Abstract
1. A study has been made of the effects of changing the external calcium concentration, [Ca]o, and the temperature on both the number of quanta available for release by the nerve impulse (n) as well as the increase in release probability of a quantum p(t) during the release period (from 0 to T) following a nerve impulse at synapses in amphibian striated muscle.
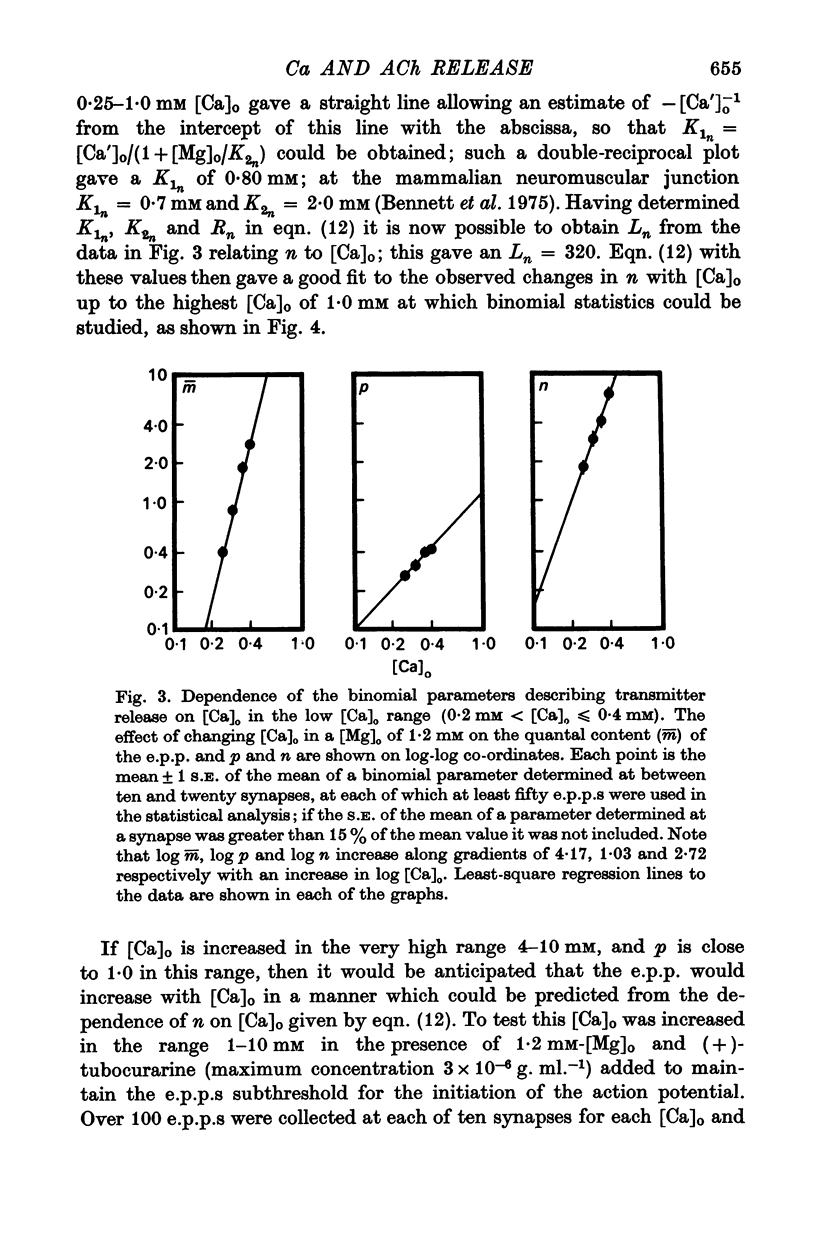
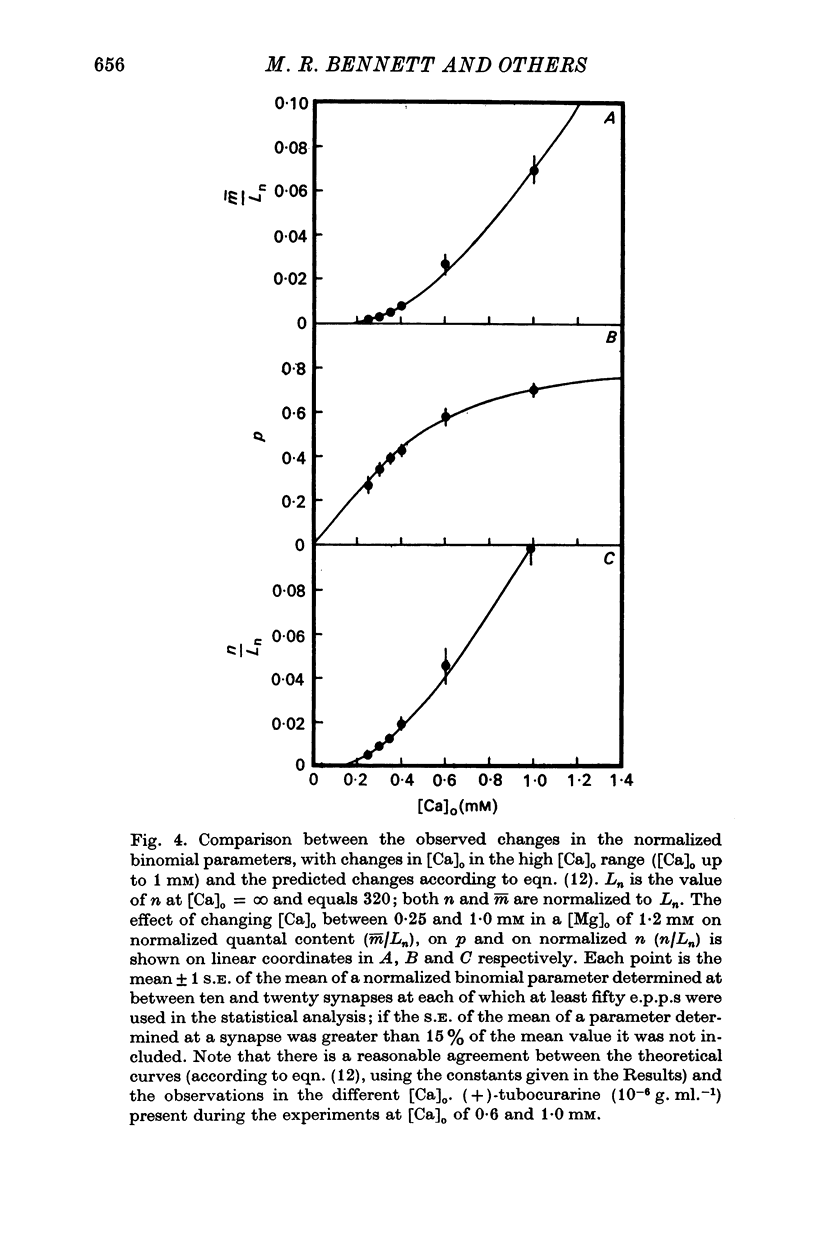
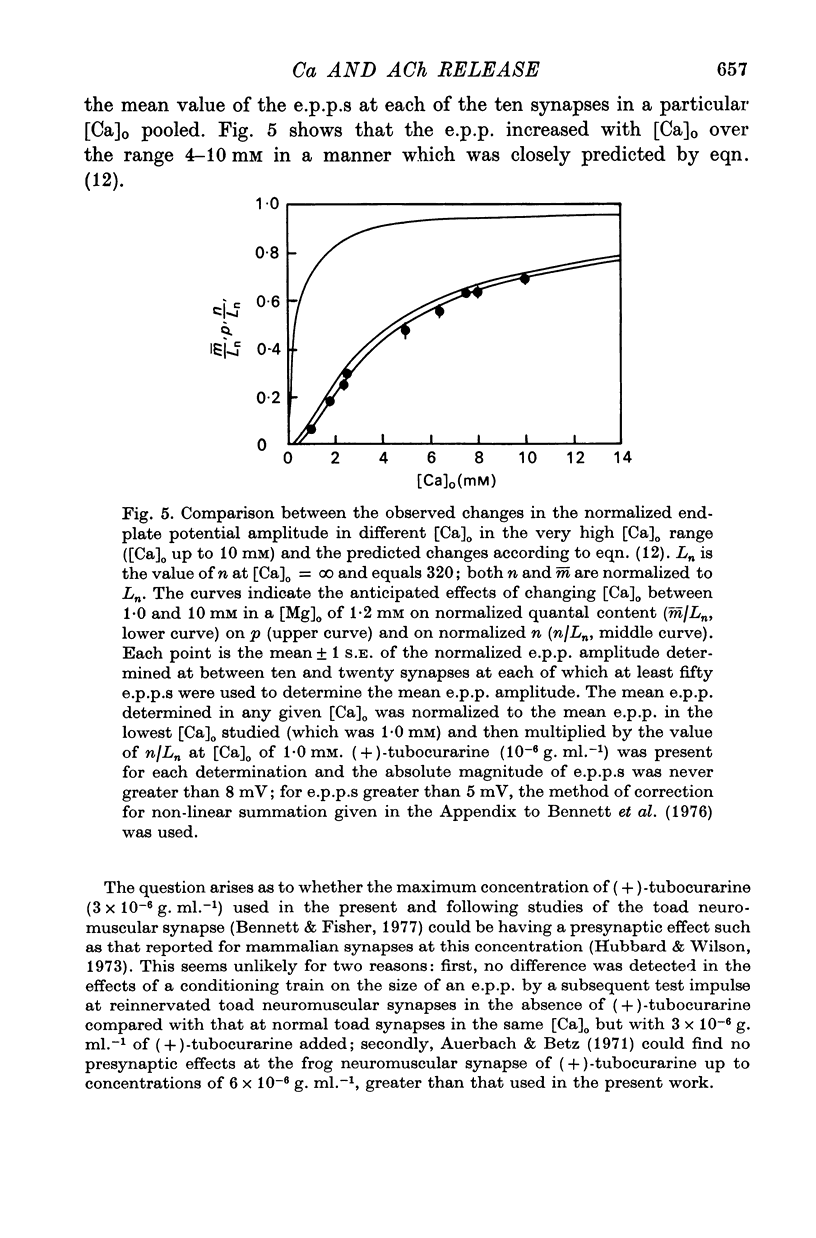
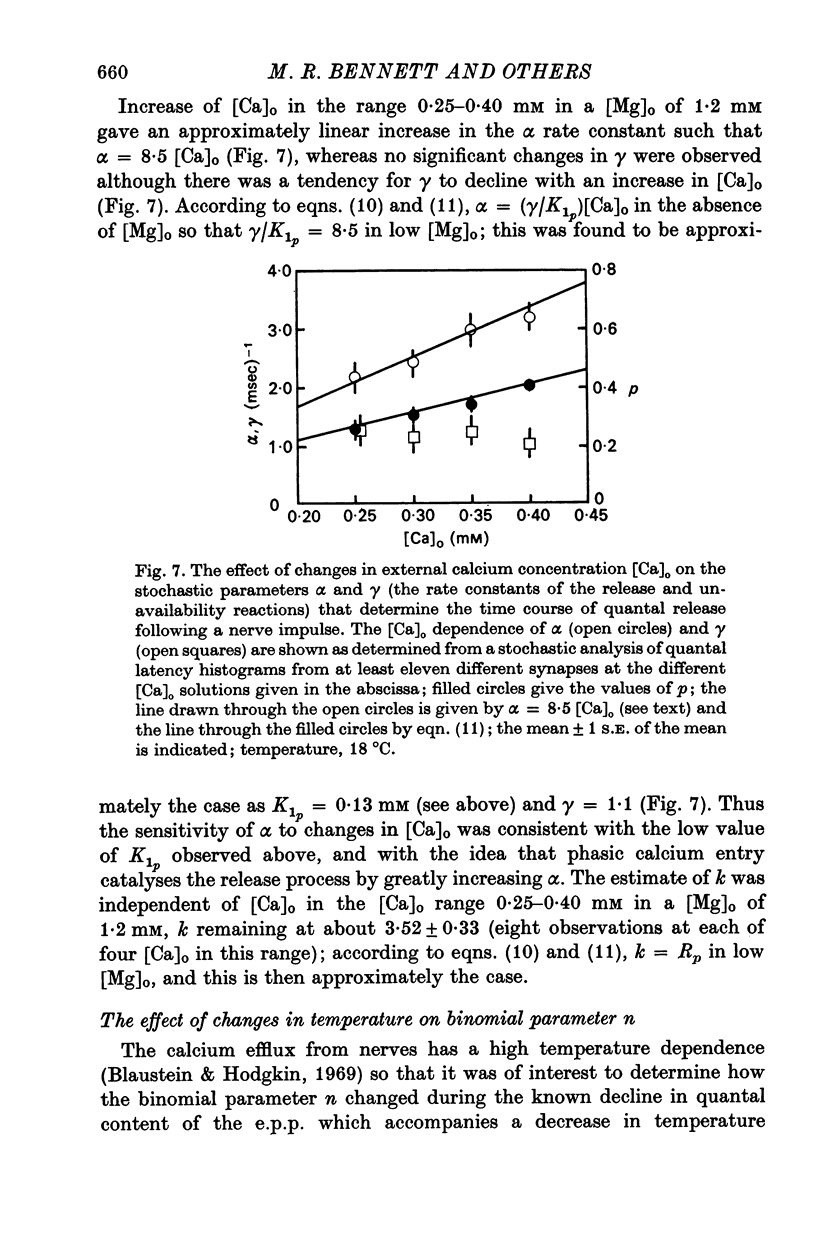
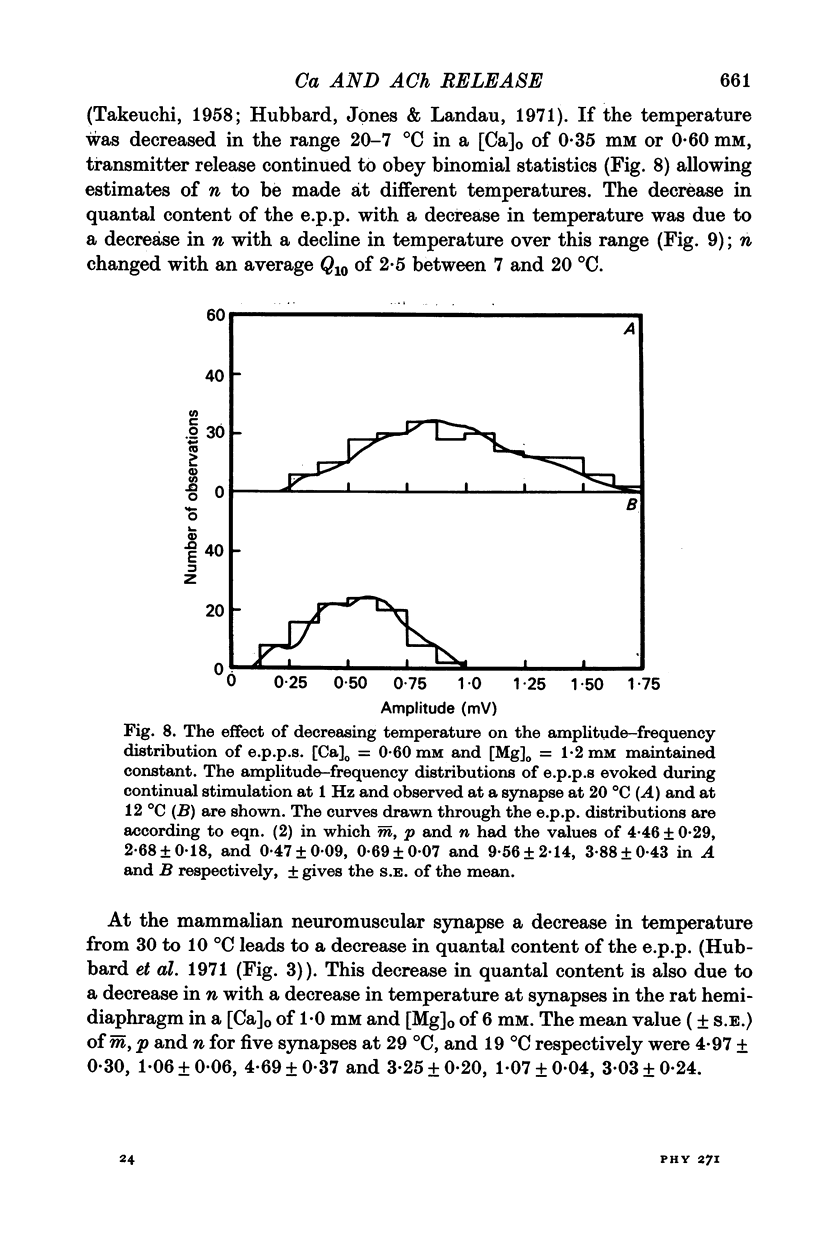
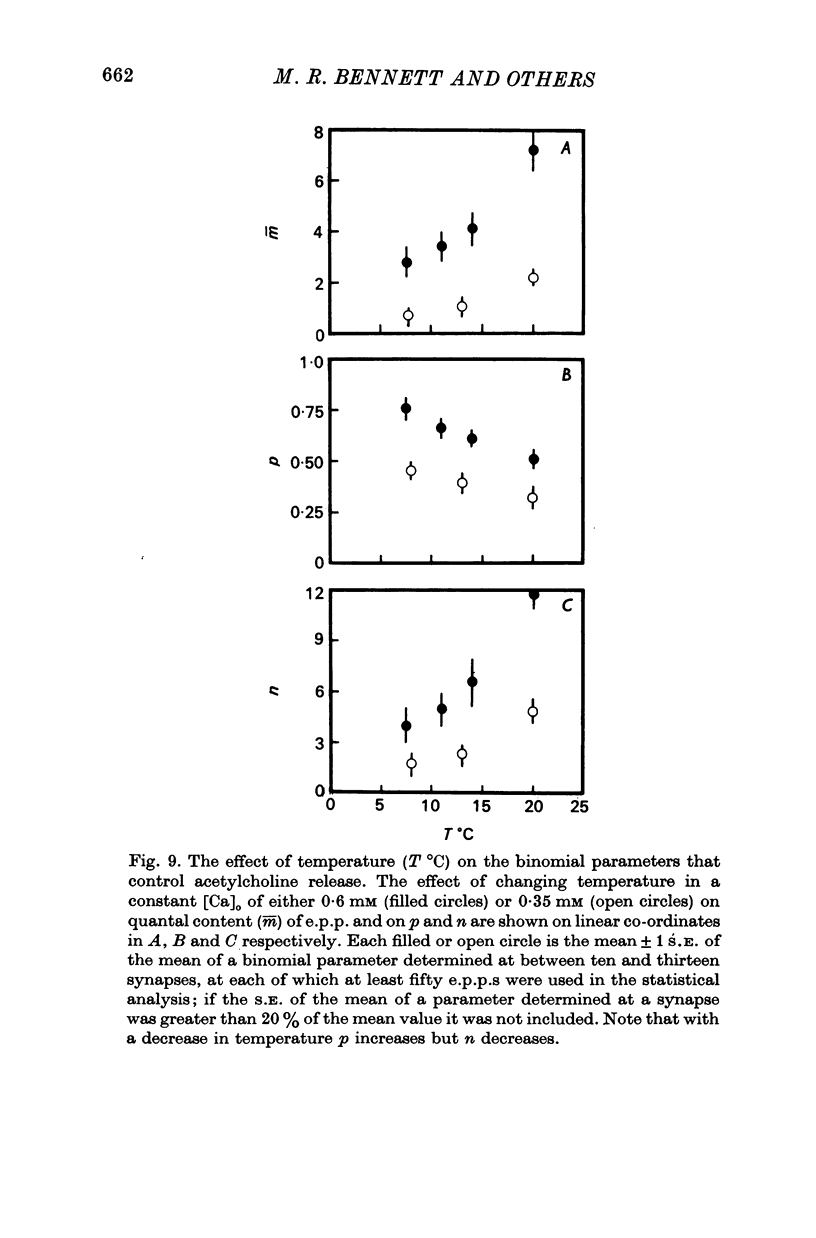
2. When [Ca]o was increased in the low range from 0·25 to 0·4 mM at 18 °C, the average quantal content of the e.p.p. (m̄) increased as the fourth power of [Ca]o and this was primarily due to a third power dependence of n on [Ca]o; the dissociation constants and power dependence of n on calcium determined in the [Ca]o range from 0·25 to 1·0 mM were successfully used to predict the changes in size of the e.p.p. in the very high [Ca]o range from 1 to 10 mM. When the temperature was increased from 7 to 18 °C in a [Ca]o of 0·6 mM or 0·35 mM, n increased with a Q10 of 2·5.
3. When [Ca]o was increased in the range from 0·25 to 1·0 mM at 18 °C, the probability that a quantum initially available for release is released during the release period (p(T)) was very sensitive to [Ca]o, increasing as the third power of [Ca]o and with a dissociation constant of 0·13 mM. When the temperature was increased from 7 to 18 °C in a [Ca]o of 0·6 mM or 0·35 mM, p(T) decreased.
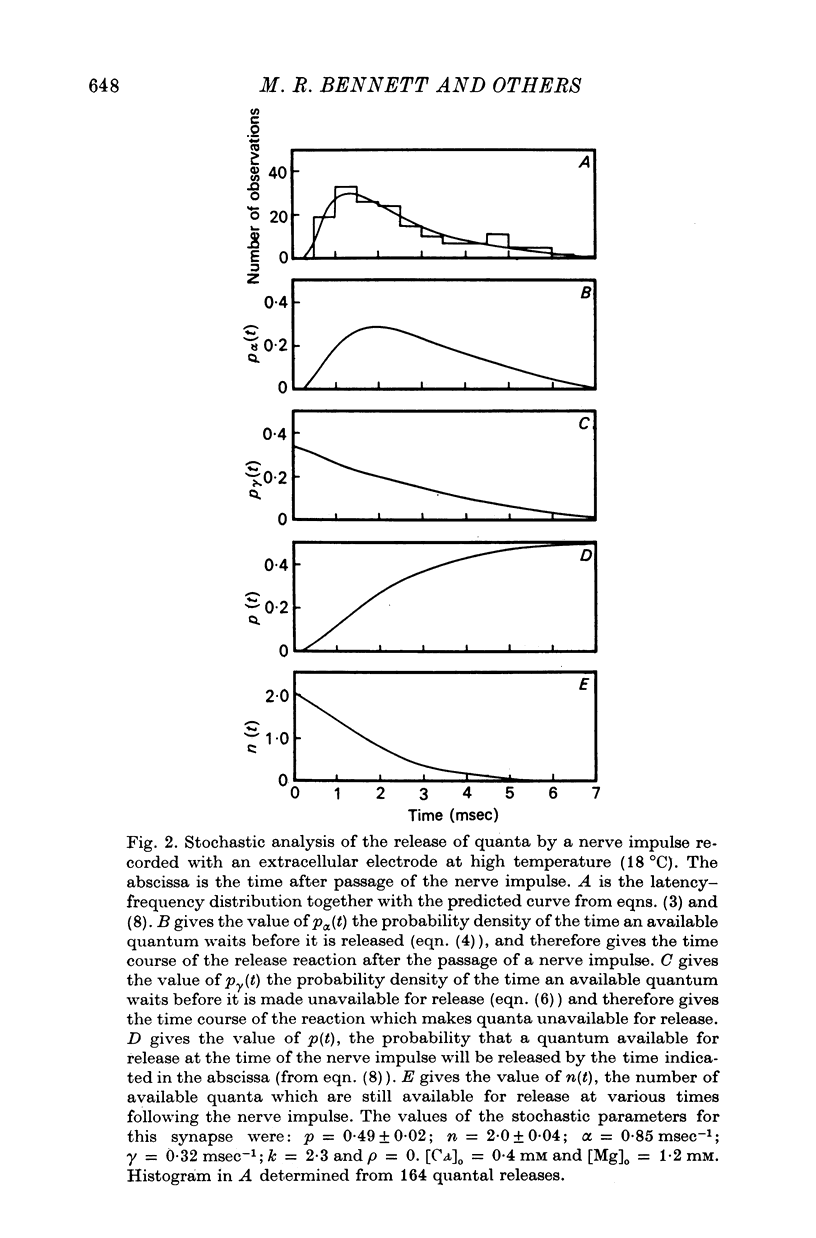
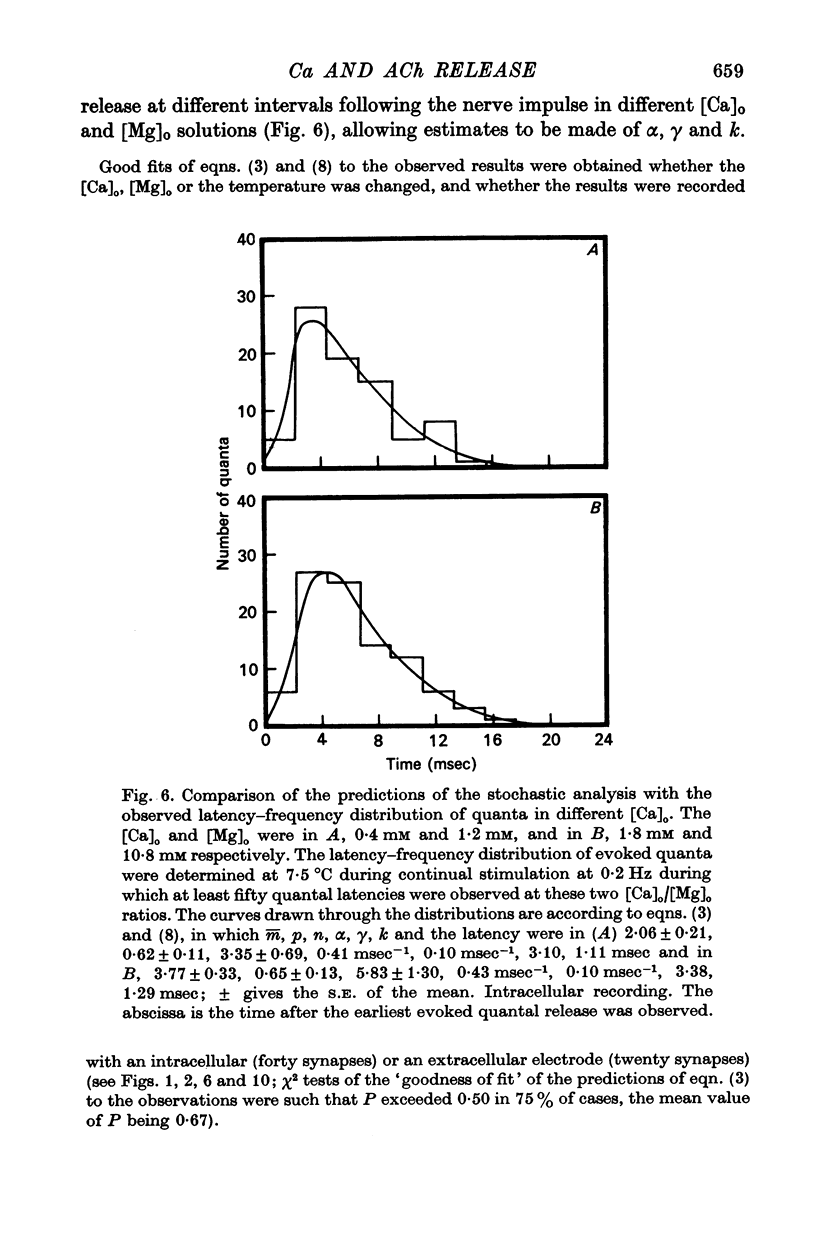
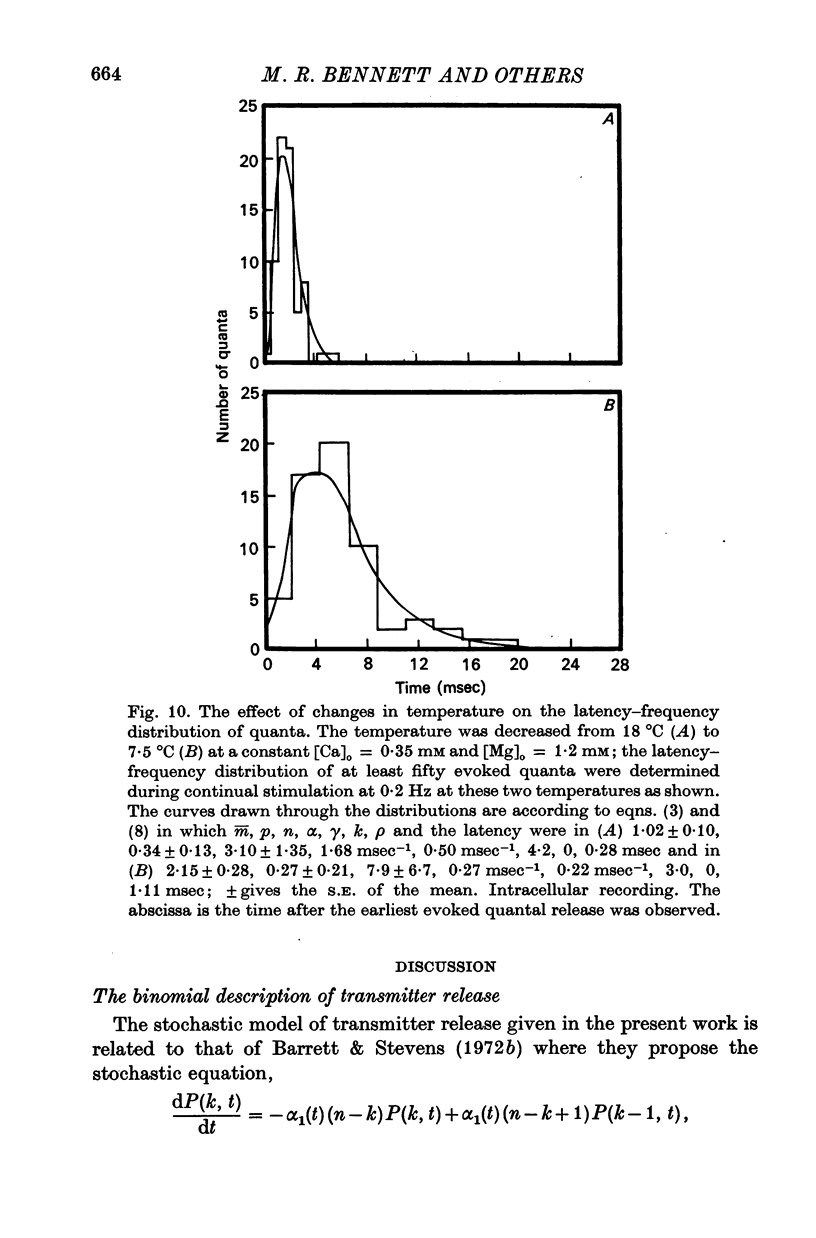
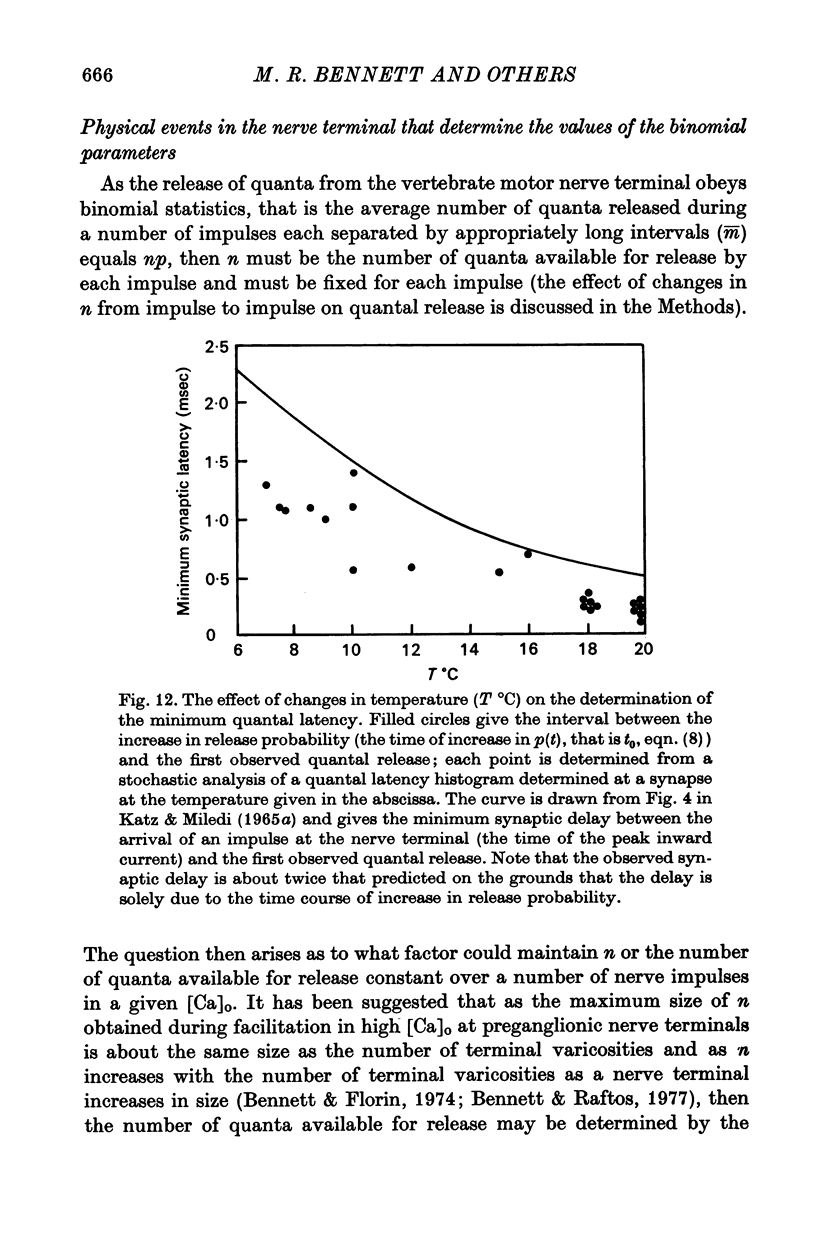
4. The histograms of latencies of individual quanta following a nerve impulse was very temperature dependent: the time to peak of the histograms (i.e. the interval in which most quanta fell) had a Q10 of over 4 as did the time constant of decline of the histograms in the temperature range from 7 to 18 °C.
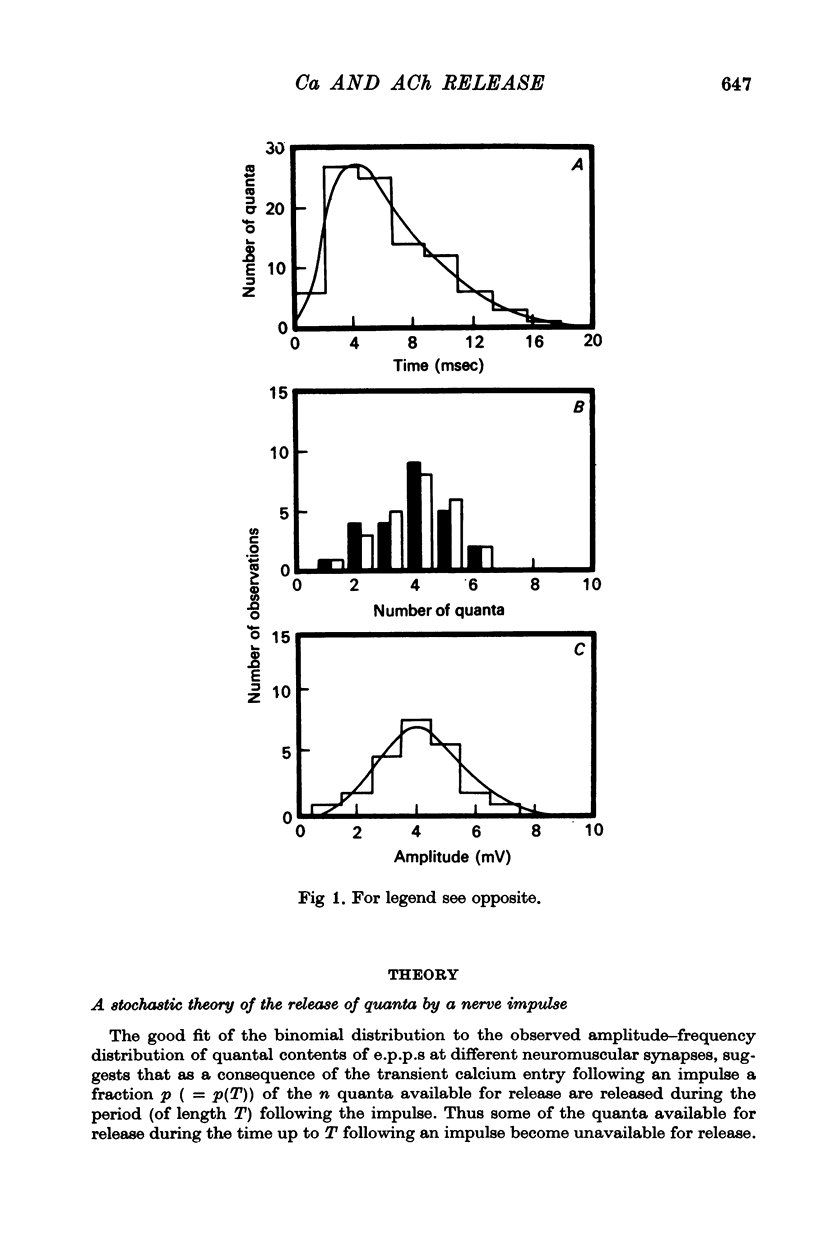
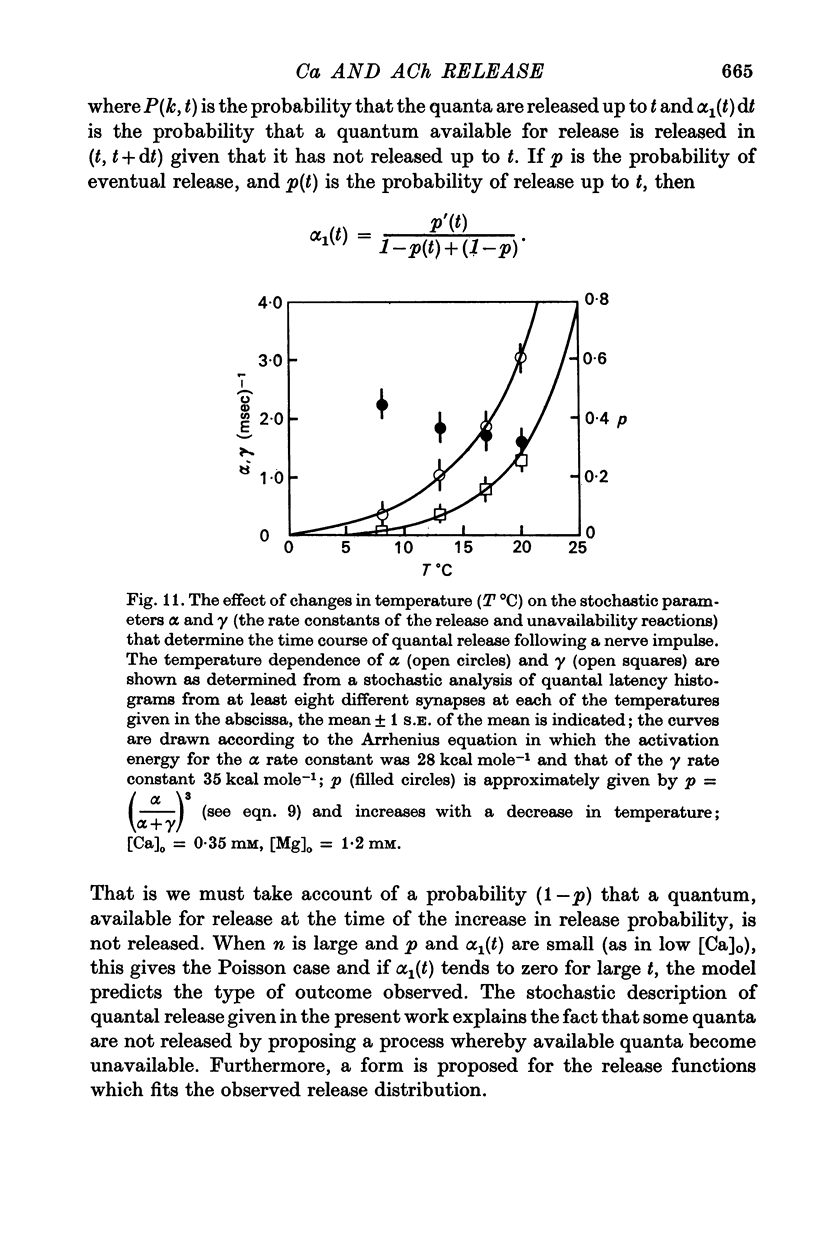
5. The average number of quanta released up to time t during the release period following a nerve impulse, namely np(t), was well described by a stochastic process in which p(t) was determined by two reactions; one of these reactions released available quanta from the nerve terminal whilst the other made some of the available quanta unavailable for release by the nerve impulse.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Fisher C. The effects of calcium ions on the binomial parameters that control acetylcholine release during trains of nerve impulses at amphibian neuromuscular synapses. J Physiol. 1977 Oct;271(3):673–698. doi: 10.1113/jphysiol.1977.sp012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Hall R. The effect of calcium ions on the binomial statistic parameters which control acetylcholine release at synapses in striated muscle. J Physiol. 1975 May;247(2):429–446. doi: 10.1113/jphysiol.1975.sp010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Feldman M. W. Evoked neurotransmitter release: statistical effects of nonuniformity and nonstationarity. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2913–2917. doi: 10.1073/pnas.73.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The effect of temperature change upon transmitter release, facilitation and post-tetanic potentiation. J Physiol. 1971 Aug;216(3):591–609. doi: 10.1113/jphysiol.1971.sp009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. J Physiol. 1975 Aug;250(1):121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- TAKEUCHI N. The effect of temperature on the neuromuscular junction of the frog. Jpn J Physiol. 1958 Dec 20;8(4):391–404. doi: 10.2170/jjphysiol.8.391. [DOI] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]


