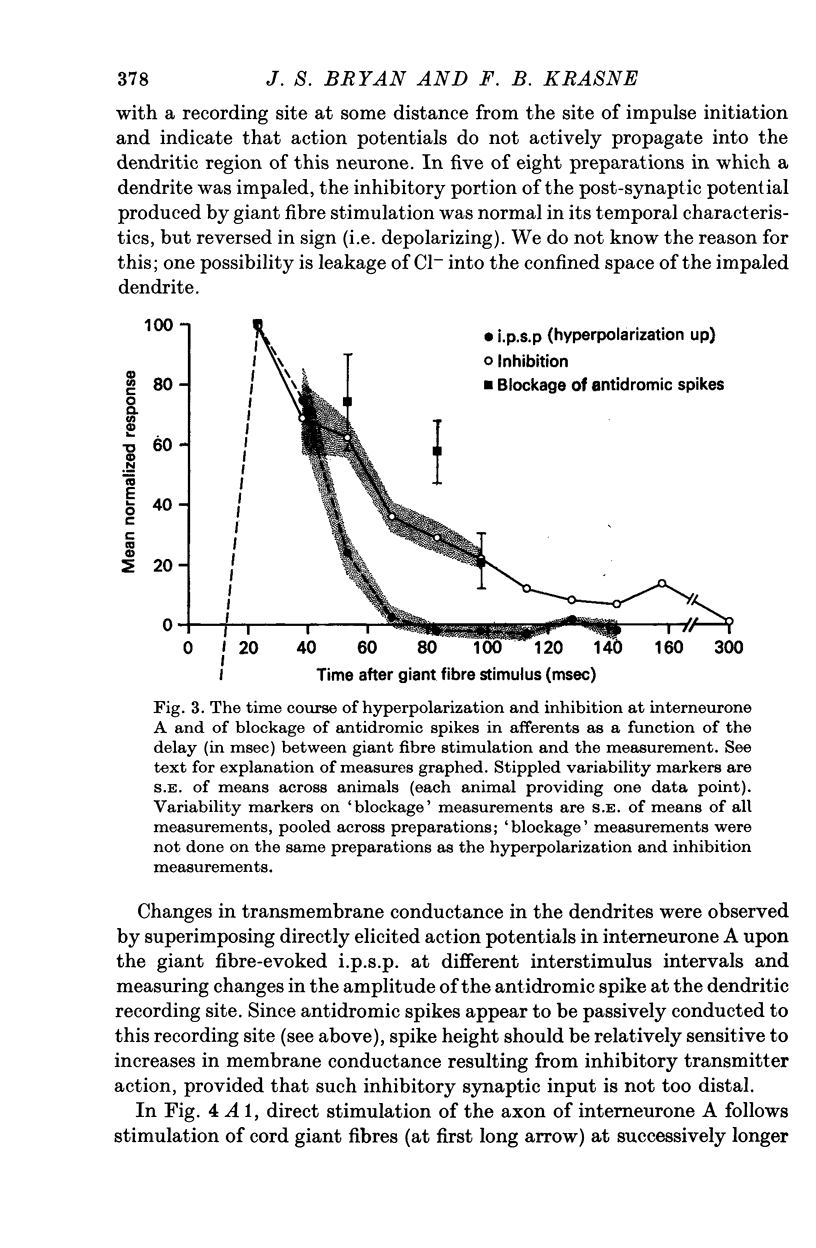
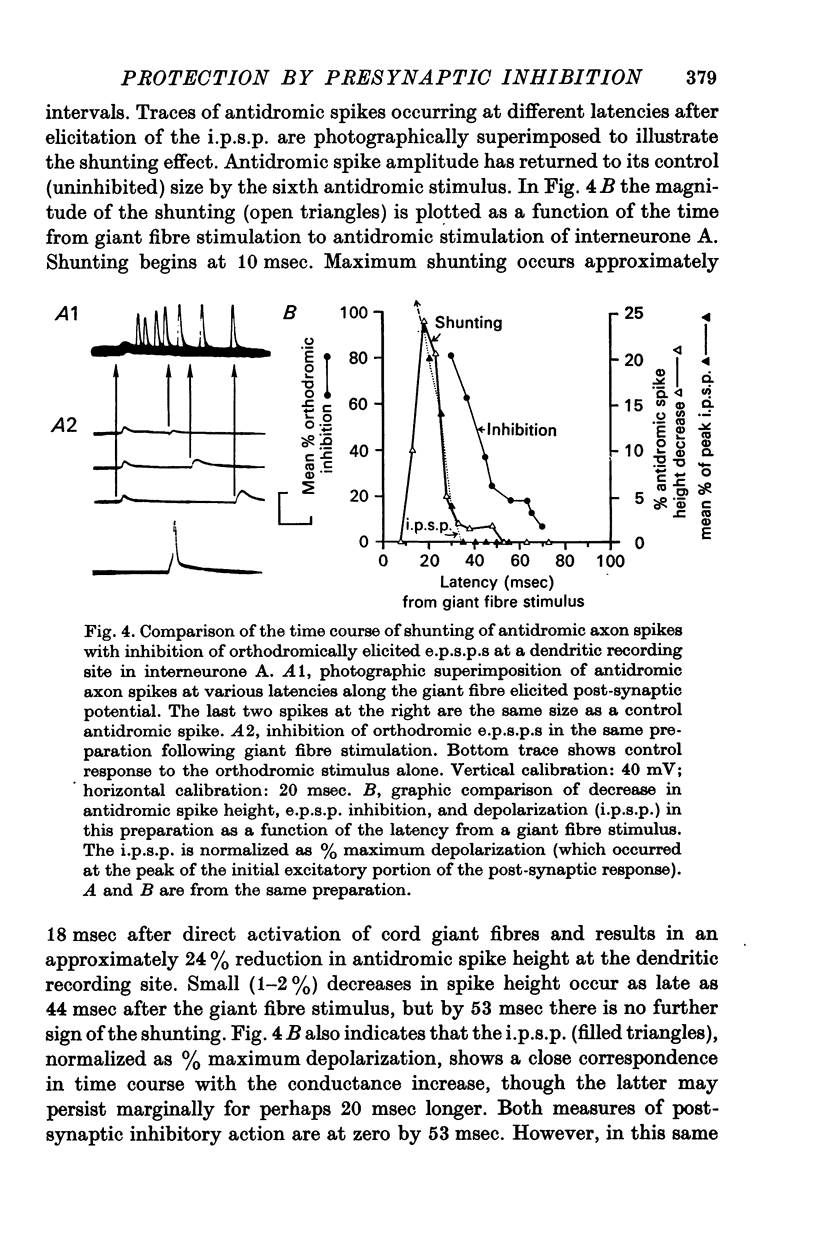
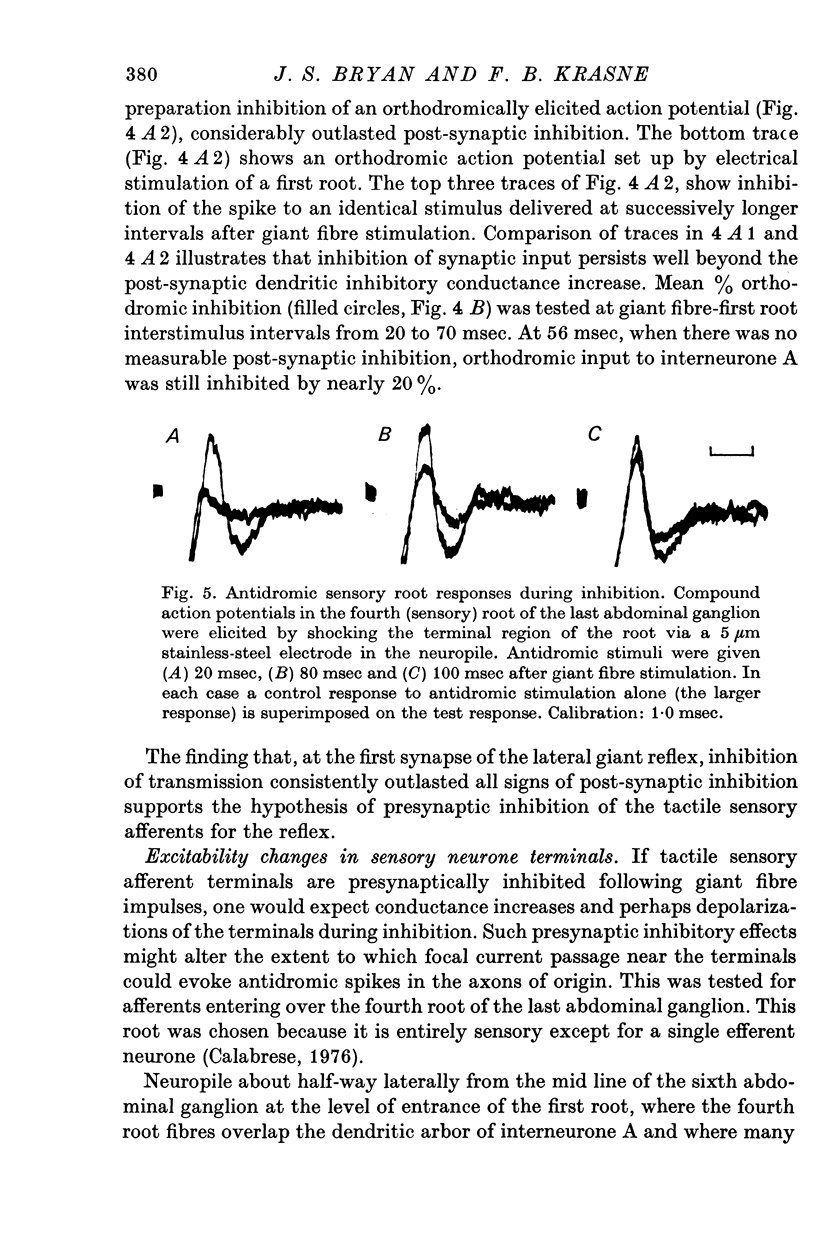
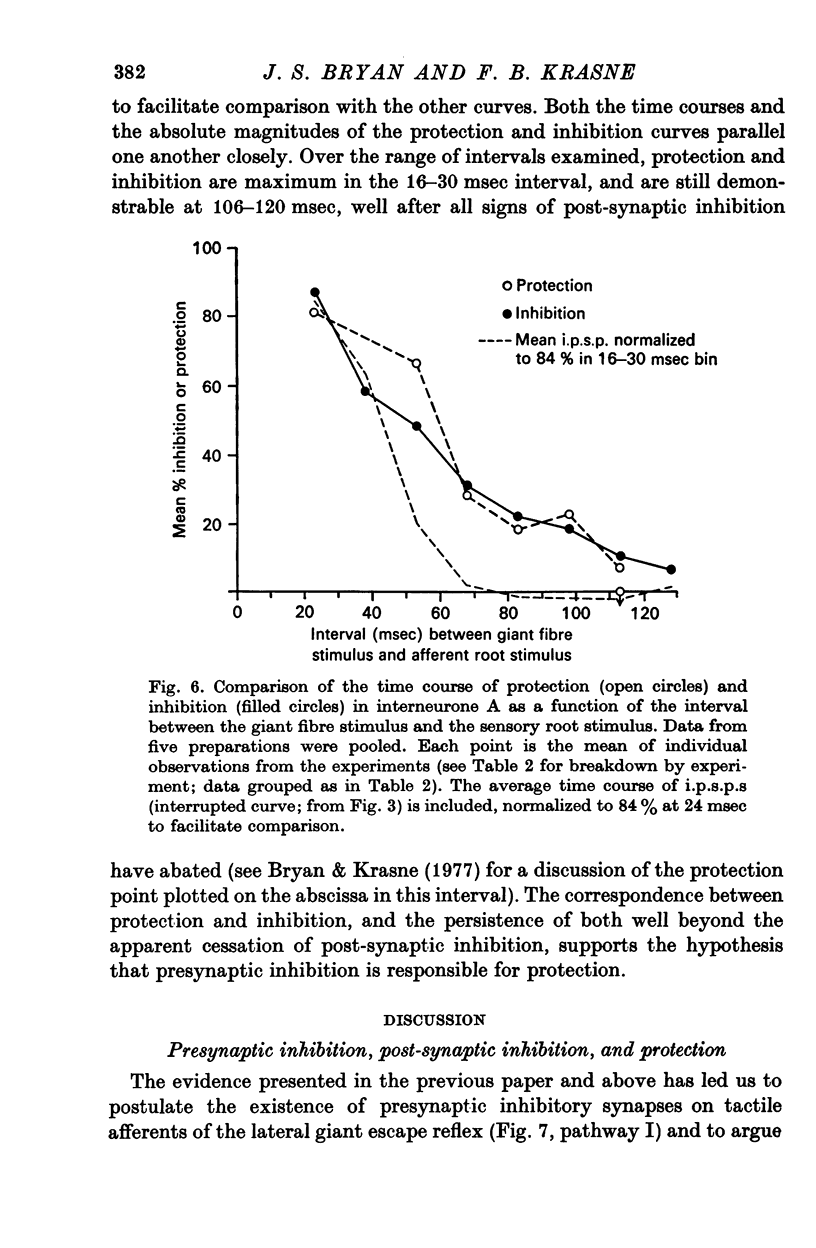
1. Mechanism of protection from habituation of the lateral giant escape reflex of the crayfish was studied. Experiments were designed to determine whether presynaptic inhibition of primary afferents for the reflex occurs following escape command neurone firing, and if so, whether it could account for protection of the first synapse from depression. 2. Synaptic transmission between afferents and interneurone A of the escape reflex is strongly inhibited following giant fibre spikes. 3. Giant fibre firing results in post-synaptic inhibition of interneurone A. However, inhibition of afferent input to interneurone A consistently outlasts both i.p.s.p.s and post-synaptic conductance increases in the neurone; the inhibition, therefore, is probably not exclusively post-synaptic. 4. Giant fibre firing results in excitability changes in sensory afferent terminals as measured by the amplitude of antidromic compound action potentials to focal electrical stimuli applied in the region of afferent terminals in the last abdominal ganglion. The time course of this effect parallels those of protection and inhibition of the first synapse. 5. The magnitude and time course of protection and inhibition of transmission to interneurone A parallel each other closely. Both processes considerably outlast measurable signs of post-synaptic inhibition. 6. We conclude that following giant fibre activity the first synapse of the lateral giant reflex is presynaptically inhibited and the presynaptic inhibition is responsible for the protection effect described in the preceding paper.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L., Bittner G. D. Matching of excitatory and inhibitory inputs to crustacean muscle fibers. J Neurophysiol. 1971 Jan;34(1):157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- Benitez L. D., Eldredge D. H., Templer J. W. Temporary threshold shifts in chinchilla: electrophysiological correlates. J Acoust Soc Am. 1972 Oct;52(4):1115–1123. doi: 10.1121/1.1913222. [DOI] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J., Dkennedy D. Habituation: occurrence at a neuromuscular junction. Science. 1970 Jul 3;169(3940):92–94. doi: 10.1126/science.169.3940.92. [DOI] [PubMed] [Google Scholar]
- Bryan J. S., Krasne F. B. Protection from habituation of the crayfish lateral giant fibre escape response. J Physiol. 1977 Oct;271(2):351–368. doi: 10.1113/jphysiol.1977.sp012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese R. L., Kennedy D. Multiple sites of spike initiation in a single dendritic system. Brain Res. 1974 Dec 27;82(2):316–321. doi: 10.1016/0006-8993(74)90612-x. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel P. B., Glanzman D. L., Thompson R. F. Habituation of a monosynaptic response in vertebrate central nervous system: lateral column-motoneuron pathway in isolated frog spinal cord. J Neurophysiol. 1973 Nov;36(6):1117–1130. doi: 10.1152/jn.1973.36.6.1117. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Ishii Y. Neurophysiological studies on hearing in goldfish. J Neurophysiol. 1967 Nov;30(6):1377–1403. doi: 10.1152/jn.1967.30.6.1377. [DOI] [PubMed] [Google Scholar]
- Green D. G., Dowling J. E., Siegel I. M., Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol. 1975 Apr;65(4):483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S. Synaptic potential in the motor giant axon of the crayfish. J Gen Physiol. 1958 Jul 20;41(6):1119–1128. doi: 10.1085/jgp.41.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi S. S., Atwood H. L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 1974 Nov;63(2 Pt 1):599–613. doi: 10.1083/jcb.63.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D., Calabrese R. L., Wine J. J. Presynaptic inhibition: primary afferent depolarization in crayfish neurons. Science. 1974 Nov 1;186(4162):451–454. doi: 10.1126/science.186.4162.451. [DOI] [PubMed] [Google Scholar]
- Kennedy D. Crayfish interneurons. Physiologist. 1971 Feb;14(1):5–30. [PubMed] [Google Scholar]
- Klinke R., Galley N. Efferent innervation of vestibular and auditory receptors. Physiol Rev. 1974 Apr;54(2):316–357. doi: 10.1152/physrev.1974.54.2.316. [DOI] [PubMed] [Google Scholar]
- Krasne F. B., Bryan J. S. Habituation: regulation through presynaptic inhibition. Science. 1973 Nov 9;182(4112):590–592. doi: 10.1126/science.182.4112.590. [DOI] [PubMed] [Google Scholar]
- Krasne F. B. Excitation and habituation of the crayfish escape reflex: the depolarizing response in lateral giant fibres of the isolated abdomen. J Exp Biol. 1969 Feb;50(1):29–46. doi: 10.1242/jeb.50.1.29. [DOI] [PubMed] [Google Scholar]
- Marmont G., Wiersma C. A. On the mechanism of inhibition and excitation of crayfish muscle. J Physiol. 1938 Aug 15;93(3):173–193. doi: 10.1113/jphysiol.1938.sp003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mittenthal J. E., Wine J. J. Connectivity patterns of crayfish giant interneurons: visualization of synaptic regions with cobalt dye. Science. 1973 Jan 12;179(4069):182–184. doi: 10.1126/science.179.4069.182. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Roberts A. Recurrent inhibition in the giant-fibre system of the crayfish and its effect on the excitability of the escape response. J Exp Biol. 1968 Jun;48(3):545–567. doi: 10.1242/jeb.48.3.545. [DOI] [PubMed] [Google Scholar]
- Roberts B. L., Russell I. J. The activity of lateral-line efferent neurones in stationary and swimming dogfish. J Exp Biol. 1972 Oct;57(2):435–448. doi: 10.1242/jeb.57.2.435. [DOI] [PubMed] [Google Scholar]
- Russell I. J. The role of the lateral-line efferent system in Xenopus laevis. J Exp Biol. 1971 Jun;54(3):621–641. doi: 10.1242/jeb.54.3.621. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Kennedy D. Structure and function of identified nerve cells in the crayfish. Endeavour. 1969 Sep;28(105):107–113. [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C. L. Relative contribution of synaptic and non-synaptic influences to response decrements in a post-synaptic neurone. J Exp Biol. 1973 Oct;59(2):315–321. doi: 10.1242/jeb.59.2.315. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine J. J. Crayfish neurons with electrogenic cell bodies: correlations with function and dendritic properties. Brain Res. 1975 Feb 21;85(1):92–98. doi: 10.1016/0006-8993(75)91011-2. [DOI] [PubMed] [Google Scholar]
- Woodson P. B., Schlapfer W. T., Tremblay J. P., Barondes S. H. Resting and stimulated values of model parameters governing transmitter release at a synapse in Aplysia californica. Brain Res. 1976 Jun 4;109(1):21–40. doi: 10.1016/0006-8993(76)90378-4. [DOI] [PubMed] [Google Scholar]
- Woodson P. B., Tremblay J. P., Schlapfer W. T., Barondes S. H. Heterosynaptic inhibition modifies the presynaptic plasticities of the transmission process at the synapse in Aplysia californica. Brain Res. 1976 Jun 4;109(1):83–95. doi: 10.1016/0006-8993(76)90381-4. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. 3. Electrical junctions and dendrite spikes in fast flexor motoneurons. J Neurophysiol. 1972 Sep;35(5):638–651. doi: 10.1152/jn.1972.35.5.638. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Kennedy D., Selverston A. I. Neuronal circuit mediating escape responses in crayfish. Science. 1971 Aug 13;173(3997):645–650. doi: 10.1126/science.173.3997.645. [DOI] [PubMed] [Google Scholar]


