Abstract
1. Kinetics of inactivation of sodium channels in myelinated nerve from Rana pipiens were studied at 4·5 °C using the voltage clamp technique of Dodge & Frankenhaeuser (1958).
2. Potassium currents were blocked by cutting the internodes in 20 mM-TEA-Cl + 100 mM-KCl and by adding 12 mM-TEA-Cl to the external Ringer. Leakage and capacitative currents were subtracted electronically.
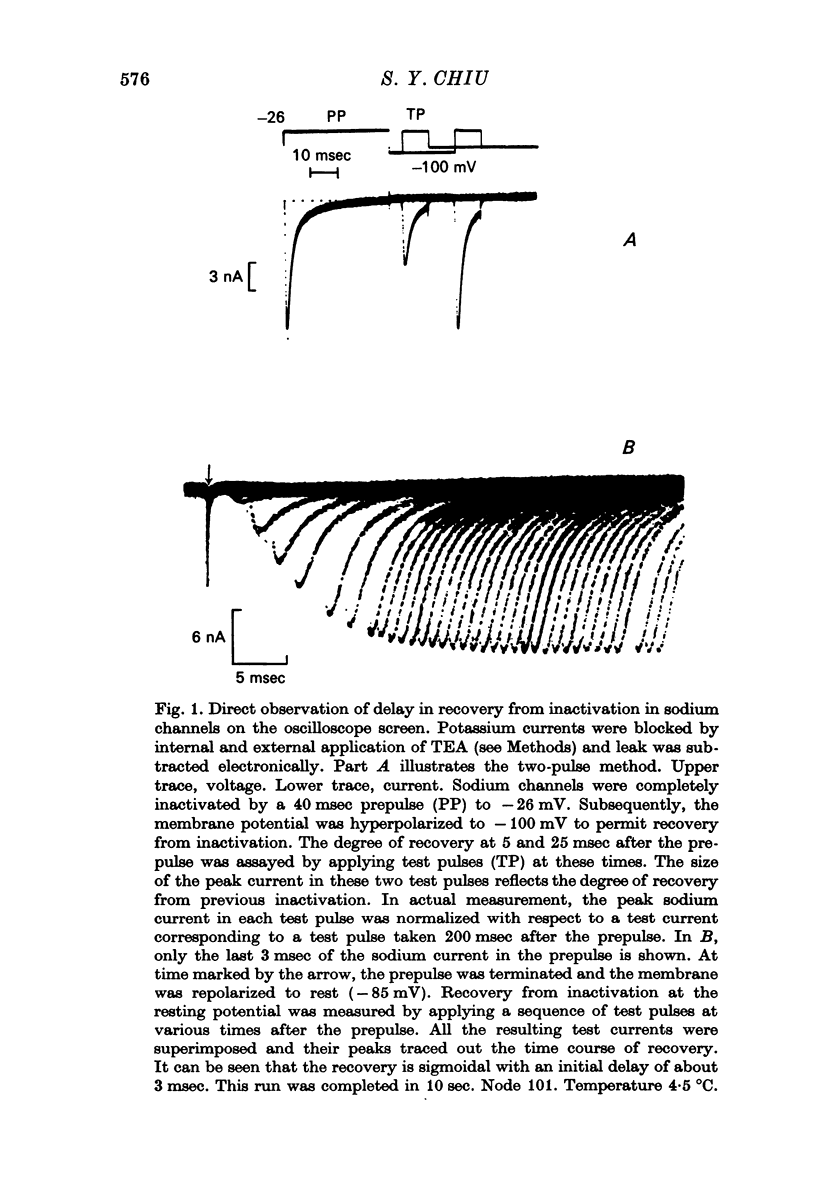
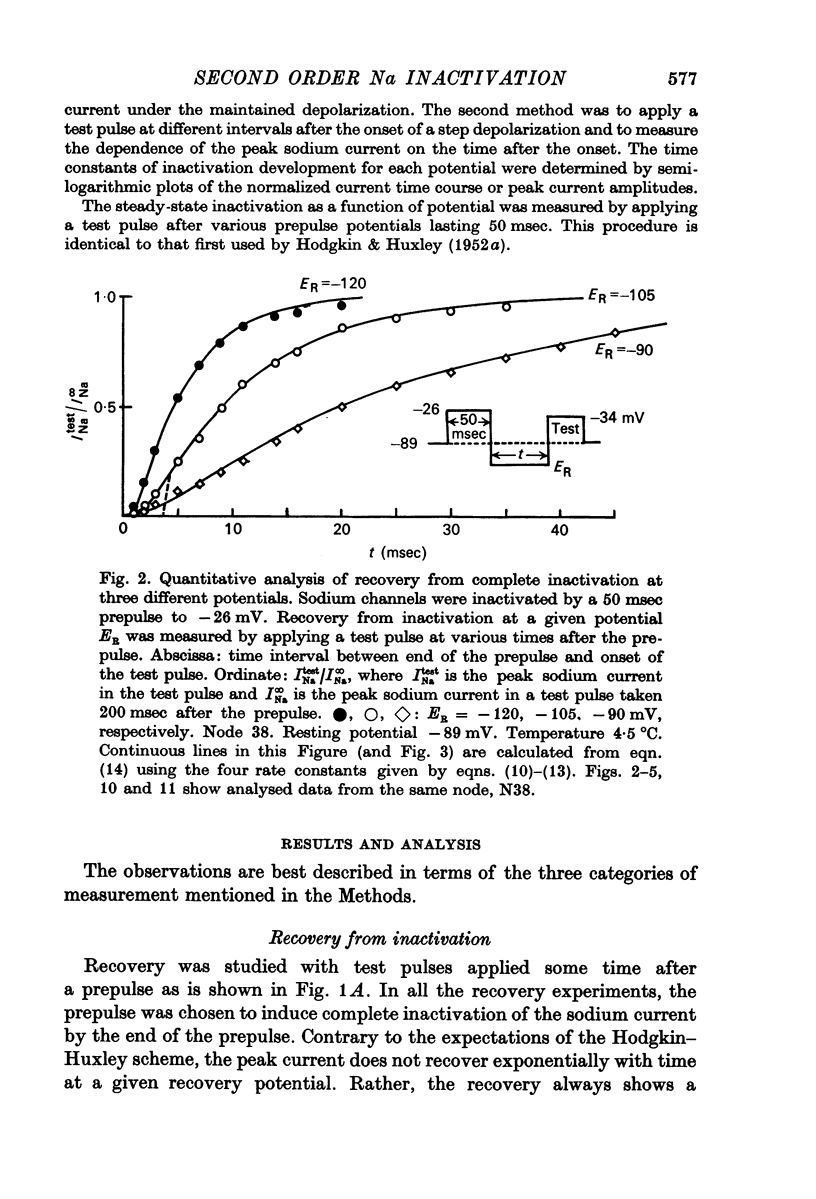
3. Kinetics of recovery from inactivation of the sodium channels were studied by inactivating the channels with a large depolarizing prepulse and allowing the channels to recover at different potentials; the extent of recovery was measured by applying a test pulse at various times after the prepulse.
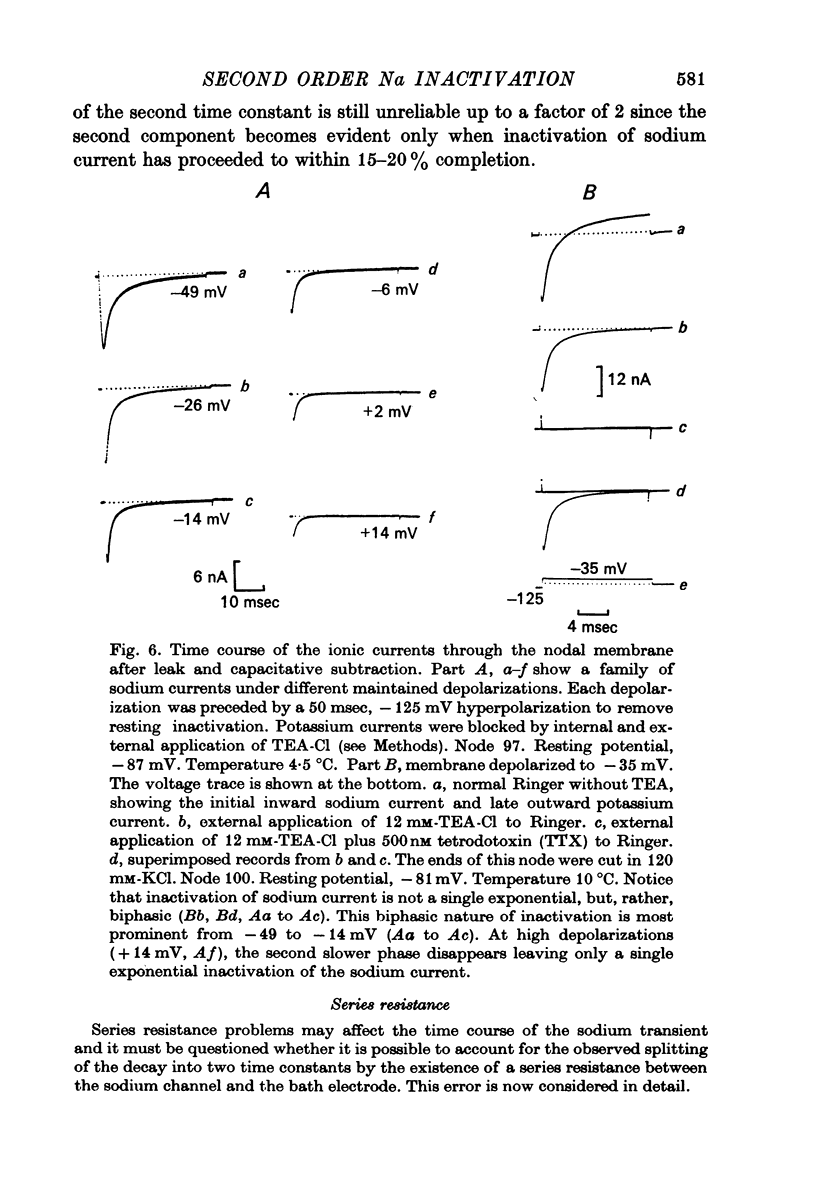
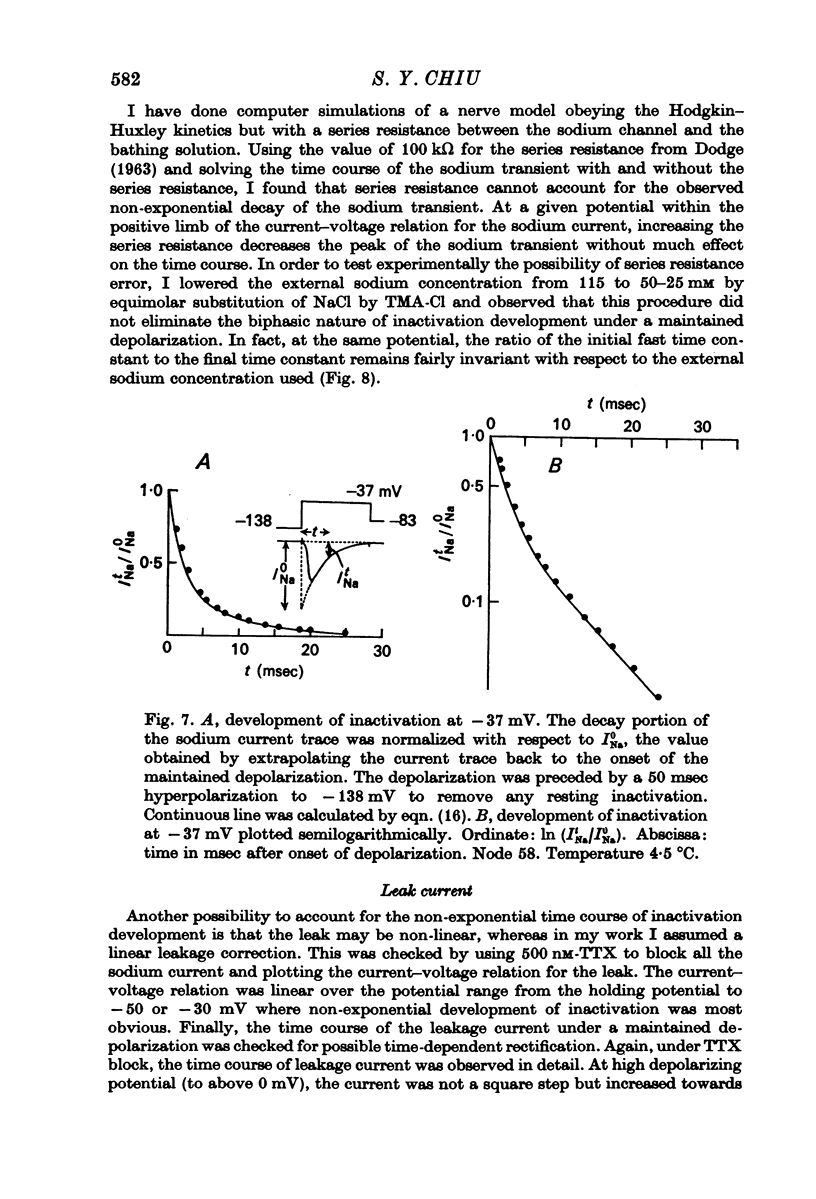
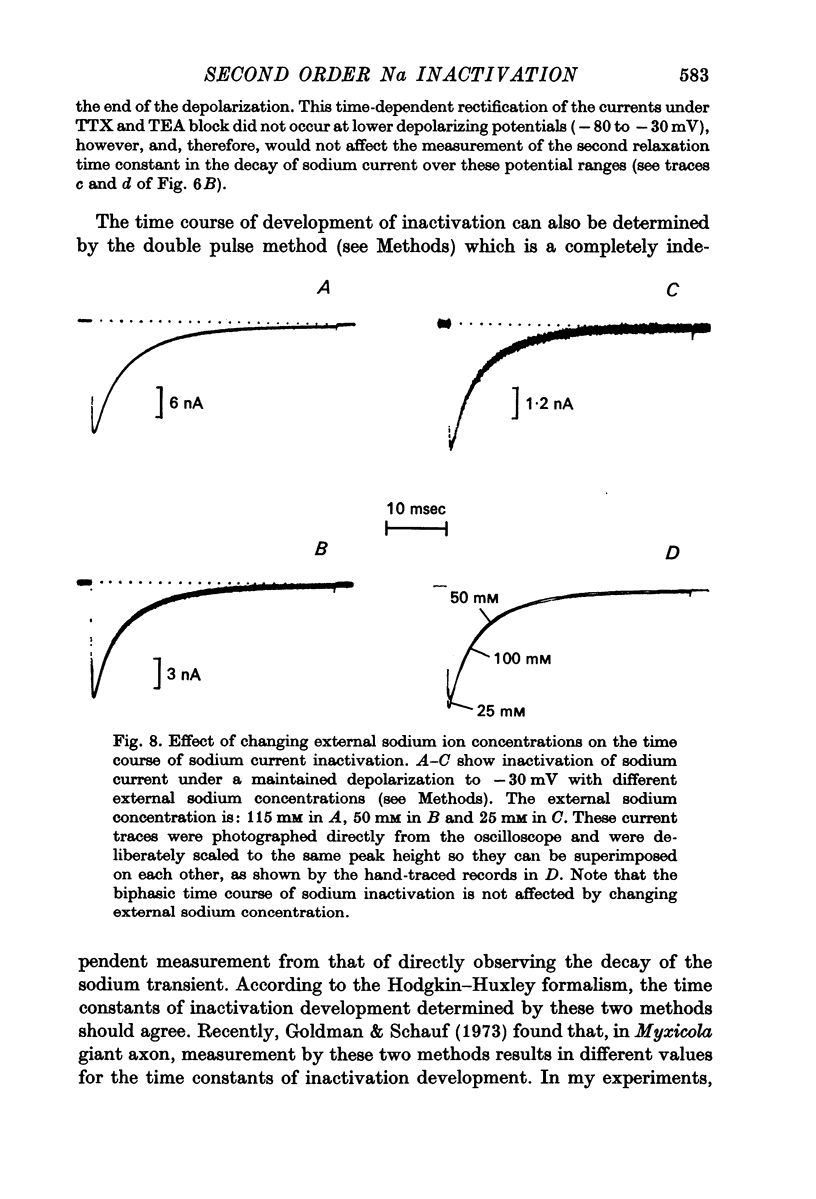
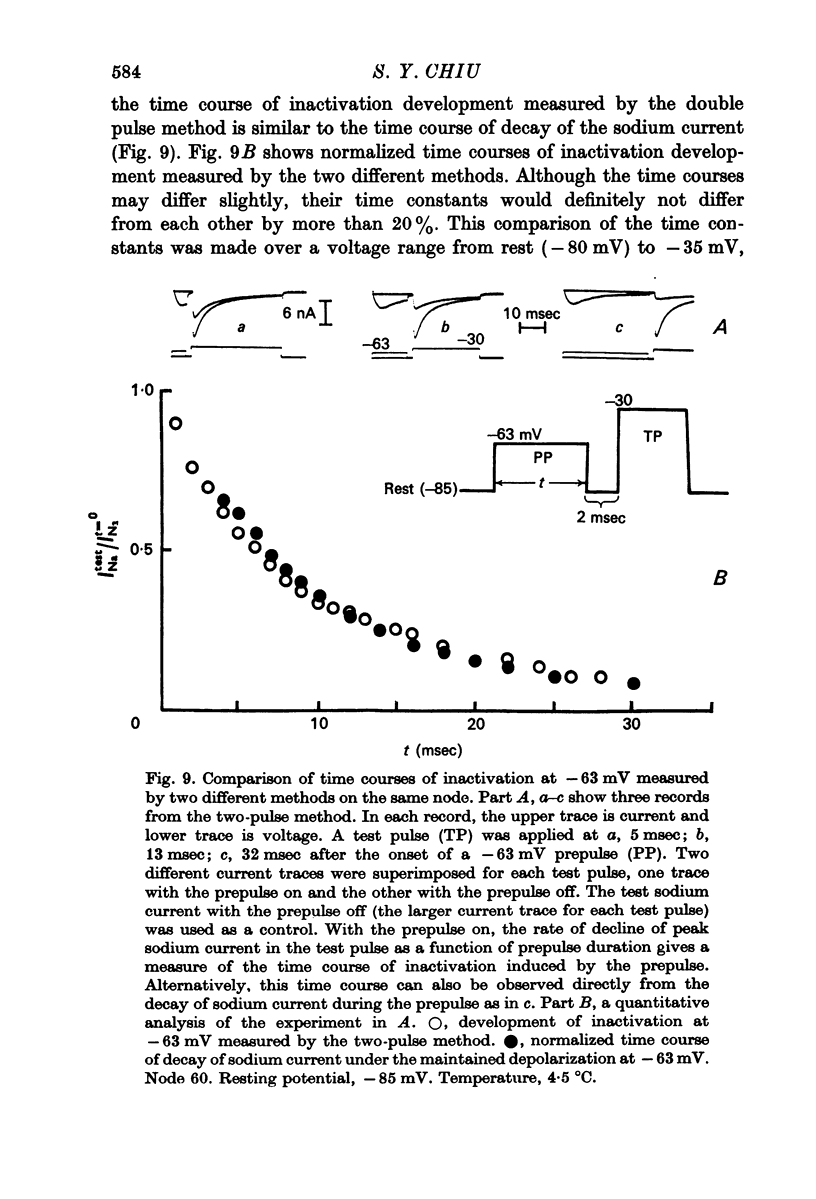
4. Kinetics of development of inactivation were studied by two different methods. The first was to measure the decay of sodium current under a maintained depolarization. The second method was to measure the decay of the peak sodium current in a test pulse as a function of time after the onset of a maintained depolarization. These two methods yielded similar results for the kinetics of inactivation development.
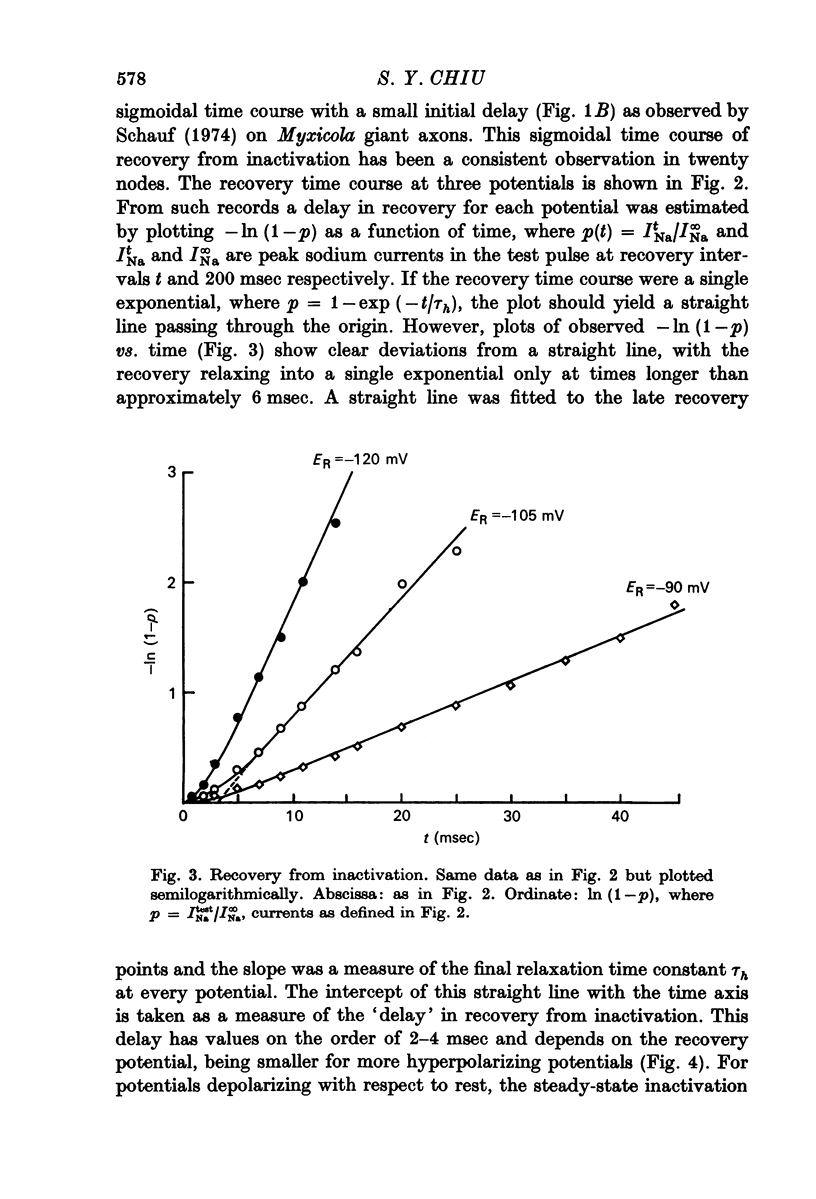
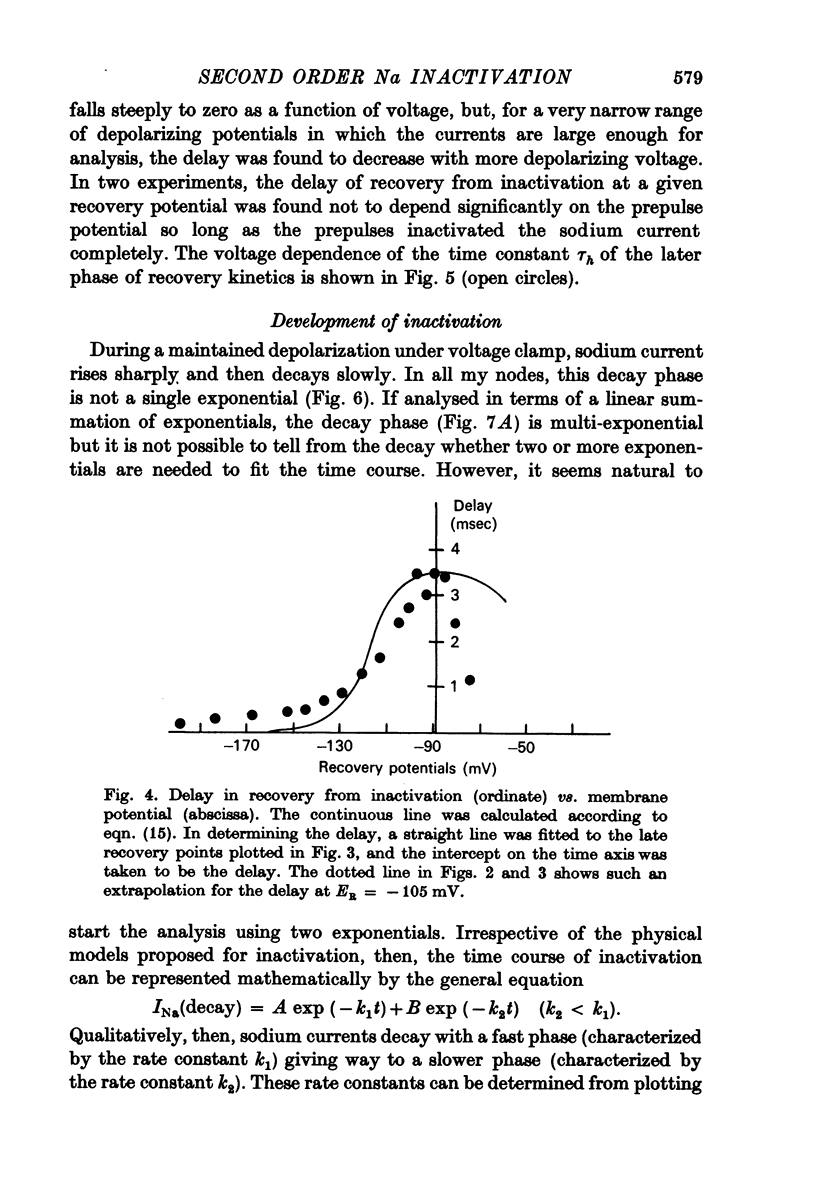
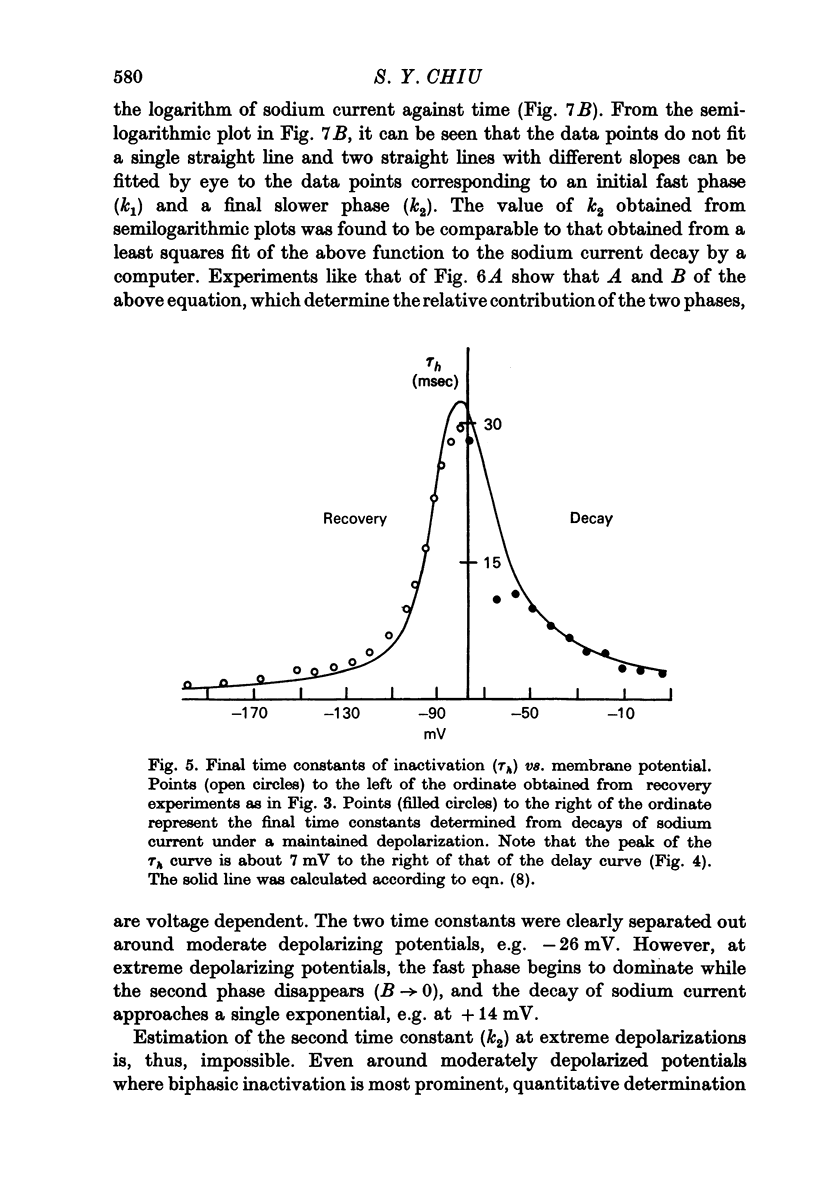
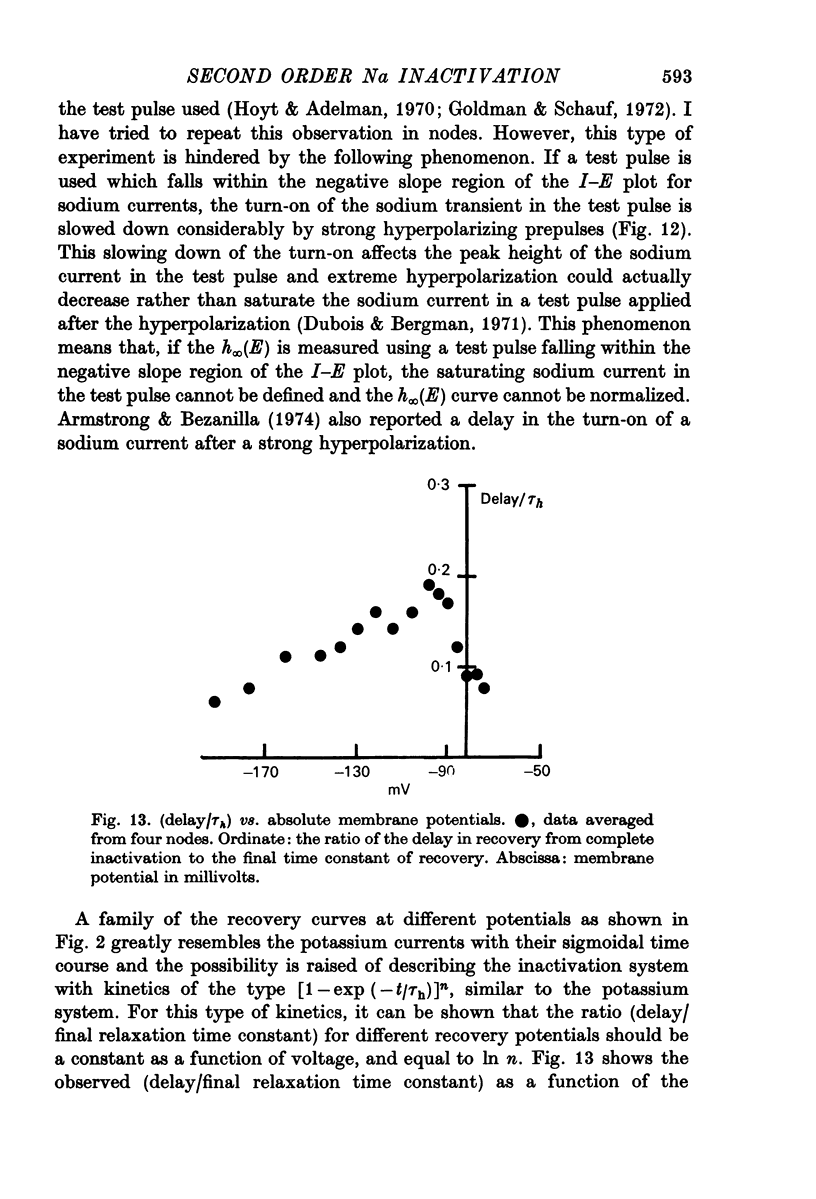
5. Contrary to expectations of the Hodgkin—Huxley formalism, the time course of recovery from and development of inactivation is not strictly exponential. Rather, recovery from complete inactivation shows an initial delay which depends on recovery potentials. Development of inactivation at a fixed potential exhibits at least two exponentials.
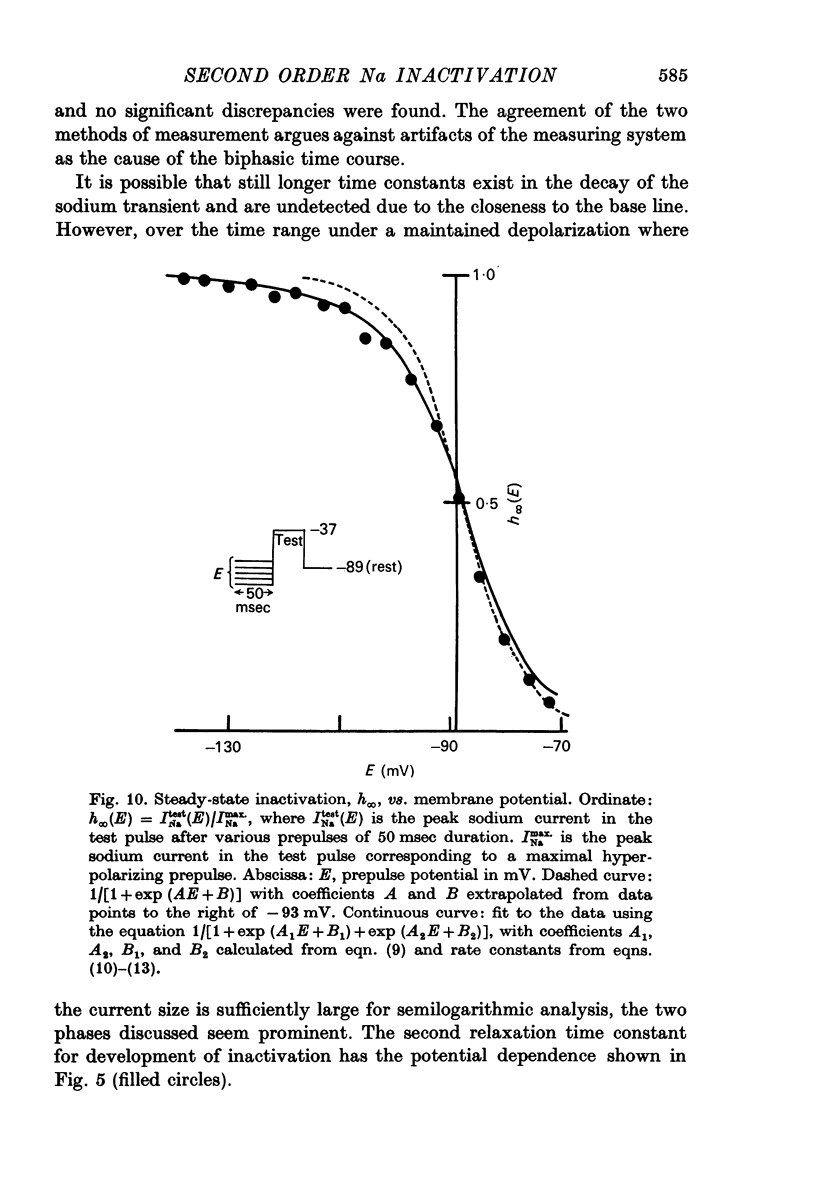
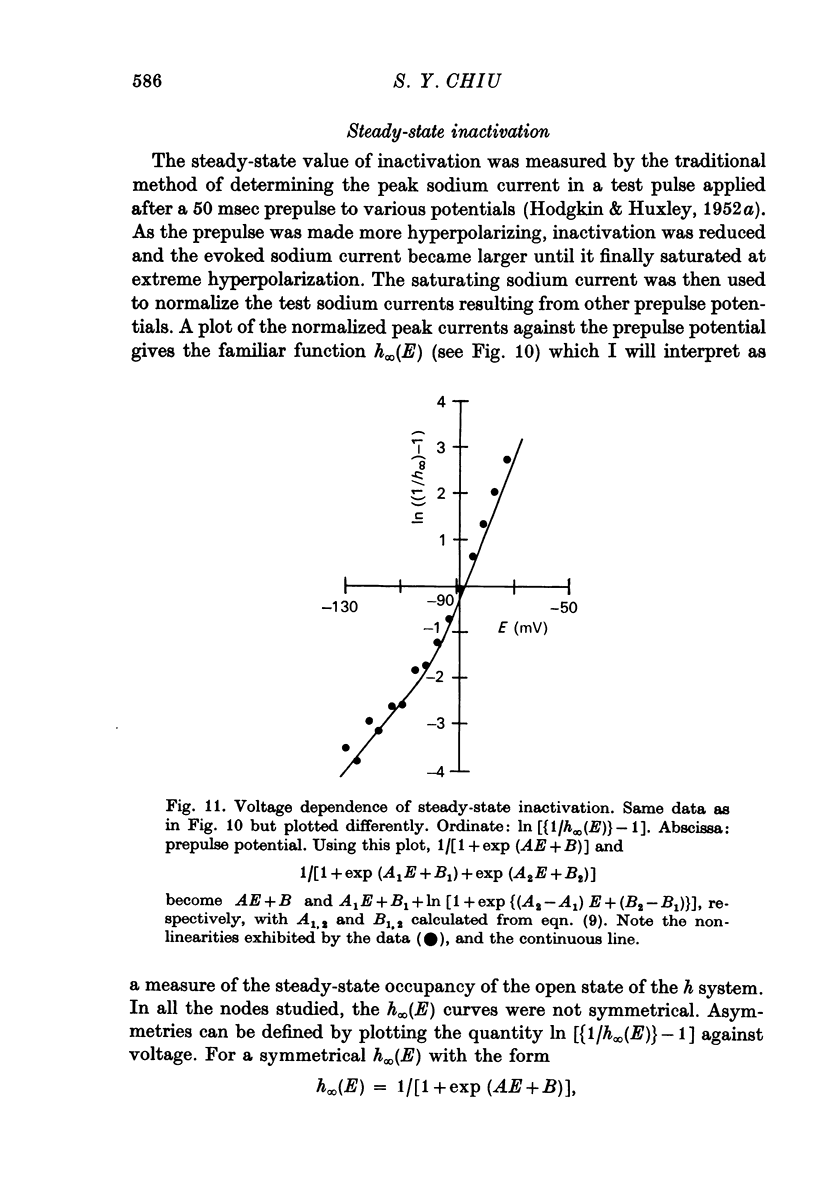
6. The steady-state inactivation curve h∞(E) is asymmetrical and is fitted better by 1/[1+exp (A1E+B1) +exp (A2E+B2)] than by 1/[1+exp (AE+B)].
7. Most of the above kinetic observation on inactivation can be fitted by the following modification of the h system of the Hodgkin—Huxley formalism: [Formula: see text]
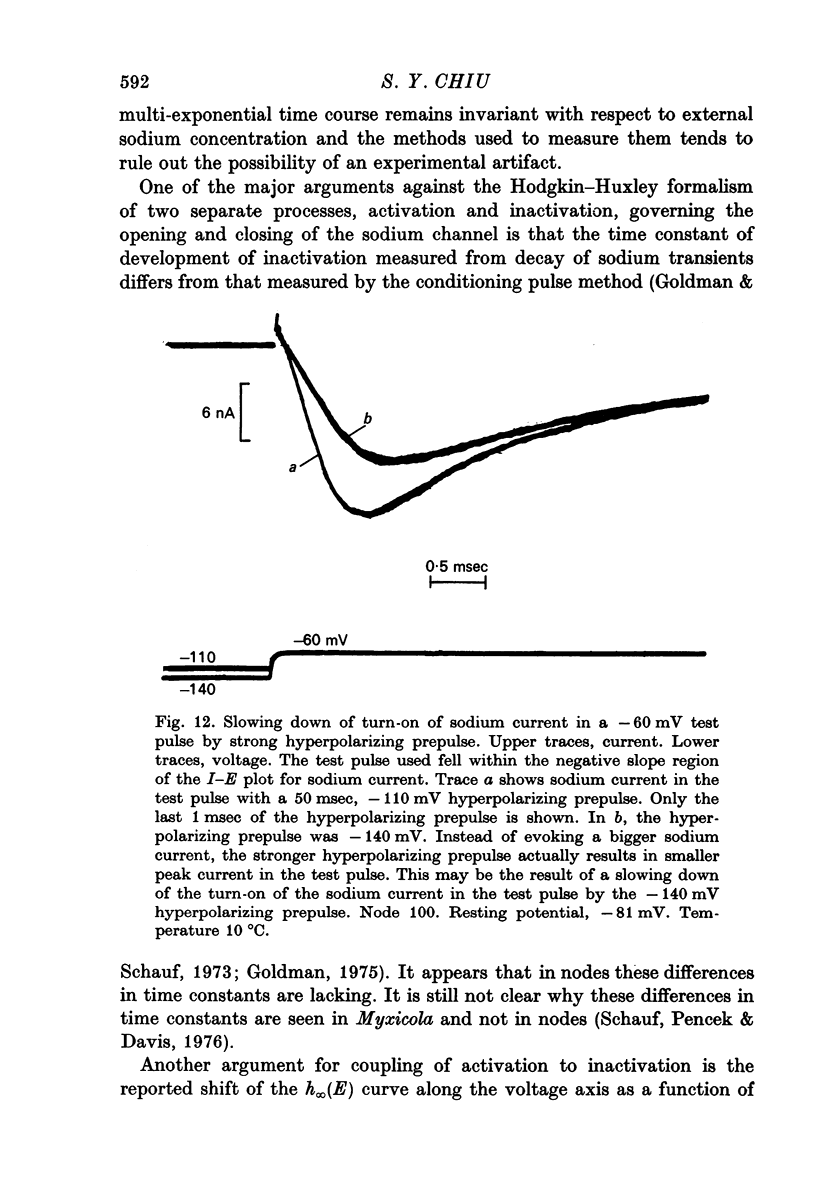
8. In the analysis it was not necessary to modify the concept of two separate processes, activation and inactivation, governing the opening and closing of the sodium channels.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Conductance sodium de la membrane nodale: inhibition compétitive calcium-sodium. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 7;272(23):2924–2927. [PubMed] [Google Scholar]
- Goldman L. Quantitative description of the sodium conductance of the giant axon of Myxicola in terms of a generalized second-order variable. Biophys J. 1975 Feb;15(2 Pt 1):119–136. doi: 10.1016/s0006-3495(75)85796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Gating in sodium channels of nerve. Annu Rev Physiol. 1976;38:139–152. doi: 10.1146/annurev.ph.38.030176.001035. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C., Adelman W. J., Jr Sodium inactivation. Experimental test of two models. Biophys J. 1970 Jul;10(7):610–617. doi: 10.1016/S0006-3495(70)86323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Comparison of two-pulse sodium inactivation with reactivation in Myxicola giant axons. Biophys J. 1976 Mar;16(3):245–248. doi: 10.1016/S0006-3495(76)85684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Further studies of activation-inactivation coupling in Myxicola axons. Insensitivity to changes in calcium concentration. Biophys J. 1975 Nov;15(11):1111–1116. doi: 10.1016/S0006-3495(75)85887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Activation-inactivation coupling in Myxicola giant axons injected with tetraethylammonium. Biophys J. 1976 Sep;16(9):985–989. doi: 10.1016/S0006-3495(76)85749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


