Abstract
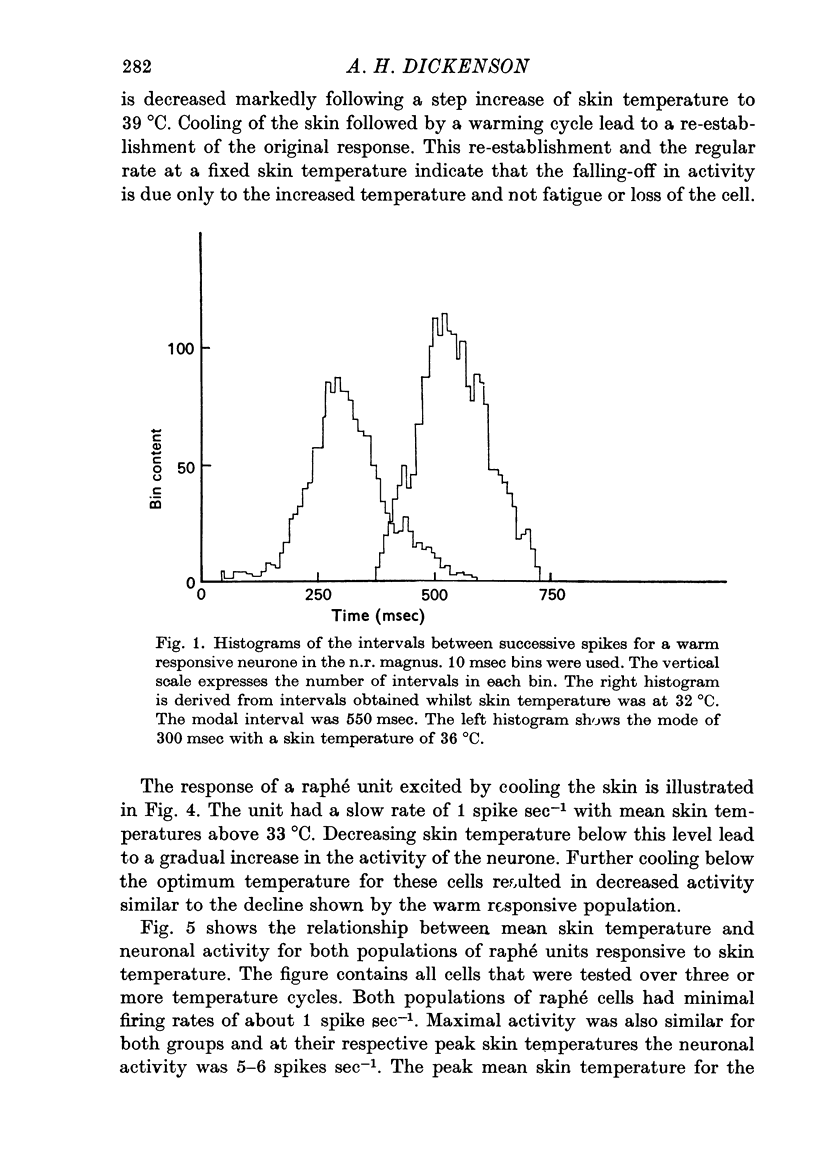
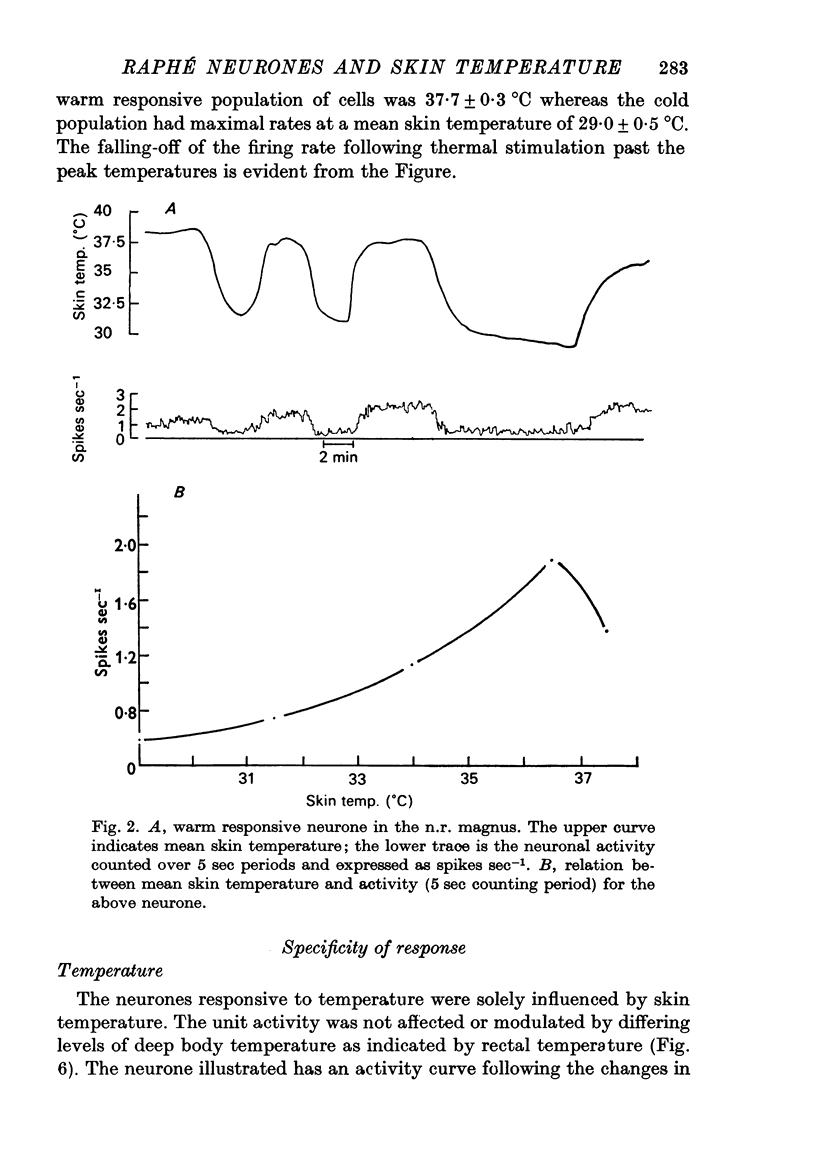
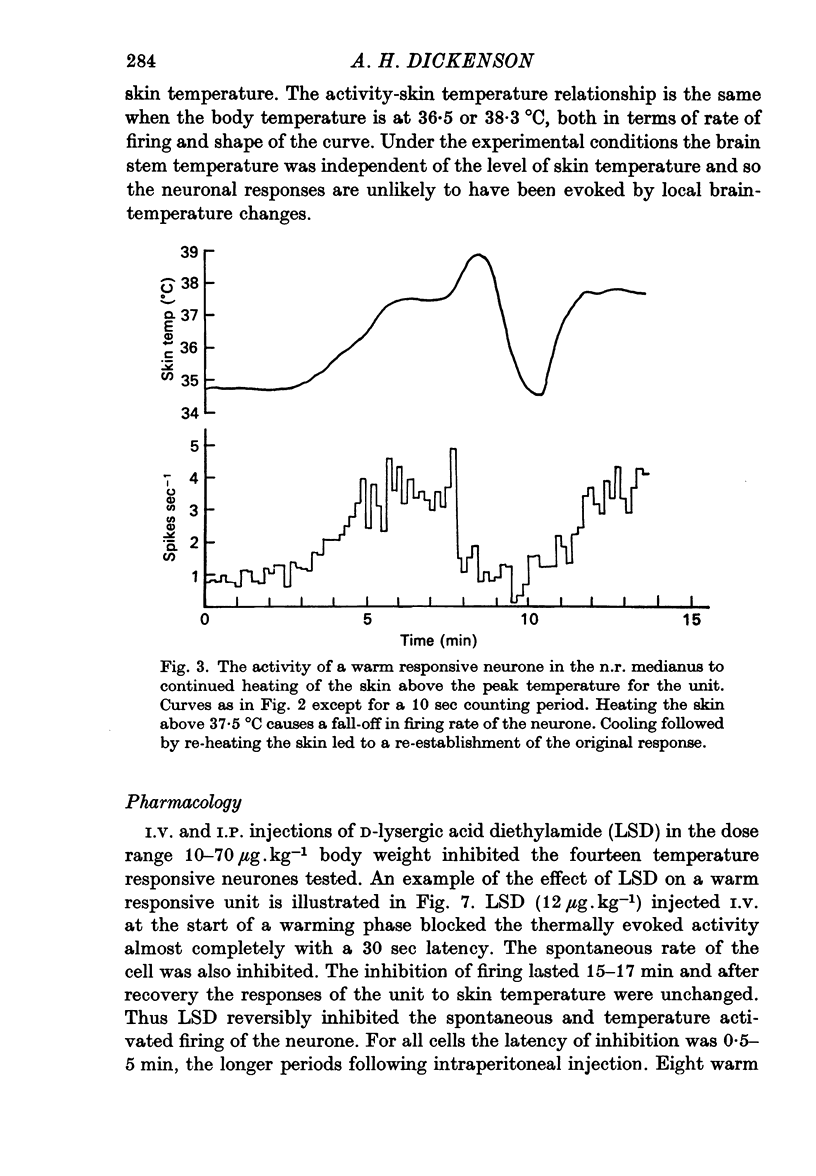
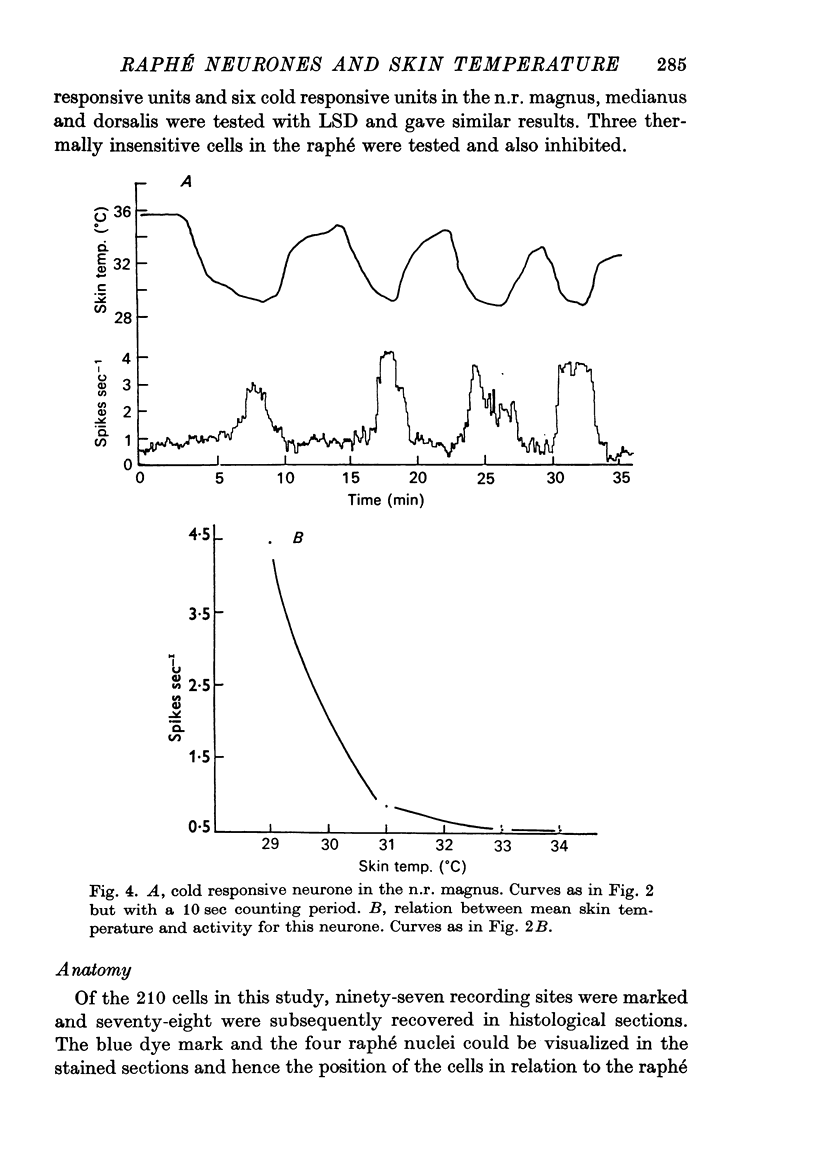
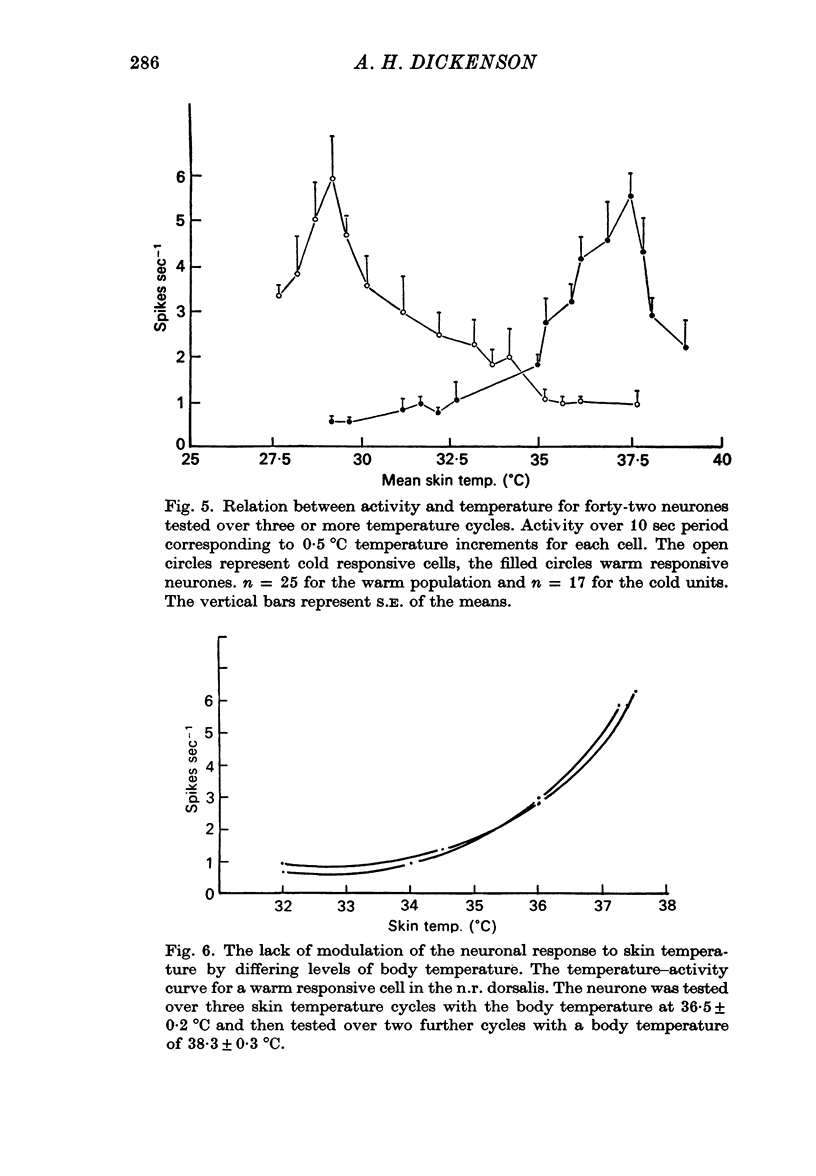
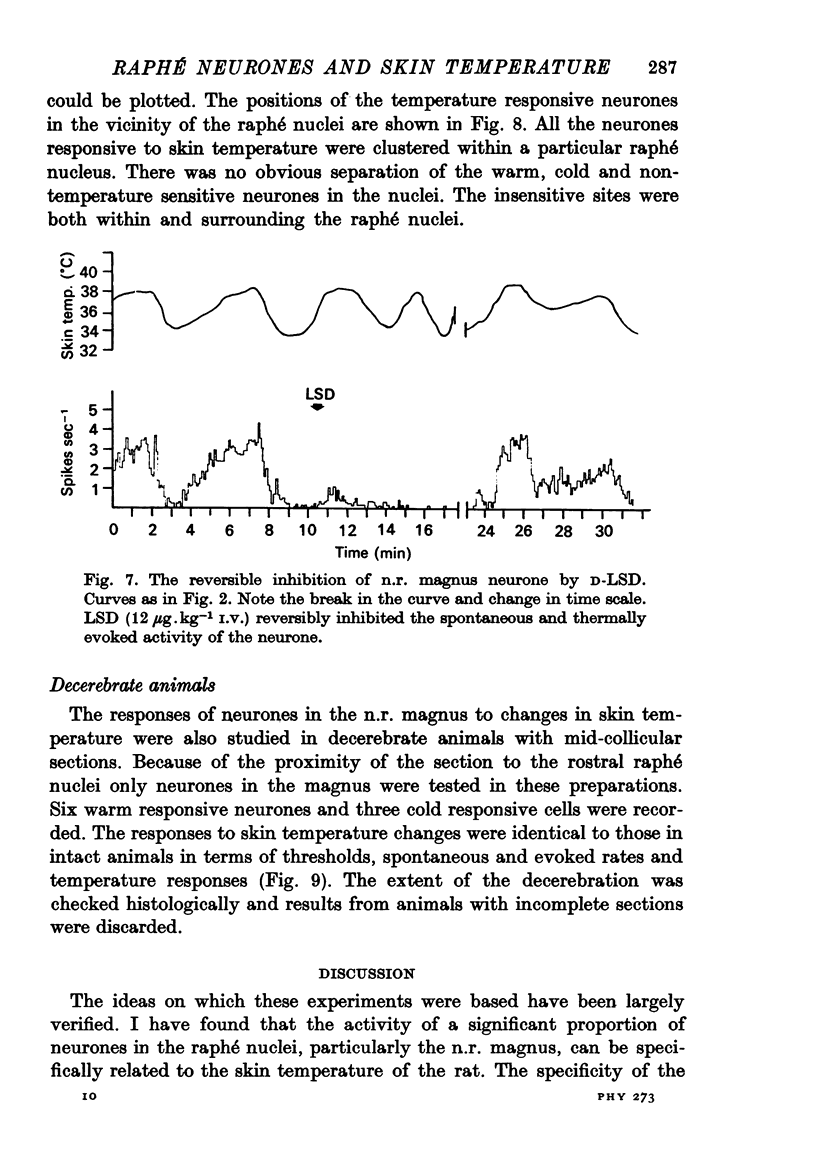
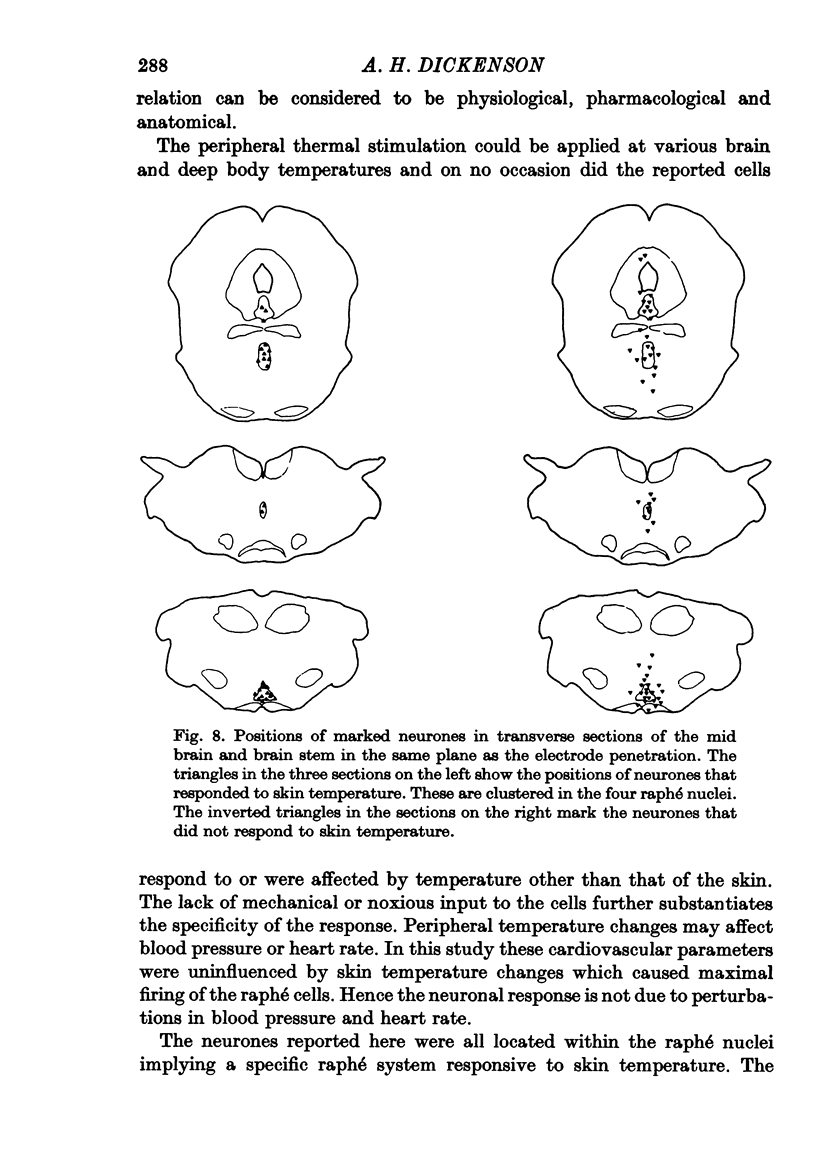
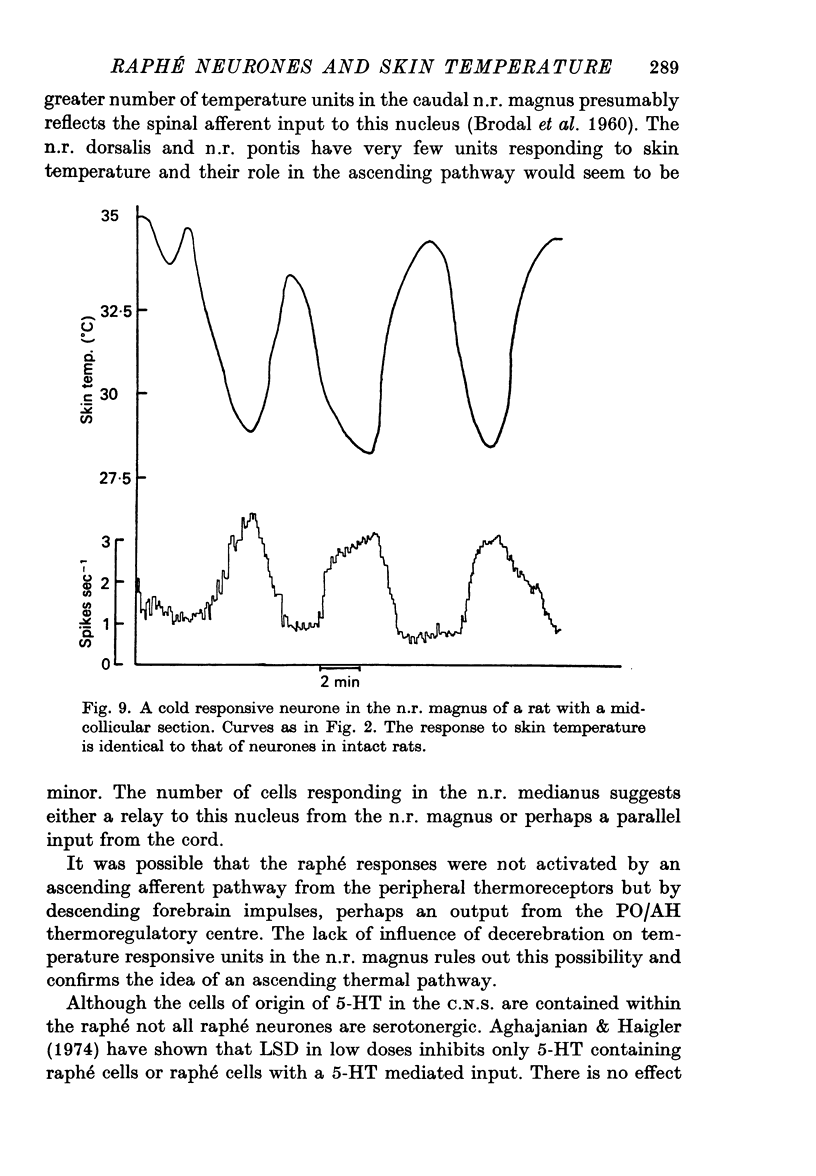
1. The responses of single neurones in the nuclei raphés magnus, medianus, dorsalis and pontis to changes in skin temperature were recorded in rats anaesthetized with urethane. Skin temperature was altered by means of a water-perfused jacket. 2. Of 210 neurones studied, thirty-five were specifically excited by warming the skin whilst twenty were cold responsive. The greatest proportion of cells responding to skin temperature were in the nucleus raphé magnus, whilst few neurones in the raphé dorsalis and pontis were influenced. 3. The warm units had peak activity at a mean skin temperature of 37.7 degrees C whilst the cold cells had a corresponding maximal rate at 29.0 degreet C. Mechanical and noxious peripheral stimulation, blood pressure changes and temperatures other than that of skin did not affect the neurones. 4. The neurones influenced by skin temperature were histologically verified as being within the raphé system. 5. LSD inhibited all neurones tested, indicating that the cells were serotonergic. 6. The responses to skin temperature were unchanged in rats with midcollicular sections suggesting an ascending thermal system. 7. The results suggest that any involvement of 5-HT in central thermo-regulation is in terms of an afferent thermal pathway mediated by serotonergic raphé neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Foote W. E., Sheard M. H. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970 Feb;171(2):178–187. [PubMed] [Google Scholar]
- Aghajanian G. K., Haigler H. J. Mode of action of LSD on serotonergic neurons. Adv Biochem Psychopharmacol. 1974;10:167–177. [PubMed] [Google Scholar]
- Bobillier P., Seguin S., Petitjean F., Salvert D., Touret M., Jouvet M. The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976 Sep 3;113(3):449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Bramwell G. J. Factors affecting the activity of 5-HT-containing neurones. Brain Res. 1974 Oct 25;79(3):515–519. doi: 10.1016/0006-8993(74)90450-8. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Leonard C. M., Pfaff D. W. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974 Jul;156(2):179–205. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- Cronin M. J., Baker M. A. Heat-sensitive midbrain raphe neurons in the anesthetized cat. Brain Res. 1976 Jun 25;110(1):175–181. doi: 10.1016/0006-8993(76)90219-5. [DOI] [PubMed] [Google Scholar]
- DOWBEN R. M., ROSE J. E. A metal-filled microelectrode. Science. 1953 Jul 3;118(3053):22–24. doi: 10.1126/science.118.3053.22. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., MYERS R. D. A NEW CONCEPT OF TEMPERATURE REGULATION BY AMINES IN THE HYPOTHALAMUS. Nature. 1963 Dec 28;200:1325–1325. doi: 10.1038/2001325a0. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., MYERS R. D. EFFECTS ON TEMPERATURE OF AMINES INJECTED INTO THE CEREBRAL VENTRICLES. A NEW CONCEPT OF TEMPERATURE REGULATION. J Physiol. 1964 Sep;173:226–231. doi: 10.1113/jphysiol.1964.sp007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Hellon R. F., Mitchell D. Convergence in a thermal afferent pathway in the rat. J Physiol. 1975 Jun;248(2):359–376. doi: 10.1113/jphysiol.1975.sp010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F. Monoamines, pyrogens and cations: their actions on central control of bodytemperature. Pharmacol Rev. 1974 Dec;26(4):289–321. [PubMed] [Google Scholar]
- Hori T., Harada Y. Responses of Midbrain raphe neurons to local temperature. Pflugers Arch. 1976 Jul 30;364(2):205–207. doi: 10.1007/BF00585193. [DOI] [PubMed] [Google Scholar]
- Jahns R. Different projections of cutaneous thermal inputs to single units of the midbrain raphe nuclei. Brain Res. 1976 Jan 16;101(2):355–361. doi: 10.1016/0006-8993(76)90276-6. [DOI] [PubMed] [Google Scholar]
- Knox G. V., Campbell C., Lomax P. Cutaneous temperature and unit activity in the hypothalamic thermoregulatory centers. Exp Neurol. 1973 Sep;40(3):717–730. doi: 10.1016/0014-4886(73)90106-4. [DOI] [PubMed] [Google Scholar]
- Lewin J. E. A counter for recording the rate of firing of neurones. J Physiol. 1972 Apr;222(2):132P–133P. [PubMed] [Google Scholar]
- Lister W. C., Woodget L. L. Precision stereotaxic equipment. J Physiol. 1972 Apr;222(2):130P–132P. [PubMed] [Google Scholar]
- Palkovits M., Jacobowitz D. M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. II. Hindbrain (mesencephalon, rhombencephalon). J Comp Neurol. 1974 Sep 1;157(1):29–42. doi: 10.1002/cne.901570104. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Jacobs B. L. Serotonin-containing neurons: lack of response to changes in body temperature in rats. Brain Res. 1976 Nov 12;116(3):523–530. doi: 10.1016/0006-8993(76)90501-1. [DOI] [PubMed] [Google Scholar]


