Abstract
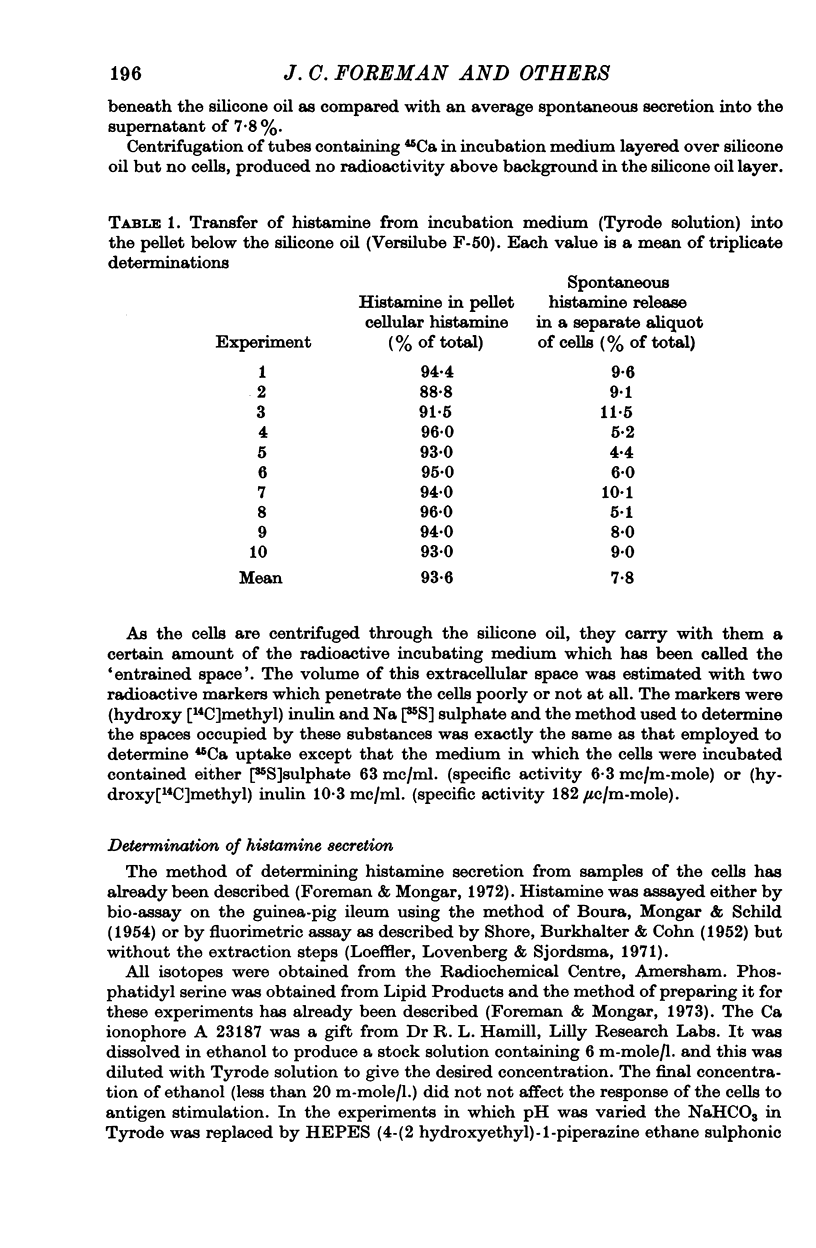
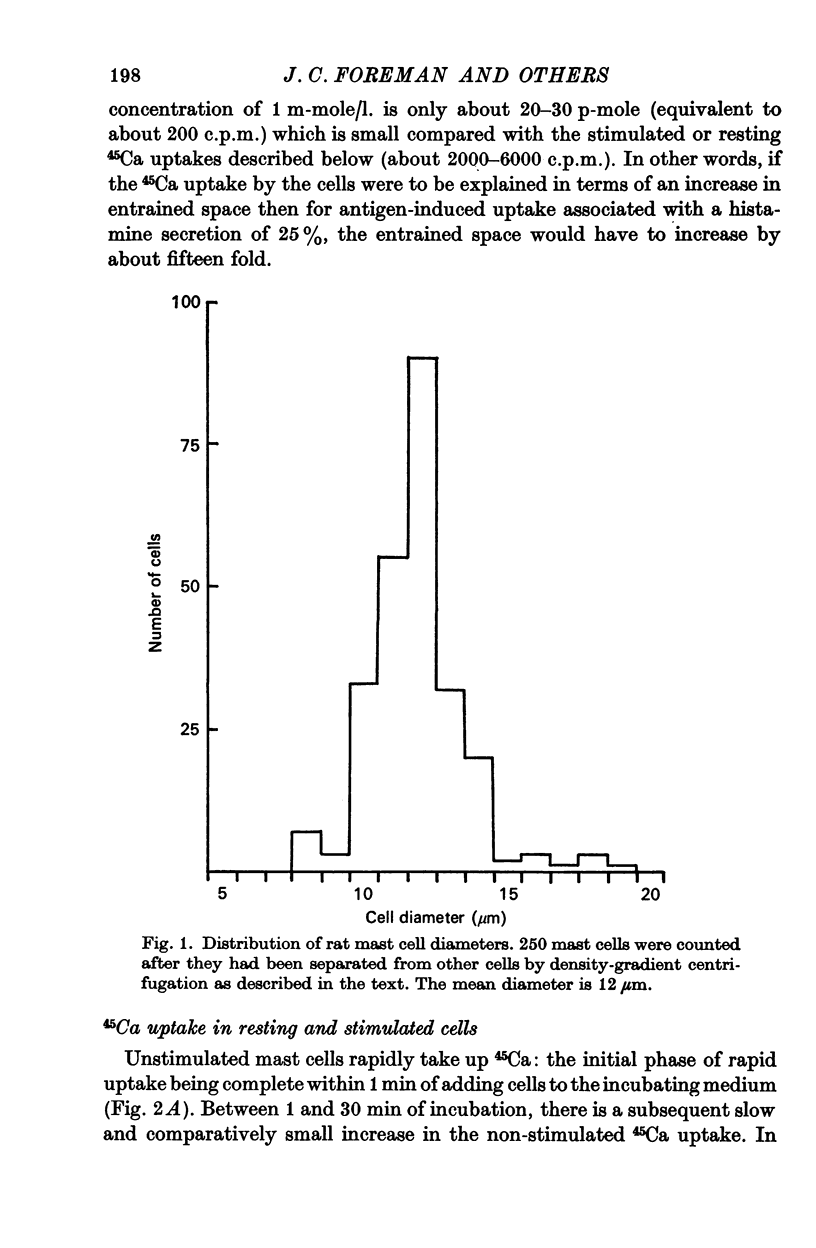
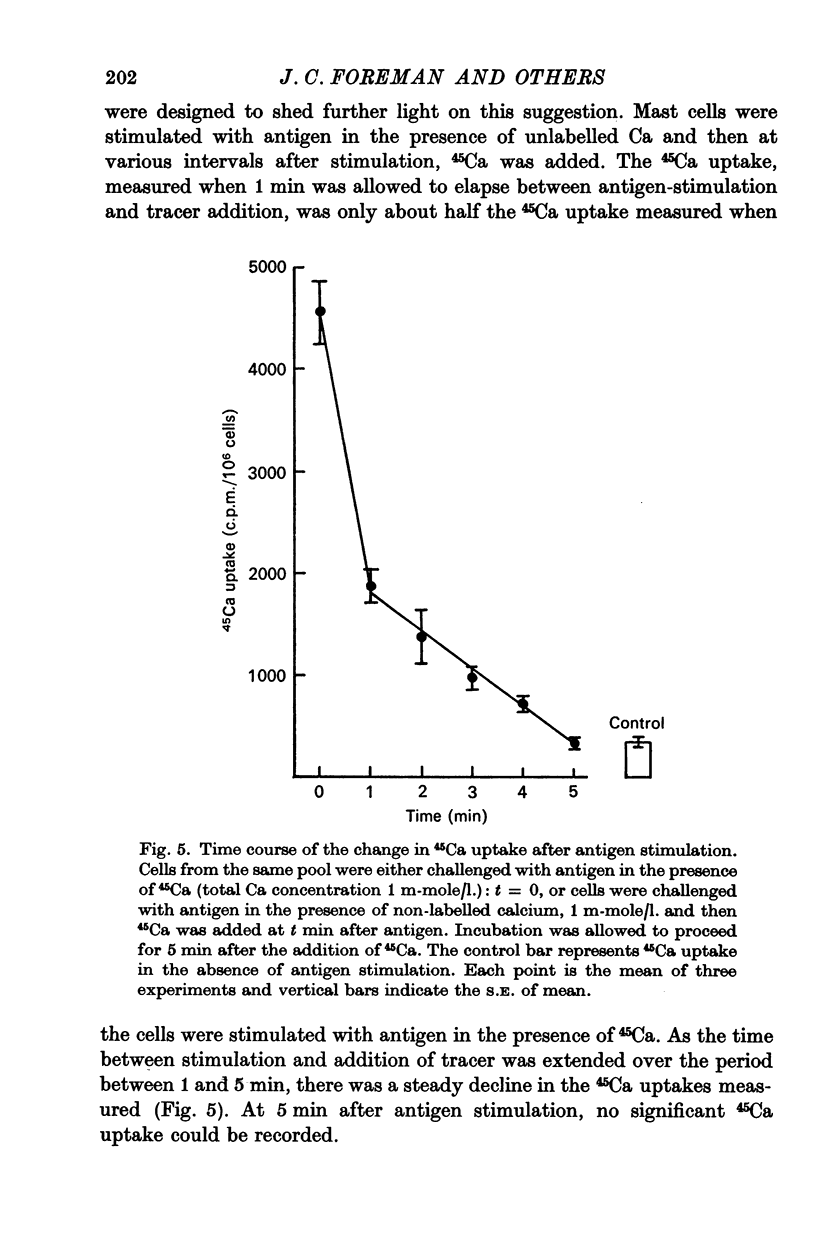
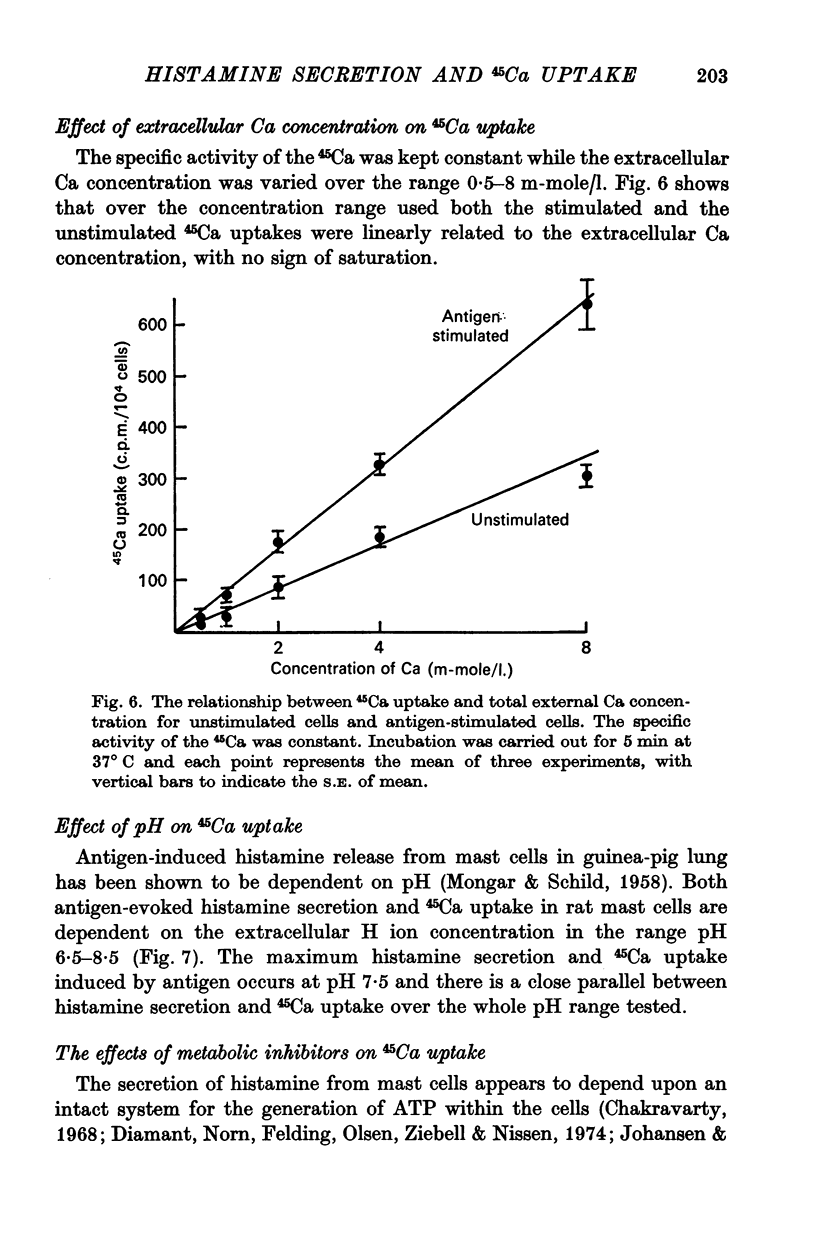
1. Unstimulated mast cells from the peritoneal cavity of the rat take up 45Ca: the initial phase of rapid uptake being complete after 1 min incubation of the cells with the isotope. Stimulation of the mast cells with an antigen-antibody reaction, dextran or concanavalin A induces an increase in the uptake of 45Ca which is accompanied by a release of granular material: this increase in 45Ca uptake is also complete in 1 min. The majority of the stimulated 45Ca uptake cannot be explained in terms of binding of Ca to released granular material, or to an enlargement in either the extracellular compartment or the cell surface area.
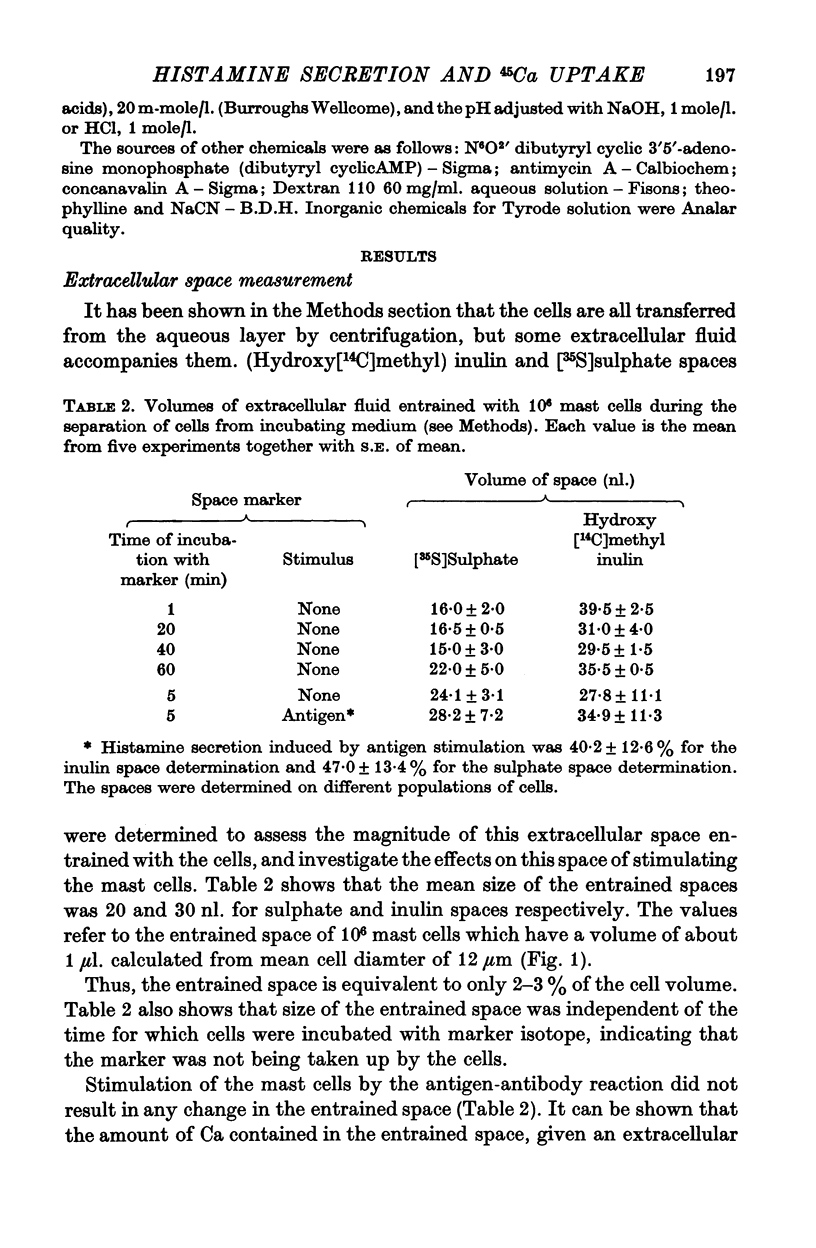
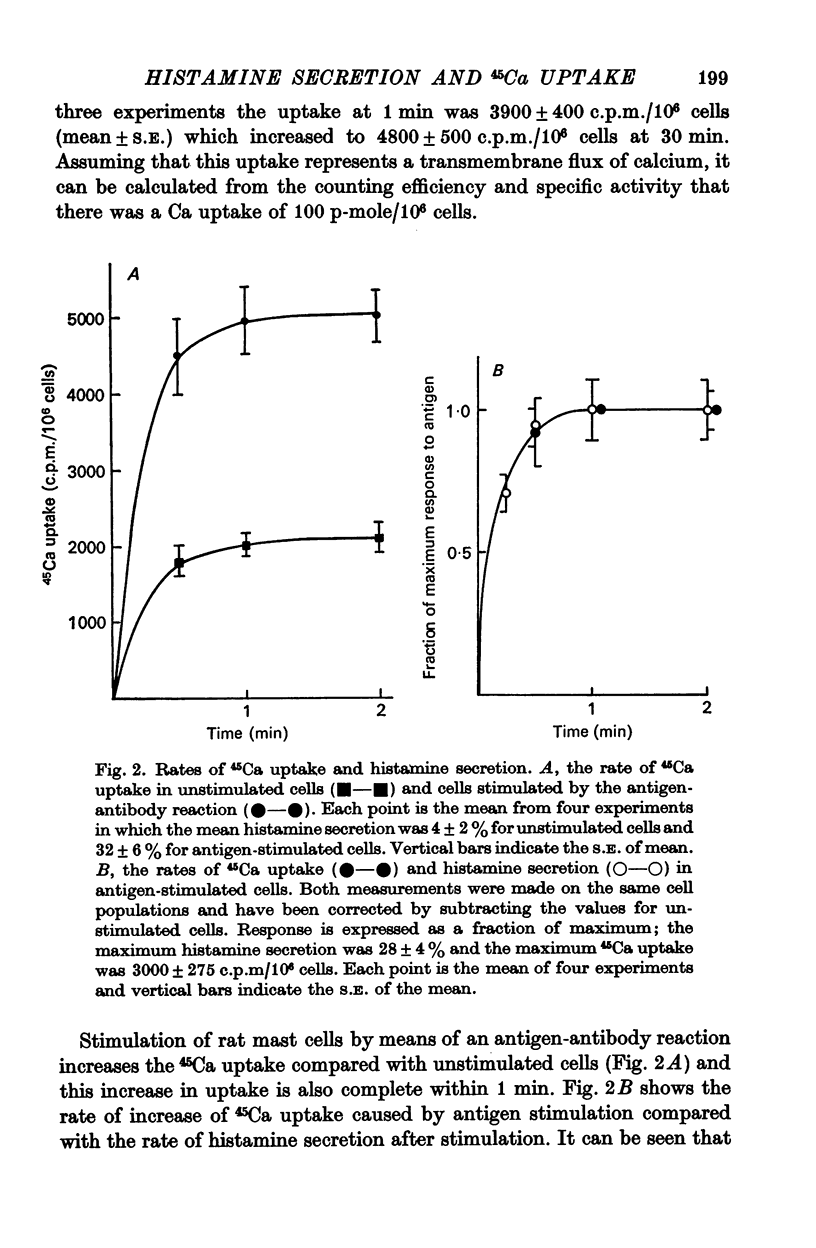
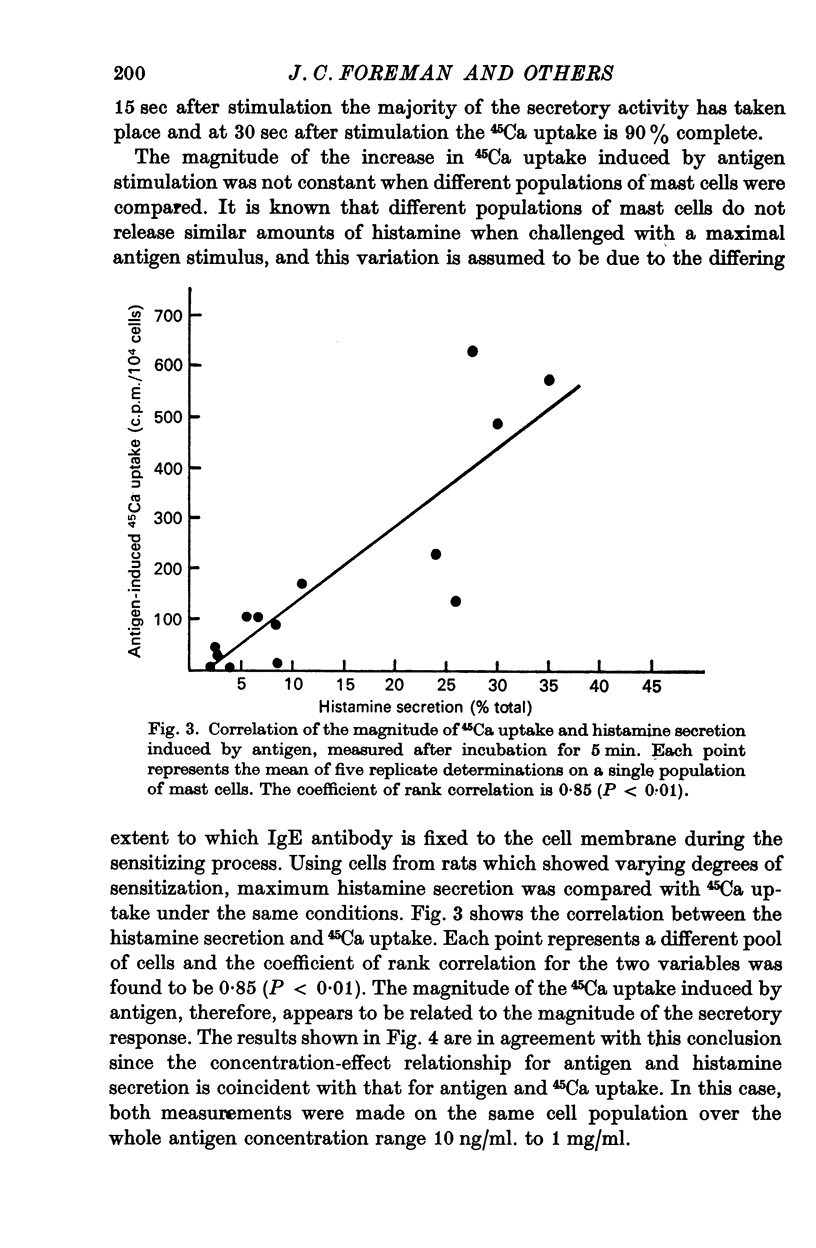
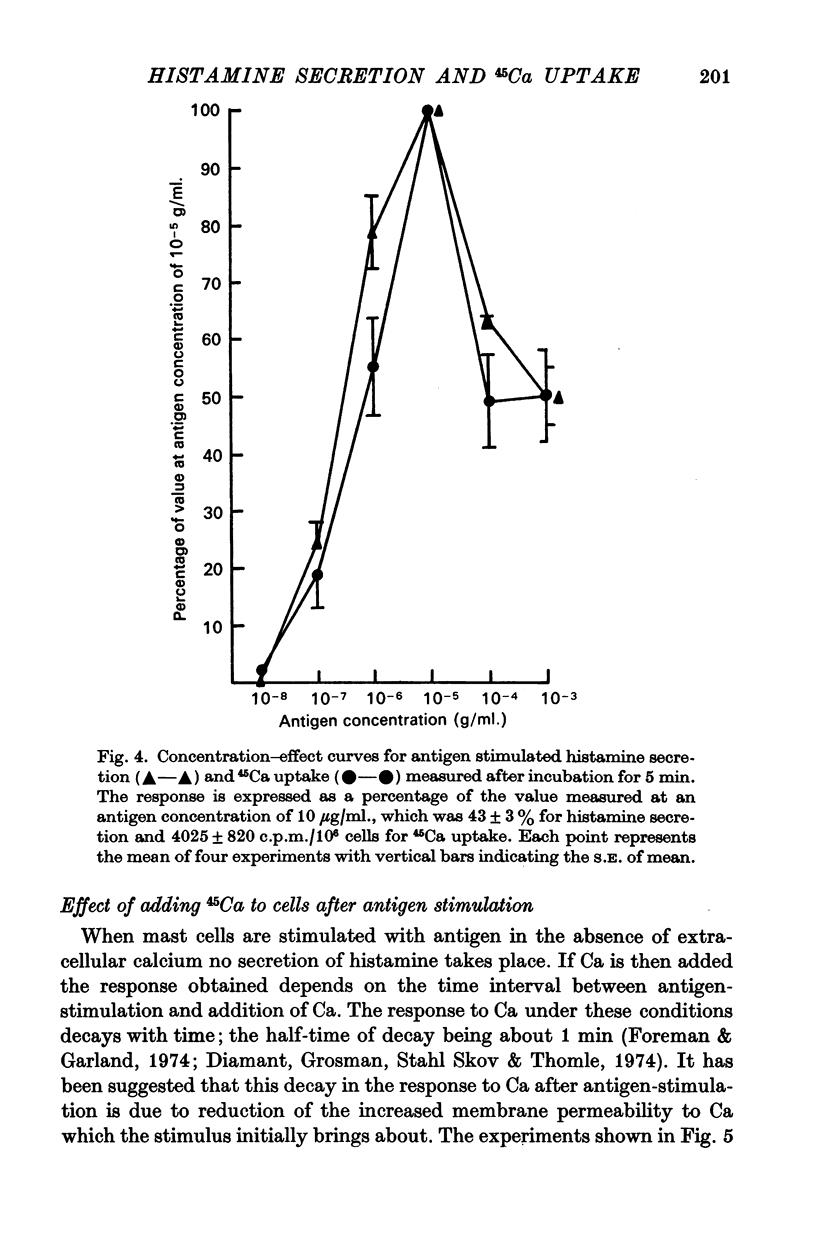
2. The magnitude of the increase in 45Ca uptake caused by stimulating the mast cells increases when the degree of histamine secretion increases.
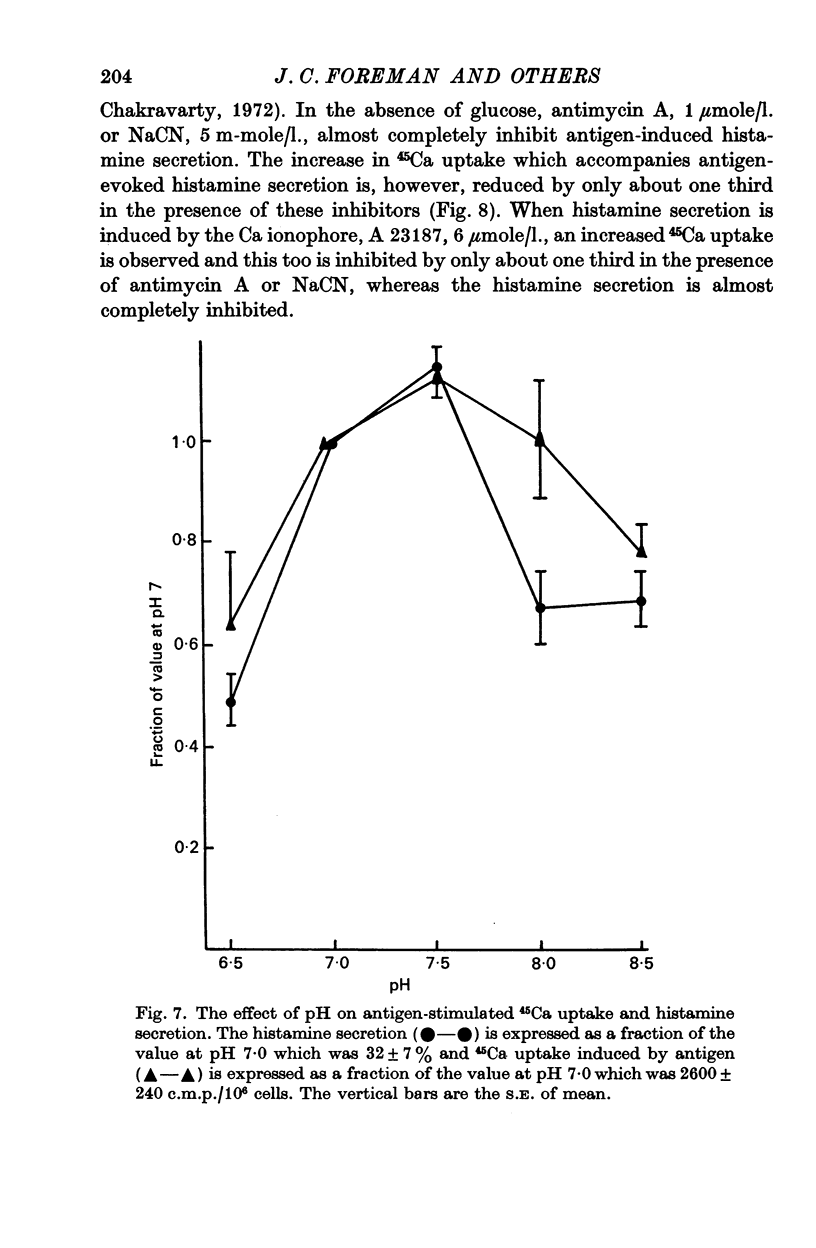
3. The increased 45Ca uptake induced by stimulation of the mast cells and the degree of histamine secretion are both dependent on extracellular H ion concentration. Changes of pH cause similar changes in 45Ca uptake and secretion with maxima at pH 7.5.
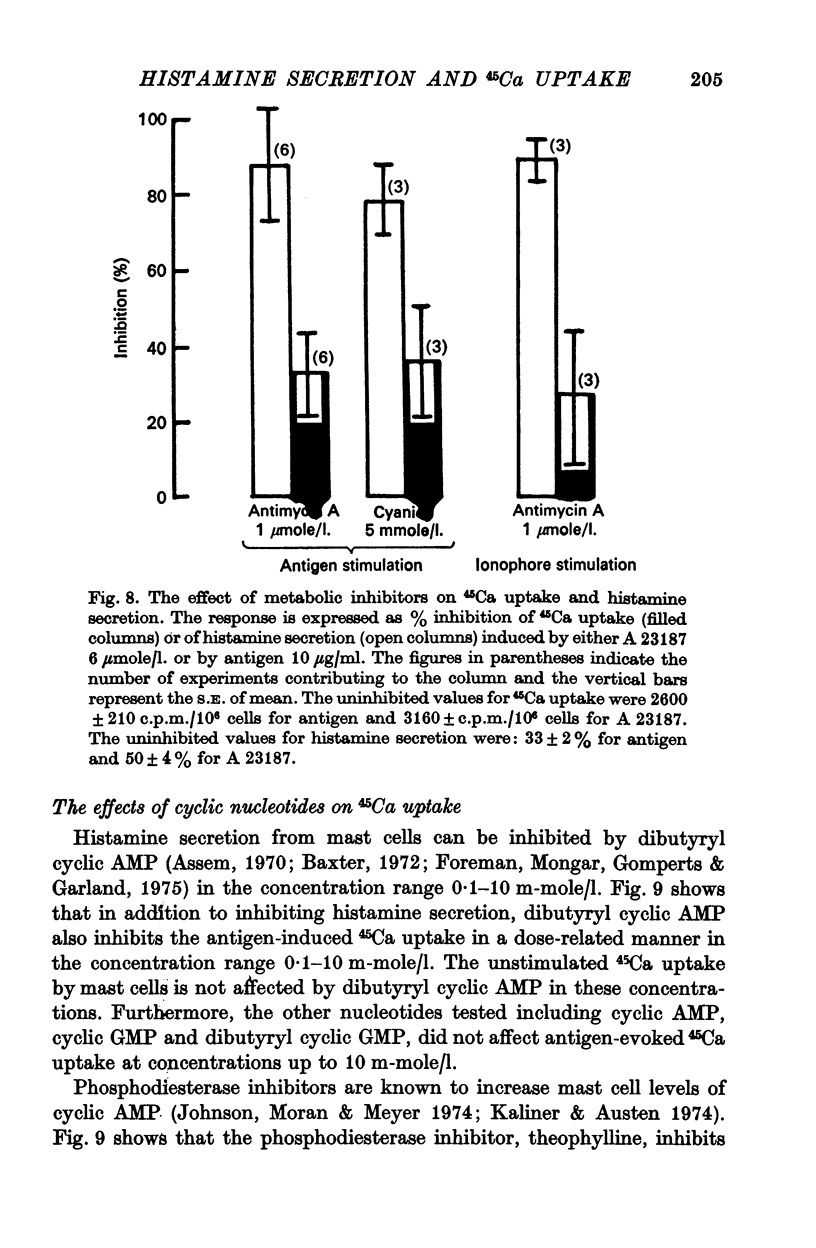
4. Two thirds of the 45Ca uptake induced by an antigen-antibody reaction or by the Ca ionophore A 23187 is unaffected by inhibiting glycolysis and oxidative phosphorylation. Histamine secretion on the other hand is practically abolished by this metabolic inhibition. Thus, 45Ca uptake proceeds in the absence of the discharge of granules.
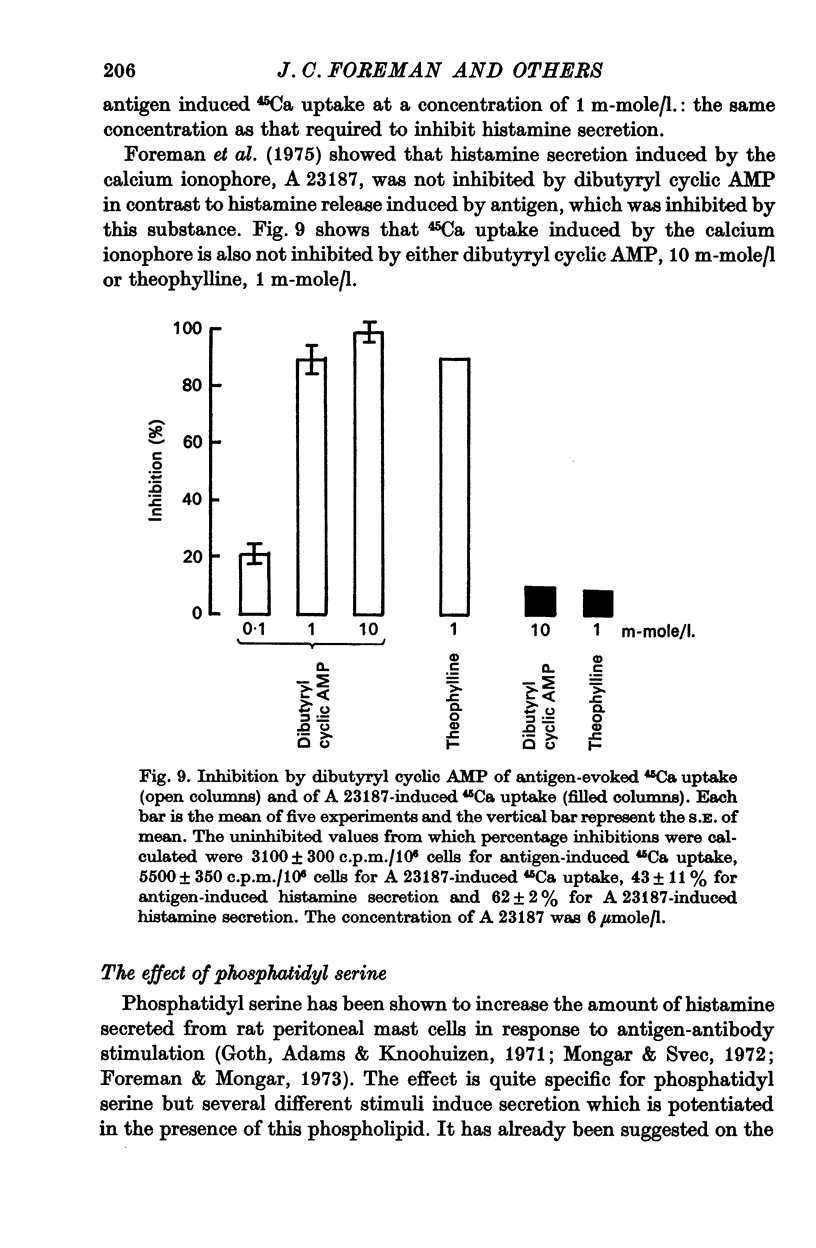
5. Dibutyryl cyclic AMP or theophylline inhibit both the increase in 45Ca uptake and the histamine secretion caused by stimulating mast cells with an antigen-antibody reaction. Cyclic AMP, cyclic GMP and dibutyryl cyclic GMP have no effect on uptake or secretion.
6. The Ca ionophore, A 23187, induces uptake of 45Ca and histamine secretion, neither effect being inhibited by either dibutyryl cyclic AMP or theophylline.
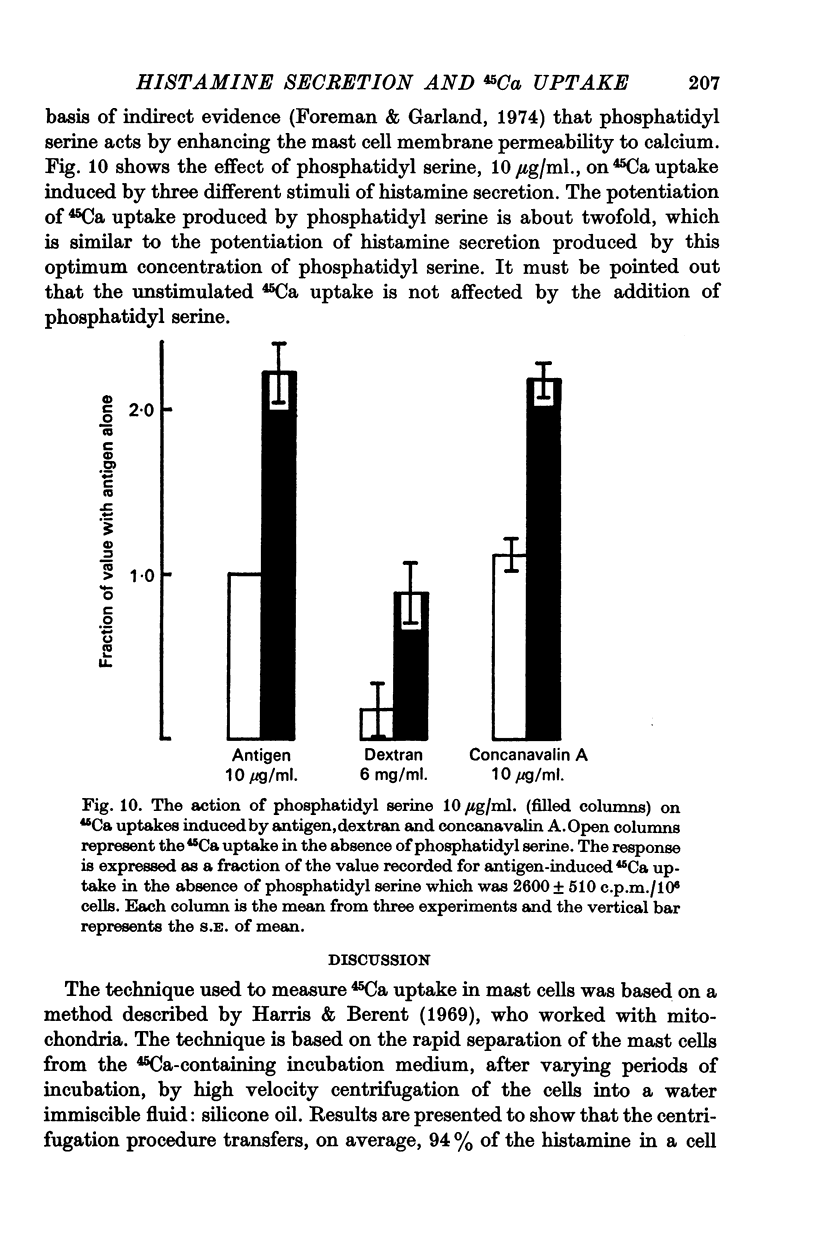
7. Phosphatidyl serine increases both 45Ca uptake and the histamine release induced by an antigen-antibody reaction, dextran or concanavalin A.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURA A., MONGAR J. L., SCHILD H. O. Improved automatic apparatus for pharmacological assays on isolated preparations. Br J Pharmacol Chemother. 1954 Mar;9(1):24–30. doi: 10.1111/j.1476-5381.1954.tb00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. H. Histamine release from rat mast cells by dextran: effects of adrenergic agents, theophylline and other drugs. Proc Soc Exp Biol Med. 1972 Nov;141(2):576–581. doi: 10.3181/00379727-141-36826. [DOI] [PubMed] [Google Scholar]
- Bergendorff A., Uvnäs B. Storage properties of rat mast cell granules in vitro. Acta Physiol Scand. 1973 Feb;87(2):213–222. doi: 10.1111/j.1748-1716.1973.tb05383.x. [DOI] [PubMed] [Google Scholar]
- CHAKRAVARTY N. The mechanism of histamine release in anaphylactic reaction in guinea pig and rat. Acta Physiol Scand. 1960 Mar 18;48:146–166. doi: 10.1111/j.1748-1716.1960.tb01854.x. [DOI] [PubMed] [Google Scholar]
- Chakravarty N. Further observations on the inhibition of histamine release by 2-deoxyglucose. Acta Physiol Scand. 1968 Apr;72(4):425–432. doi: 10.1111/j.1748-1716.1968.tb03867.x. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Stanworth D. R. Characterization of calcium-ion-activated adenosine triphosphatase in the plasma membrane of rat mast cells. Biochem J. 1976 Jun 15;156(3):691–700. doi: 10.1042/bj1560691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B., Grosman N., Stahl Skov P., Thomle S. Effect of divalent cations and metabolic energy on the anaphylactic histamine release from rat peritoneal mast cells. Int Arch Allergy Appl Immunol. 1974;47(3):412–424. doi: 10.1159/000231234. [DOI] [PubMed] [Google Scholar]
- Diamant B., Norn S., Felding P., Olsen N., Ziebell A., Nissen J. ATP level and CO2 production of mast cells in anaphylaxis. Int Arch Allergy Appl Immunol. 1974;47(6):894–908. doi: 10.1159/000231280. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster L. J., Copenhaver J. H., Jr Phosphatidyl serine requirement of (Na+-K+)-activated adenosine triphosphatase from rat kidney and brain. Biochim Biophys Acta. 1967 Apr 4;137(2):406–408. doi: 10.1016/0005-2760(67)90120-8. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Garland L. G. Desensitization in the process of histamine secretion induced by antigen and dextran. J Physiol. 1974 Jun;239(2):381–391. doi: 10.1113/jphysiol.1974.sp010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Proceedings: 45-Calcium uptake in rat peritoneal mast cells. Br J Pharmacol. 1975 Oct;55(2):283P–284P. [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D., Garland L. G. A possible role for cyclic AMP in the regulation of histamine secretion and the action of cromoglycate. Biochem Pharmacol. 1975 Feb 15;24(4):538–540. doi: 10.1016/0006-2952(75)90142-2. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The interaction of calcium and strontium with phosphatidyl serine in the anaphylactic secretion of histamine. J Physiol. 1973 Apr;230(2):493–507. doi: 10.1113/jphysiol.1973.sp010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland L. G., Mongar J. L. Differential histamine release by dextran and the ionophore A23187: the actions of inhibitors. Int Arch Allergy Appl Immunol. 1976;50(1):27–42. doi: 10.1159/000231477. [DOI] [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- Gushchin I. S., Orlov S. M., Tsziu N. L. Membrannyi potentsial tuchnykh kletok i vysvobozhdenie iz nikh gistamina. Biull Eksp Biol Med. 1973 Nov;76(11):20–23. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Berent C. Calcium ion-induced uptakes and transormations of substrates in liver mitochondria. Biochem J. 1969 Dec;115(4):645–652. doi: 10.1042/bj1150645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander H. F., Bloom G. D. Quantitative analysis of mast cell structure. J Microsc. 1974 Apr;100(3):315–321. doi: 10.1111/j.1365-2818.1974.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Johansen T., Chakravarty N. Dependence of histamine release from rat mast cells on adenosine triphosphate. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(4):457–463. doi: 10.1007/BF00501133. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Moran N. C., Mayer S. E. Cyclic AMP content and histamine release in rat mast cells. J Immunol. 1974 Feb;112(2):511–519. [PubMed] [Google Scholar]
- Johnson A. R., Moran N. C. Selective release of histamine from rat mast cells by compound 48-80 and anitgen. Am J Physiol. 1969 Mar;216(3):453–459. doi: 10.1152/ajplegacy.1969.216.3.453. [DOI] [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J Immunol. 1974 Feb;112(2):664–674. [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Loeffler L. J., Lovenberg W., Sjoerdsma A. Effects of dibutyryl-3',5'-cyclic adenosine monophosphage, phosphodiesterase inhibitors and prostaglandin E1 on compound 48-80-induced histamine release from rat peritoneal mast cells in vitro. Biochem Pharmacol. 1971 Sep;20(9):2287–2297. doi: 10.1016/0006-2952(71)90228-0. [DOI] [PubMed] [Google Scholar]
- MOORE J. E., 3rd, JAMES G. W., 3rd A simple direct method for absolute basophil leucocyte count. Proc Soc Exp Biol Med. 1953 Apr;82(4):601–603. doi: 10.3181/00379727-82-20190. [DOI] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Mongar J. L., Svec P. The effect of phospholipids on anaphylactic histamine release. Br J Pharmacol. 1972 Dec;46(4):741–752. doi: 10.1111/j.1476-5381.1972.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J. Evidence for calcium inactivation during hormone release in the rat neurohypophysis. J Exp Biol. 1976 Dec;65(3):669–683. doi: 10.1242/jeb.65.3.669. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Spataro A. C., Bosmann H. B. Mechanism of action of disodium cromoglycate--mast cell calcium ion influx after a histamine-releasing stimulus. Biochem Pharmacol. 1976 Mar 1;25(5):505–510. doi: 10.1016/0006-2952(76)90378-6. [DOI] [PubMed] [Google Scholar]
- UVNAS B., THON I. L. Evidence for enzymatic histamine release from isolated rat mast cells. Exp Cell Res. 1961 Feb;23:45–57. doi: 10.1016/0014-4827(61)90062-3. [DOI] [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol. 1970 Apr;207(2):303–328. doi: 10.1113/jphysiol.1970.sp009063. [DOI] [PMC free article] [PubMed] [Google Scholar]


