Abstract
1. The light-induced electrical responses of barnacle photoreceptors spread decrementally along the cells' axons. The decay of the depolarizing and hyperpolarizing components of the visual signal was studied by recording intracellularly from single receptor axons of the median ocellus of the giant barnacle.
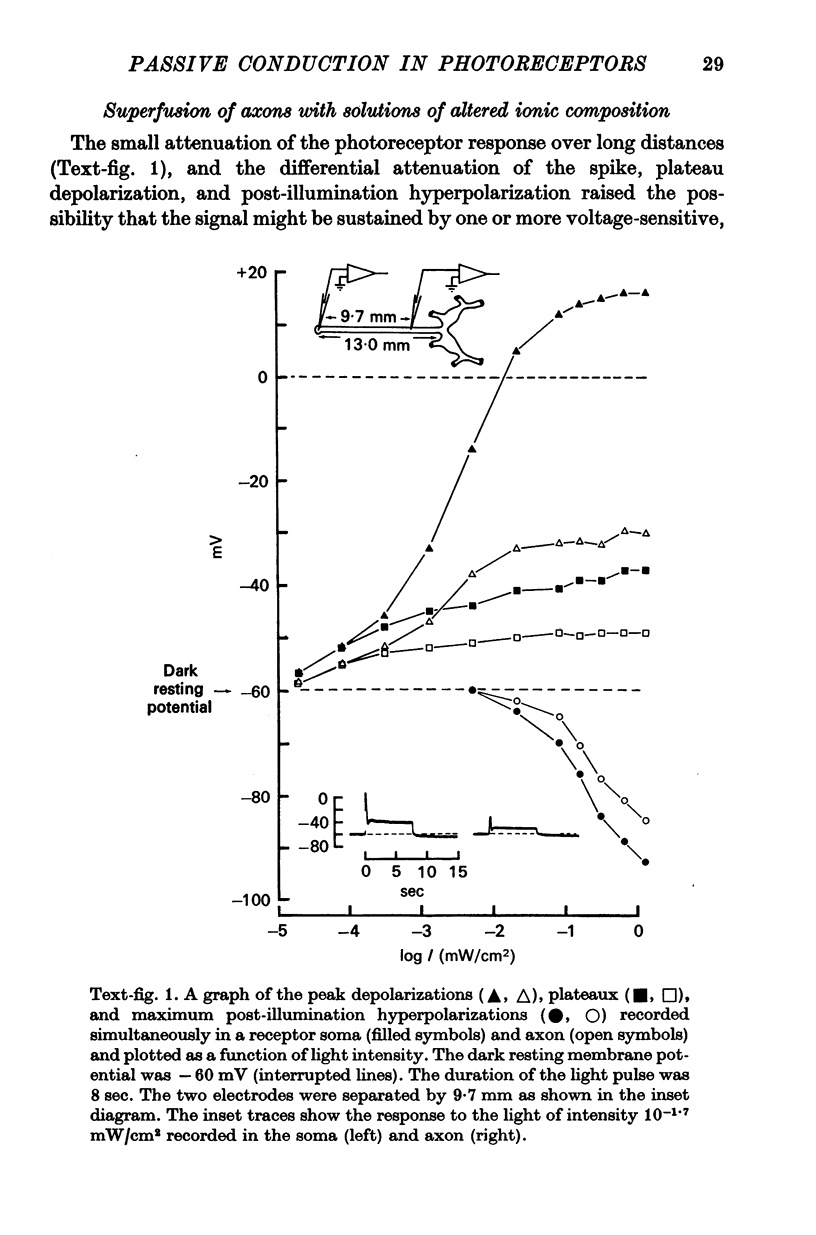
2. The resistance of the photoreceptor neurone decreases markedly when the cell is depolarized with respect to its dark resting potential of -60 mV. This rectification results in differential attenuation of the depolarizing and hyperpolarizing components of the visual signal as they spread down the axon. Consequently, the visual signal entering the synaptic region is conspicuously distorted.
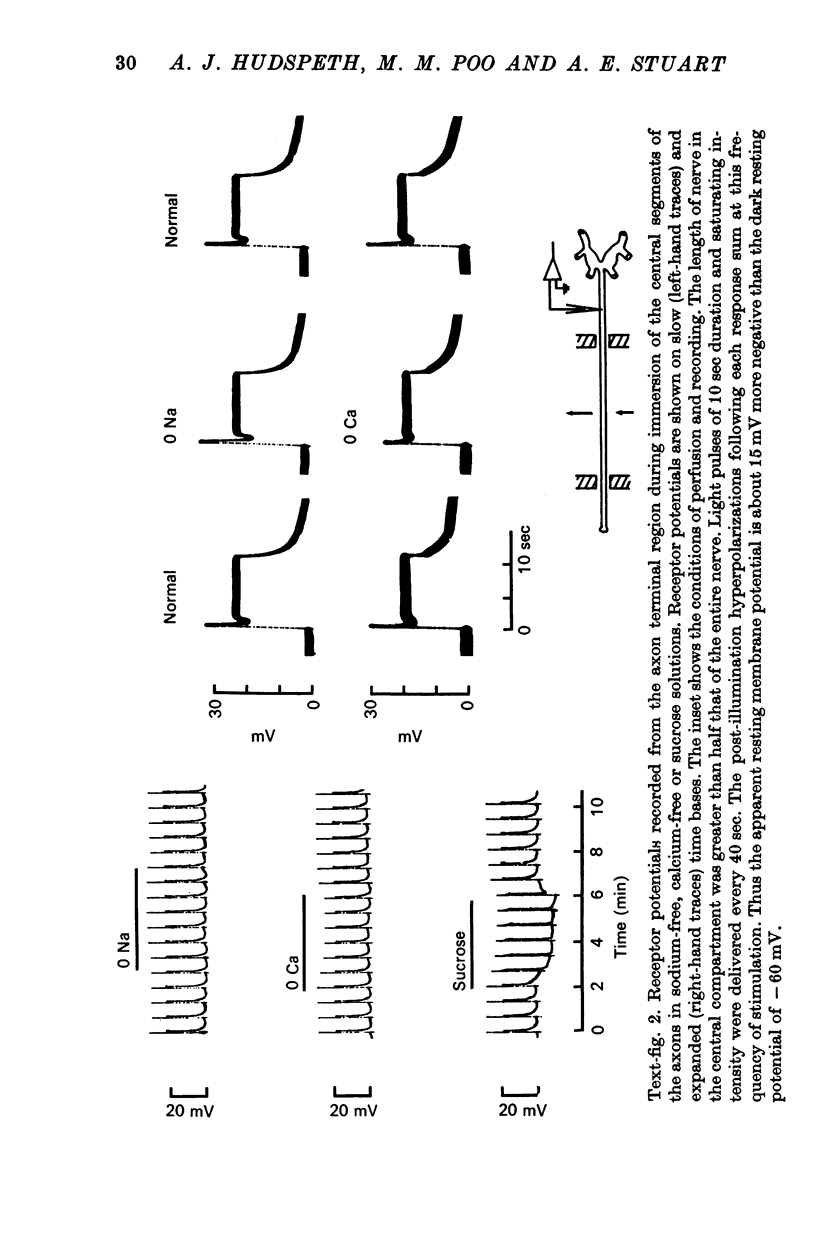
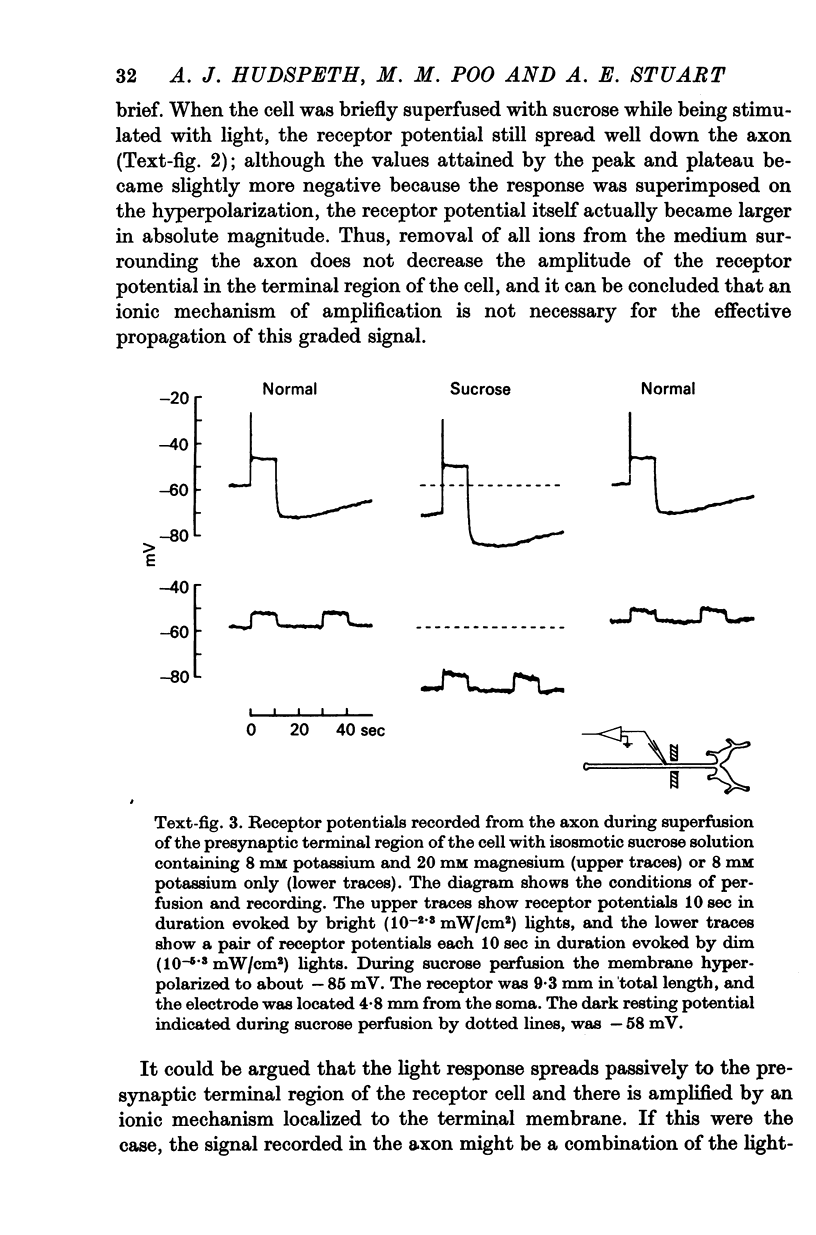
3. Bathing the photoreceptor axons in sodium-free or calcium-free saline or in isotonic sucrose does not significantly affect the spread of the visual signal to the terminals. Thus the signal is not amplified by an ionic mechanism along the axon.
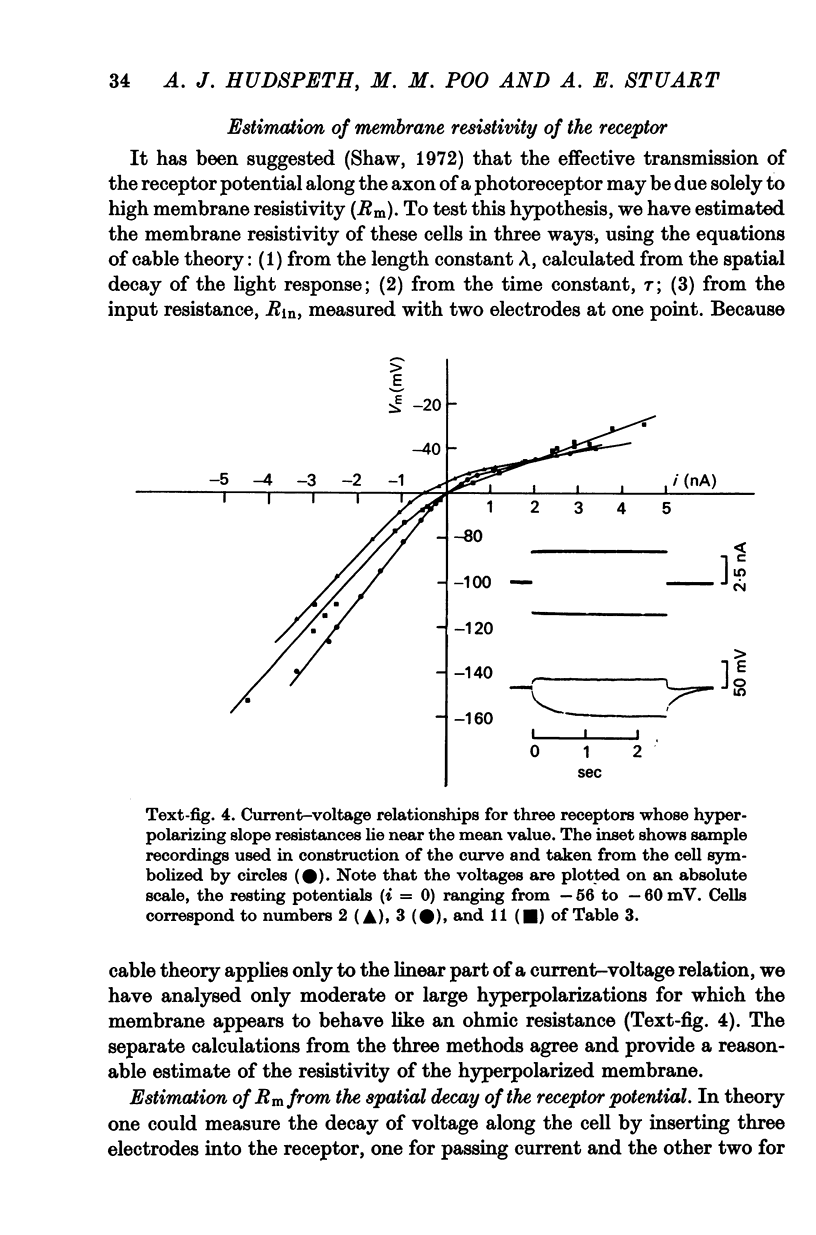
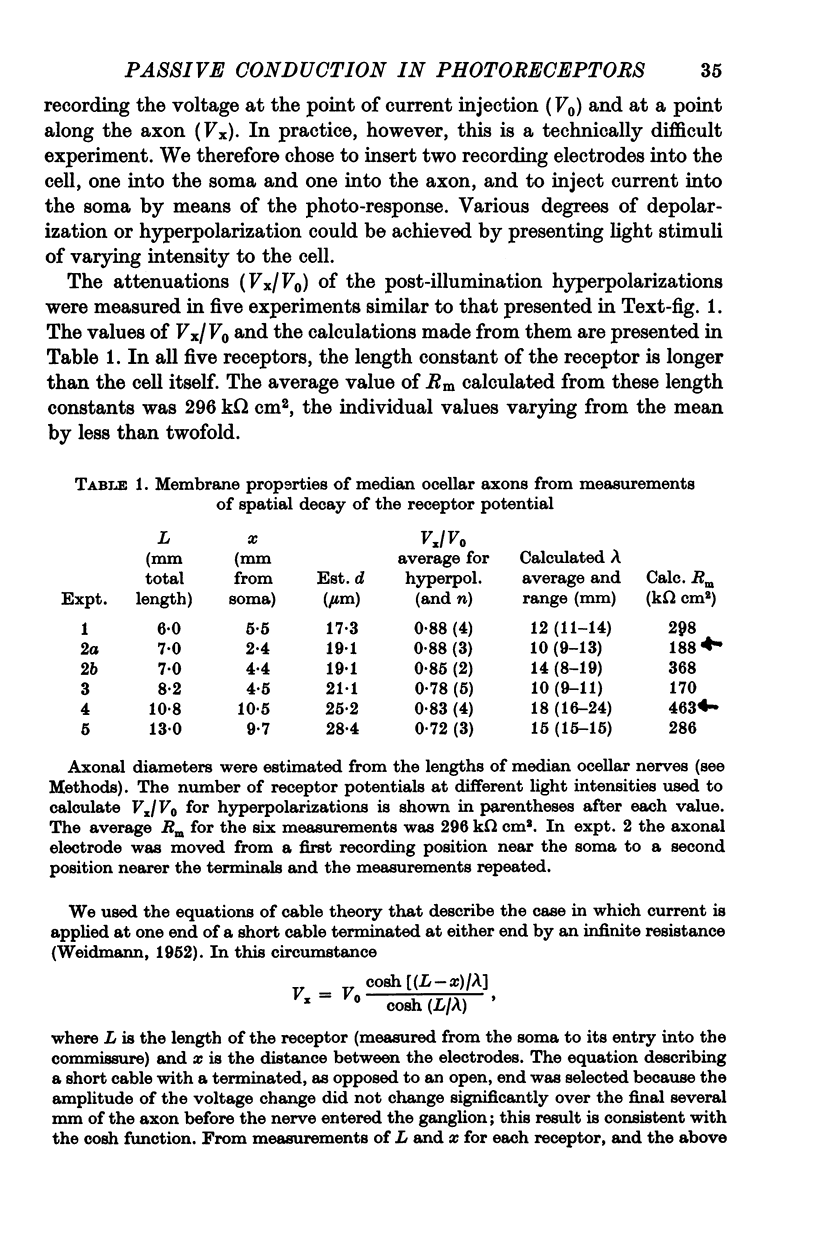
4. Membrane characteristics of the photoreceptor for hyperpolarizing voltage changes were estimated from (a) the ratio of the amplitudes of the visual signals recorded simultaneously in the axon and in the soma, (b) the time constant, and (c) the input resistance of the cell. All three independent measurements are consistent with a length constant 1 to 2 times the total length of the cell (λ = 10-18 mm) and an unusually high membrane resistivity of about 300 kΩ cm2. This resistivity enables the receptor potential to spread passively to the terminal region.
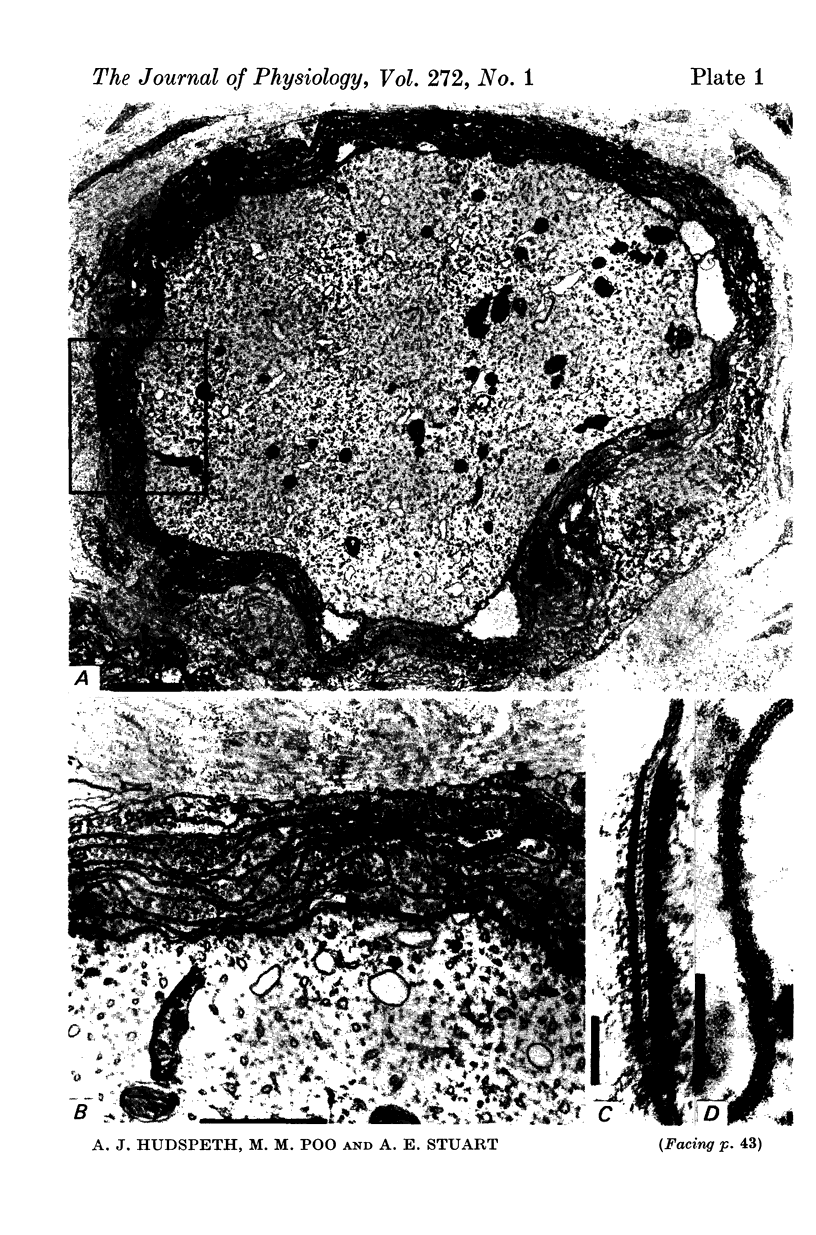
5. Electron microscopic examination of receptor axons reveals an investment of glial lamellae, but demonstrates neither unusual structures which would lead to a high apparent membrane resistivity, nor junctions between cells which would seal off the extracellular space. Thus the observed high resistivity appears to be an intrinsic property of the receptor membrane.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann F. Slow and spike potentials recorded from retinula cells of the honeybee drone in response to light. J Gen Physiol. 1968 Dec;52(6):855–875. doi: 10.1085/jgp.52.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Trinkaus J. P. Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol. 1970 Mar;44(3):592–610. doi: 10.1083/jcb.44.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. W. Electrical characteristics of a barnacle photoreceptor. Fed Proc. 1971 Jan-Feb;30(1):69–78. [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. An electrogenic sodium pump in Limulus ventral photoreceptor cells. J Gen Physiol. 1972 Jun;59(6):720–733. doi: 10.1085/jgp.59.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. I. The microanatomy. J Gen Physiol. 1969 Sep;54(3):289–309. doi: 10.1085/jgp.54.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Mirolli M. The passive electrical properties of the membrane of a molluscan neurone. J Physiol. 1972 Dec;227(1):35–49. doi: 10.1113/jphysiol.1972.sp010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Doggenweiler C. F. The fine structural organization of nerve fibers, sheaths, and glial cells in the prawn, Palaemonetes vulgaris. J Cell Biol. 1966 Aug;30(2):381–403. doi: 10.1083/jcb.30.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Stuart A. E. Morphology and responses to light of the somata, axons, and terminal regions of individual photoreceptors of the giant barnacle. J Physiol. 1977 Oct;272(1):1–23. doi: 10.1113/jphysiol.1977.sp012031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Two light-induced processes in the photoreceptor cells of Limulus ventral eye. J Gen Physiol. 1971 Nov;58(5):544–561. doi: 10.1085/jgp.58.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Gwilliam G. F. Photoreception in a barnacle: electrophysiology of the shadow reflex pathway in Balanus cariosus. Science. 1972 Aug 4;177(4047):438–441. doi: 10.1126/science.177.4047.438. [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. H. Decremental Conduction over "Giant" Afferent Processes in an Arthropod. Science. 1972 May 12;176(4035):680–682. doi: 10.1126/science.176.4035.680. [DOI] [PubMed] [Google Scholar]
- Ripley S. H., Bush B. M., Roberts A. Crab muscle receptor which responds without impulses. Nature. 1968 Jun 22;218(5147):1170–1171. doi: 10.1038/2181170a0. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Decremental conduction of the visual signal in barnacle lateral eye. J Physiol. 1972 Jan;220(1):145–175. doi: 10.1113/jphysiol.1972.sp009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEGAS G. M., VILLEGAS R. The ultrastructure of the giant nerve fibre of the squid: axon-Schwann cell relationship. J Ultrastruct Res. 1960 Jun;3:362–373. doi: 10.1016/s0022-5320(60)90015-0. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltman B. Electrical properties and fine structure of the ampullary canals of Lorenzini. Acta Physiol Scand Suppl. 1966;264:1–60. [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]