Abstract
1. Simultaneous recordings of mechanical activity and membrane potential of individual smooth muscle cells have been made in the rabbit anococcygeus muscle and the effect of field stimulation on these examined.
2. In the absence of tone the mean resting membrane potential was - 48 mV. In the stretched muscle spontaneous tone and rhythmic activity quite frequently appeared and this was associated with depolarization of the muscle cells.
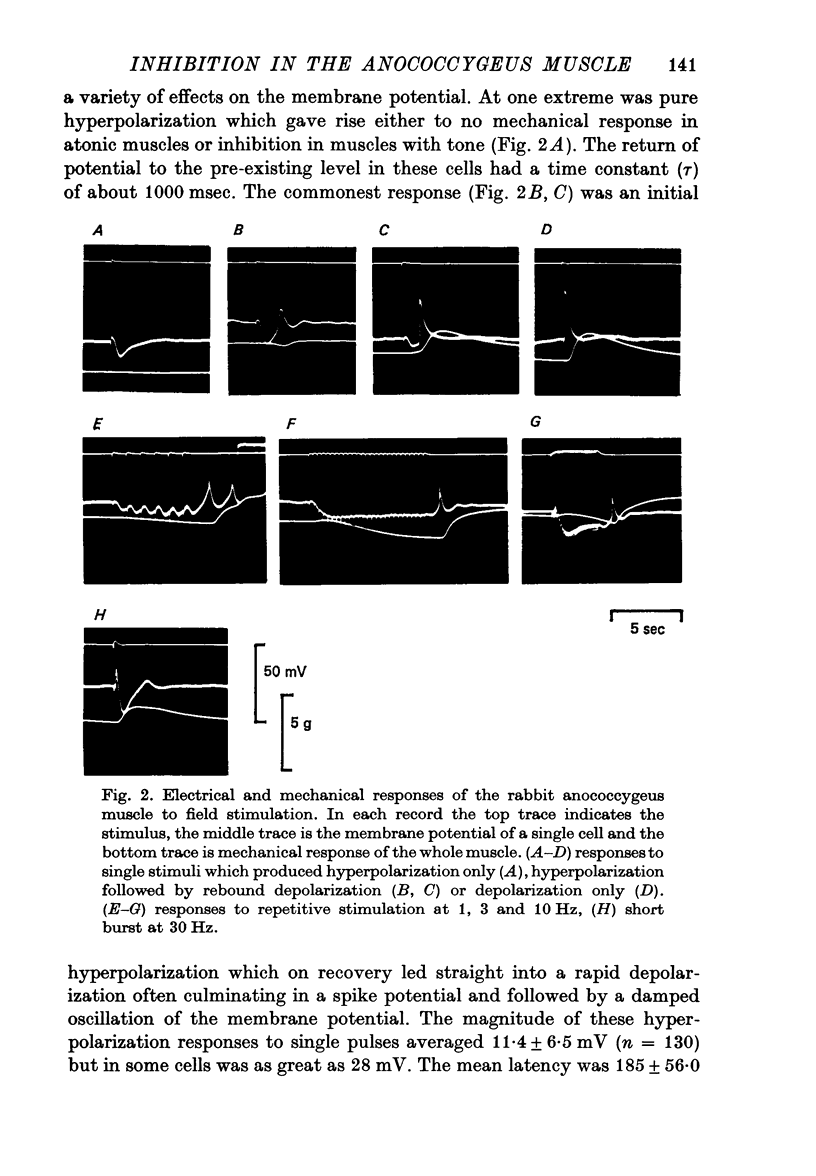
3. The response to field stimulation depended on the frequency of stimulation, the level of membrane potential and the presence of myogenic tone. The usual response to single pulses or low frequency stimulation was a hyperpolarization of up to 30 mV (mean 14±6·8 mV) after a latency of 185 msec and accompanied by muscle relaxation. Higher frequencies (over 8 Hz) produced an initial depolarization often with a spike potential and followed by hyperpolarization. The mechanical response in these instances was contraction or contraction followed by relaxation. At all frequencies rebound depolarization and an associated contraction followed the end of stimulation).
4. Phentolamine (5×10-6 M) and guanethidine (10-6 M) blocked the initial depolarization and contraction but had no effect on hyperpolarization, muscle relaxation or rebound depolarization and contraction.
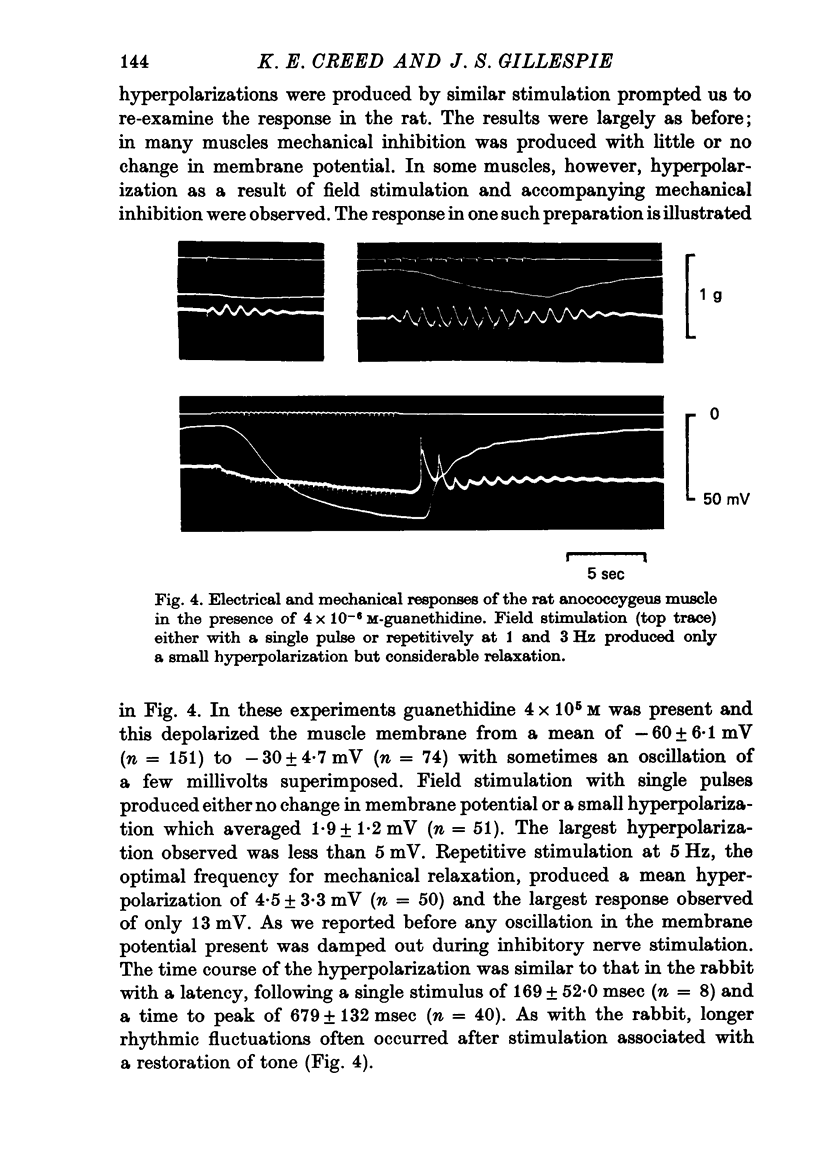
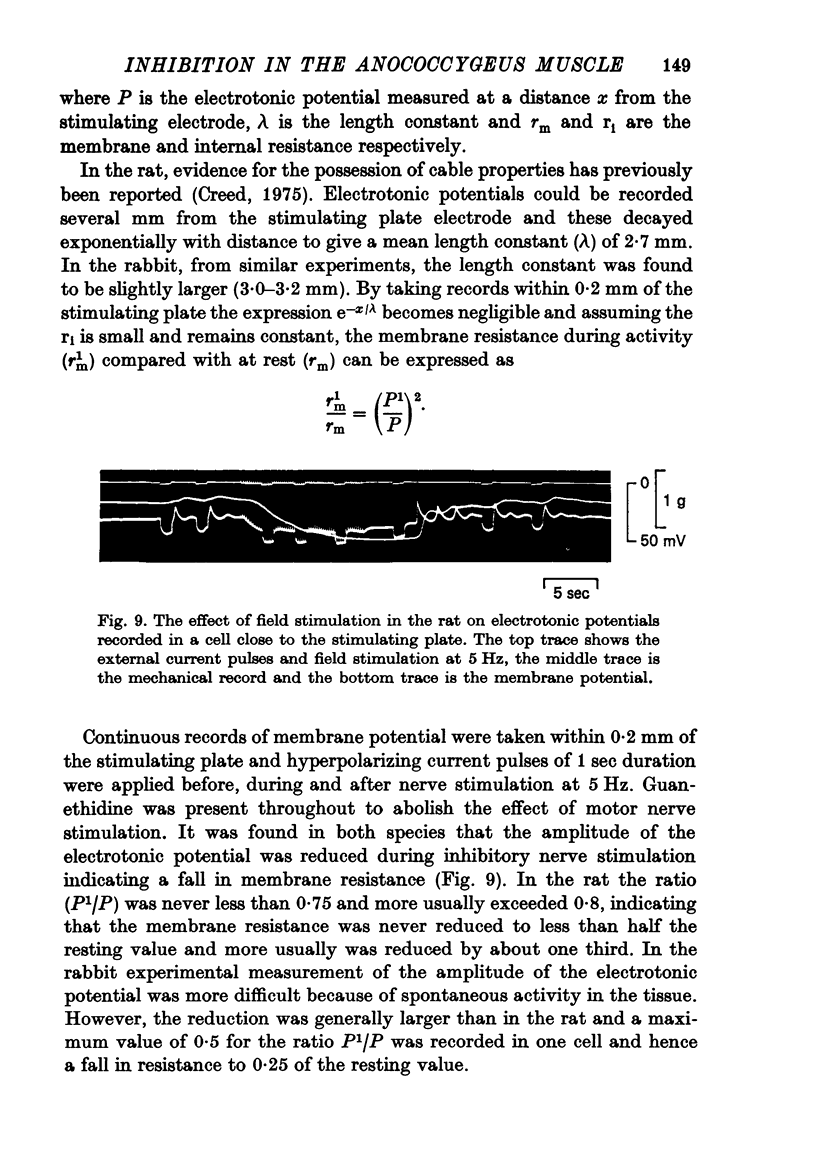
5. The effect of field stimulation in the presence of guanethidine (4×10-5 M) was re-examined in the rat anococcygeus. Single pulses were ineffective, repetitive stimulation produced muscle relaxation but no hyperpolarization comparable to the rabbit. Any oscillations in membrane potential were damped during field stimulation and sometimes a small hyperpolarization was produced with a maximum amplitude of 13 mV and a mean of 1·9±1·2 mV.
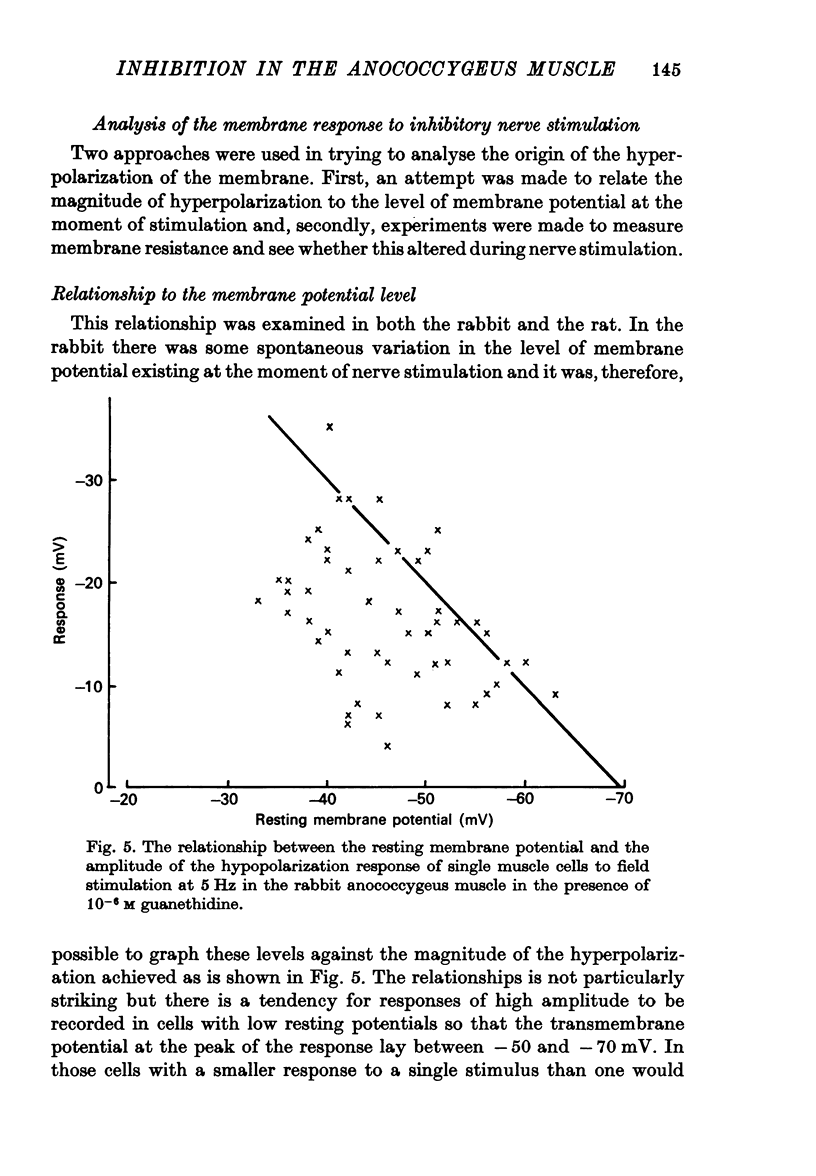
6. The transmembrane potential at the peak of hyperpolarization in the rabbit was rarely more than -70 mV. Passive displacement of the membrane potential by current pulses altered the amplitude of the hyperpolarization and suggested that there was a reversal potential at between -80 and -90 mV.
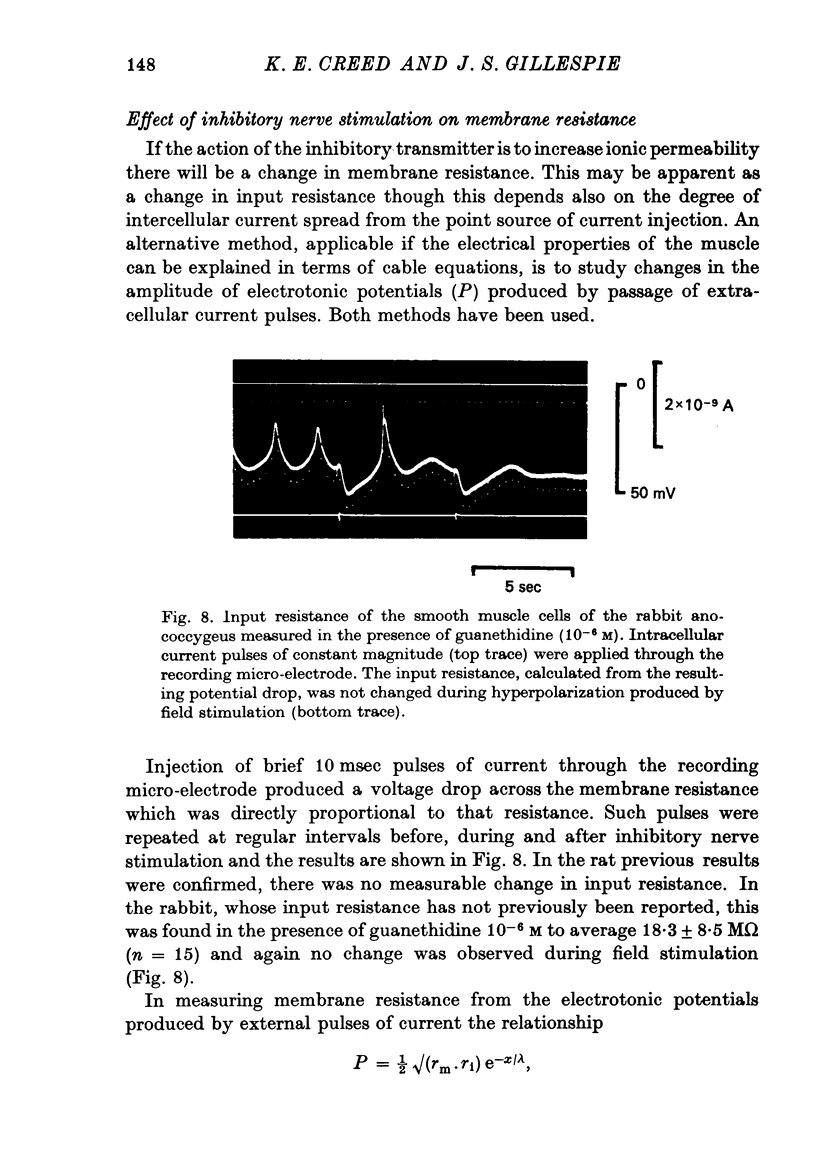
7. No change in input resistance could be measured during inhibitory nerve stimulation in either the rabbit or the rat but measurements based on electrotonic potentials indicated a reducation in membrane resistance, small in the rat but greater in the rabbit.
8. These experiments suggest that in both species muscle relaxation is associated with an increase in ionic permeability and a move, at least in the rabbit muscle, towards an equilibrium potential of -80 to -90 mV. In view of the much smaller effect in the rat it is not clear whether this is the cause or at least the sole cause of the muscle relaxation.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., McCaffery H. The rabbit anococcygeus muscle and its response to field stimulation and to some drugs. J Physiol. 1977 Dec;273(1):121–135. doi: 10.1113/jphysiol.1977.sp012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle cells of the rat anococcygeus muscle. J Physiol. 1975 Feb;245(1):49–62. doi: 10.1113/jphysiol.1975.sp010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969 Dec;205(3):549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Lüllmann-Rauch R. On the ultrastructure of the rat anococcygeus muscle. Cell Tissue Res. 1974;149(1):91–104. doi: 10.1007/BF00209052. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., McGrath J. C. The response of the cat anococcygeus muscle to nerve or drug stimulation and a comparison with the rat anococcygeus. Br J Pharmacol. 1974 Jan;50(1):109–118. doi: 10.1111/j.1476-5381.1974.tb09597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part VII. Anatomical Observations. J Physiol. 1896 Oct 19;20(4-5):372–406. doi: 10.1113/jphysiol.1896.sp000629. [DOI] [PMC free article] [PubMed] [Google Scholar]


