Abstract
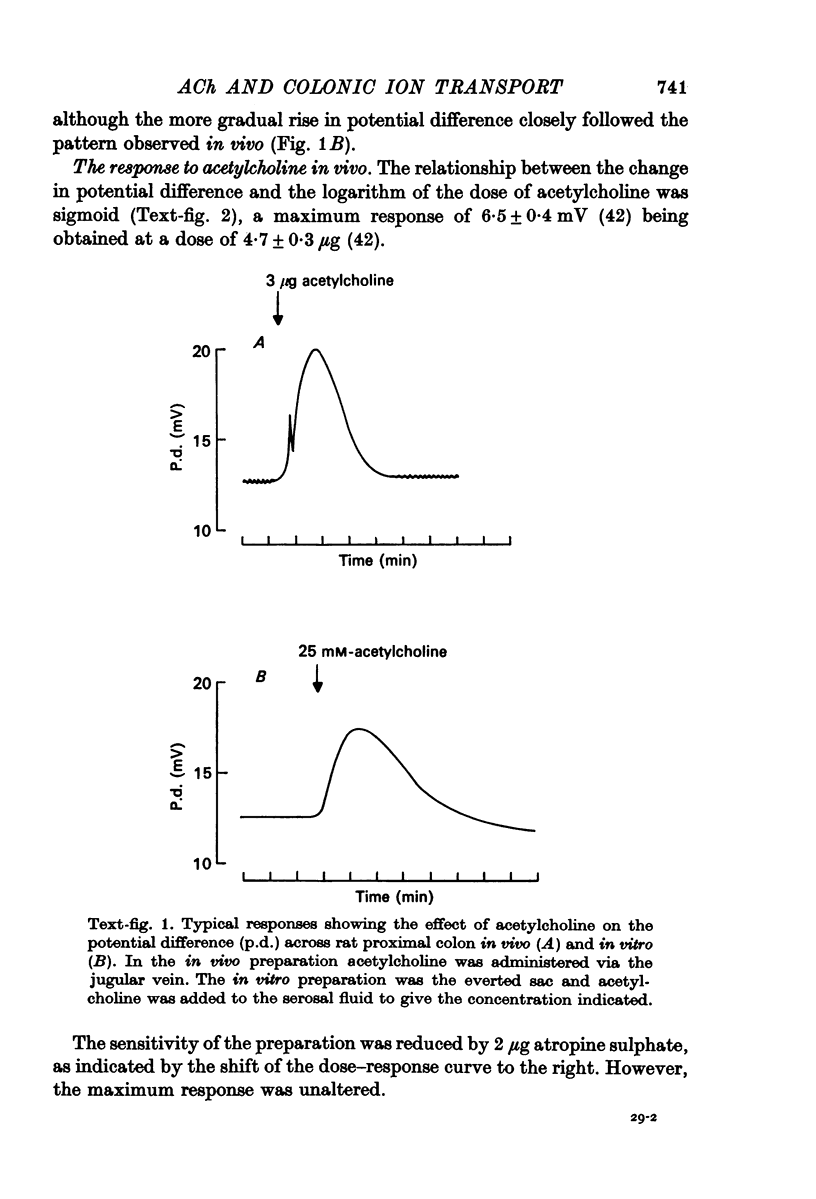
1. Acetylcholine increases the potential difference across rat proximal colon both in vivo and in vitro.
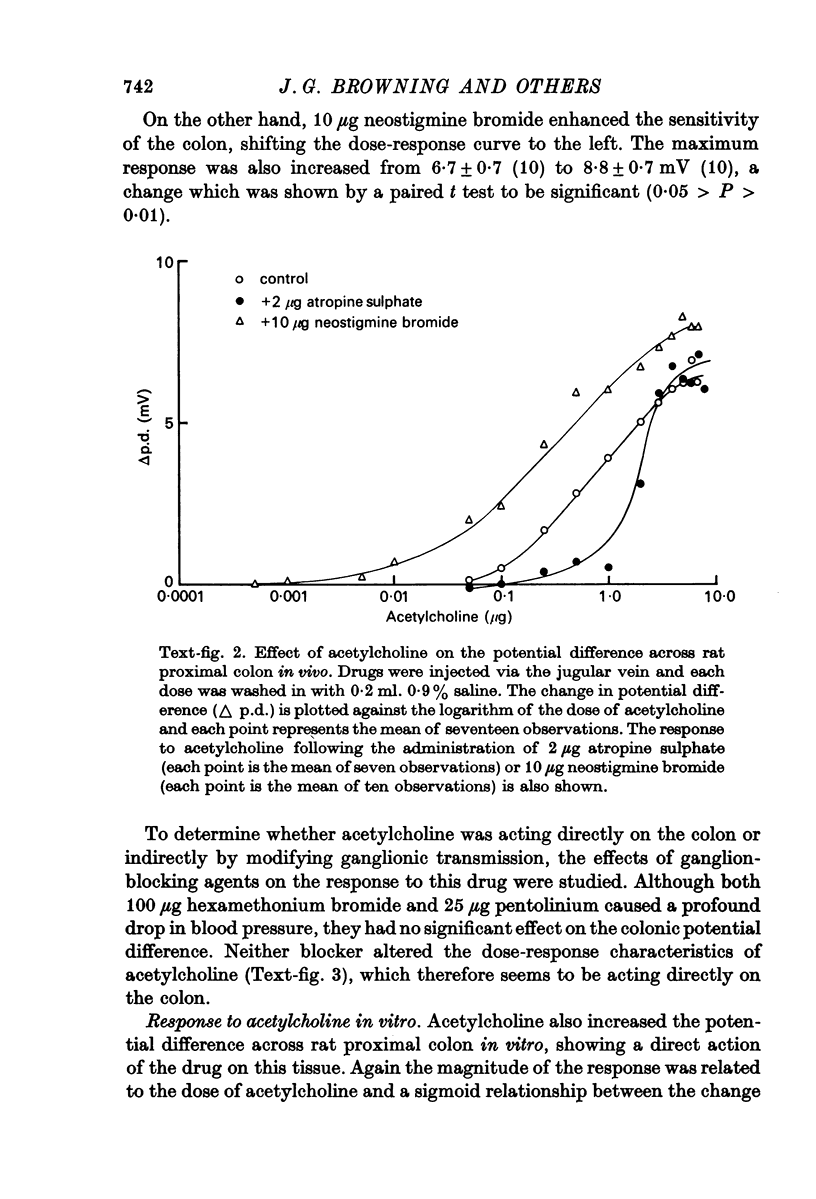
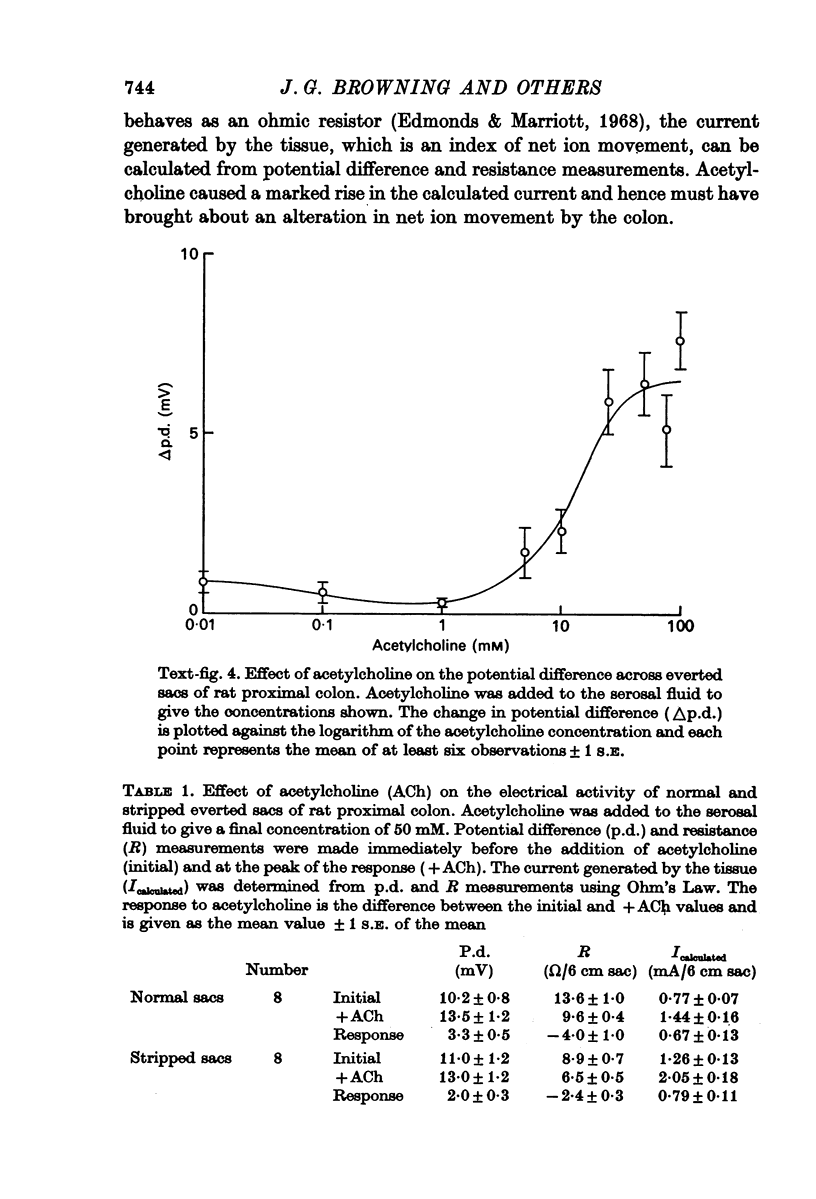
2. There is a sigmoid relationship between the change in potential difference and the logarithm of the dose of acetylcholine. The dose—response curve is shifted to the left by neostigmine and to the right by atropine, suggesting that the action of acetylcholine is mediated by a muscarinic type of receptor.
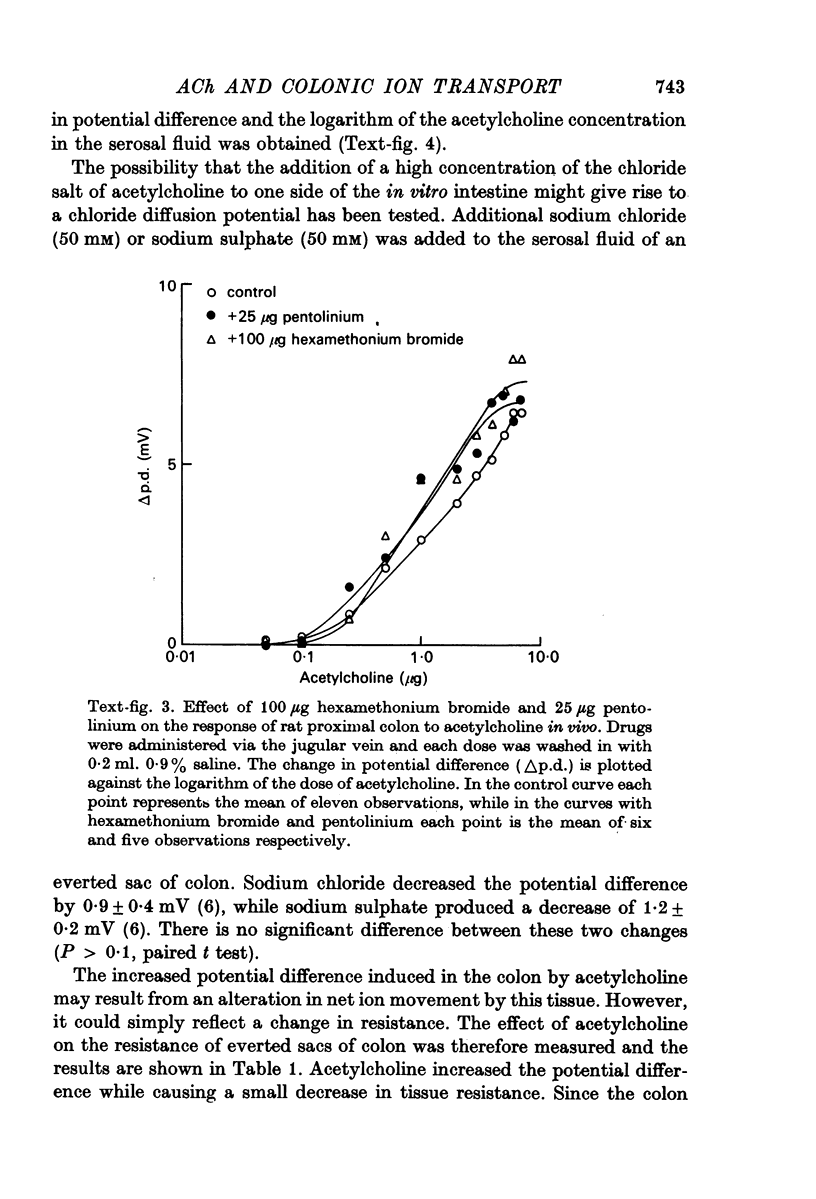
3. The dose-response curve for acetylcholine in vivo is not altered by the ganglion-blocking agents hexamethonium and pentolinium, suggesting a direct effect of this transmitter on the colon.
4. Acetylcholine causes an increase in potential difference, a small decrease in resistance and hence a rise in the current generated by both normal and stripped everted sacs of rat colon.
5. In the absence of sodium, the calculated current change produced by acetylcholine is reduced, and the removal of chloride has a similar inhibitory effect. The absence of bicarbonate does not significantly affect the response.
6. Acetylcholine virtually abolished net sodium movement and induced net chloride secretion and these changes accounted for the increased short-circuit current.
7. Acetylcholine had no effect on oxygen consumption by rings of colon.
8. Tracts staining for acetylcholinesterase were observed running from the submucous plexus towards the mucosal epithelium.
9. This study shows that acetylcholine can influence ion movement by rat colonic mucosa and suggests that the autonomic nervous system might be involved in the regulation of transport mechanisms in this tissue.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Freeman M. A., Hobbiger F. Distribution of acetylcholinesterase and butyrylcholinesterase in the myenteric plexus and longitudinal muscle of the guinea-pig intestine. Biochem Pharmacol. 1971 Jun;20(6):1123–1132. doi: 10.1016/0006-2952(71)90342-x. [DOI] [PubMed] [Google Scholar]
- BAILLIEN M., SCHOFFENIELS E. [Origin of the bioelectric potentials of the intestinal epithelium of the Greek tortoise]. Biochim Biophys Acta. 1961 Nov 11;53:537–548. doi: 10.1016/0006-3002(61)90213-x. [DOI] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLISS B. J., TODRICK A. The use of a selective acetylcholinesterase inhibitor in the estimation of pseudocholinesterase activity in rat brain. Biochem J. 1956 Jan;62(1):62–67. doi: 10.1042/bj0620062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLICKENSTAFF D. D., LEWIS L. J. Effect of atropine on intestinal absorption of water and chloride. Am J Physiol. 1952 Jul;170(1):17–23. doi: 10.1152/ajplegacy.1952.170.1.17. [DOI] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H. J., Rawlins C. L. Electrolyte transport across isolated large intestinal mucosa. Am J Physiol. 1973 Nov;225(5):1232–1239. doi: 10.1152/ajplegacy.1973.225.5.1232. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Field M., Kimberg D. V. Intestinal mucosal cyclic GMP: regulation and relation to ion transport. Am J Physiol. 1976 Jul;231(1):275–282. doi: 10.1152/ajplegacy.1976.231.1.275. [DOI] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. Influence of the thyroid gland on the accumulation of sugars in rat intestinal mucosa during absorption. J Physiol. 1965 Jul;179(2):323–332. doi: 10.1113/jphysiol.1965.sp007665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J. G., Hardcastle J., Hardcastle P. T., Sanford P. A. Proceedings: The effect of acetylcholine on the electrical activity of colonic mucosa. J Physiol. 1976 Jul;259(1):58P–59P. [PubMed] [Google Scholar]
- CURRAN P. F., SCHWARTZ G. F. Na, Cl, and water transport by rat colon. J Gen Physiol. 1960 Jan;43:555–571. doi: 10.1085/jgp.43.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Absorption and secretion by the colon. Gut. 1975 Apr;16(4):323–329. doi: 10.1136/gut.16.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge H. R. The localization of guanylate cyclase in rat small intestinal epithelium. FEBS Lett. 1975 May 1;53(2):237–242. doi: 10.1016/0014-5793(75)80028-7. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. Electrical potential and short circuit current of an in vitro preparation of rat colon mucosa. J Physiol. 1968 Feb;194(2):479–494. doi: 10.1113/jphysiol.1968.sp008419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):589–602. doi: 10.1113/jphysiol.1967.sp008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Baillien M., Schoffeniels E. Bioelectric potentials in the intestinal epithelium of the Greek tortoise. Comp Biochem Physiol. 1967 Oct;23(1):95–104. doi: 10.1016/0010-406x(67)90476-8. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Hardcastle P. T., Eggenton J. The effect of acetylcholine on the electrical activity of intestinal epithelial cells. Biochim Biophys Acta. 1973 Feb 27;298(1):95–100. doi: 10.1016/0005-2736(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Henderson J. R. The use of silver for intensifying sulfide deposits in the cholinesterase technique. Stain Technol. 1967 Mar;42(2):101–102. [PubMed] [Google Scholar]
- Hubel K. A. Intestinal ion transport: effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976 Jul;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- Isaacs P. E., Corbett C. L., Riley A. K., Hawker P. C., Turnberg L. A. In vitro behavior of human intestinal mucosa. The influence of acetyl choline on ion transport. J Clin Invest. 1976 Sep;58(3):535–542. doi: 10.1172/JCI108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Schooley R. T., Henderson A. Effects of cycloheximide on the response of intestinal mucosa to cholera enterotoxin. J Clin Invest. 1973 Jun;52(6):1376–1383. doi: 10.1172/JCI107310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. J., Frömter E., Gebler B., Knauf H., Young J. A. The effects of carbachol on water and electrolyte fluxes and transepithelial electrical potential differences of the rabbit submaxillary main duct perfused in vitro. Pflugers Arch. 1973 Jun 26;341(2):131–142. doi: 10.1007/BF00587320. [DOI] [PubMed] [Google Scholar]
- PARSONS D. S., PATERSON G. R. Movements of fluid and glucose in an everted sac preparation of rat colonic mucosa. Biochim Biophys Acta. 1960 Jun 17;41:173–175. doi: 10.1016/0006-3002(60)90393-0. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Sheerin H. E., Field M., Said S. I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974 Sep;54(3):536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R. L., Makhlouf G. M., Sachs G. Action of cholinergic drugs on Necturus gastric mucosa. Am J Physiol. 1970 Oct;219(4):1056–1060. doi: 10.1152/ajplegacy.1970.219.4.1056. [DOI] [PubMed] [Google Scholar]
- TIDBALL C. S. Active chloride transport during intestinal secretion. Am J Physiol. 1961 Feb;200:309–312. doi: 10.1152/ajplegacy.1961.200.2.309. [DOI] [PubMed] [Google Scholar]
- TIDBALL C. S., TIDBALL M. E. Changes in intestinal net absorption of a sodium chloride solution produced by atropine in normal and vagotomized dogs. Am J Physiol. 1958 Apr;193(1):25–28. doi: 10.1152/ajplegacy.1958.193.1.25. [DOI] [PubMed] [Google Scholar]
- TRIER J. S. STUDIES ON SMALL INTESTINAL CRYPT EPITHELIUM. II. EVIDENCE FOR THE MECHANISMS OF SECRETORY ACTIVITY BY UNDIFFERENTIATED CRYPT CELLS OF THE HUMAN SMALL INTESTINE. Gastroenterology. 1964 Nov;47:480–495. [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Elevation of cyclic guanosine 3',5'-monophosphate levels in dog thyroid slices caused by acetylcholine and sodium fluoride. J Biol Chem. 1972 Nov 10;247(21):7062–7066. [PubMed] [Google Scholar]