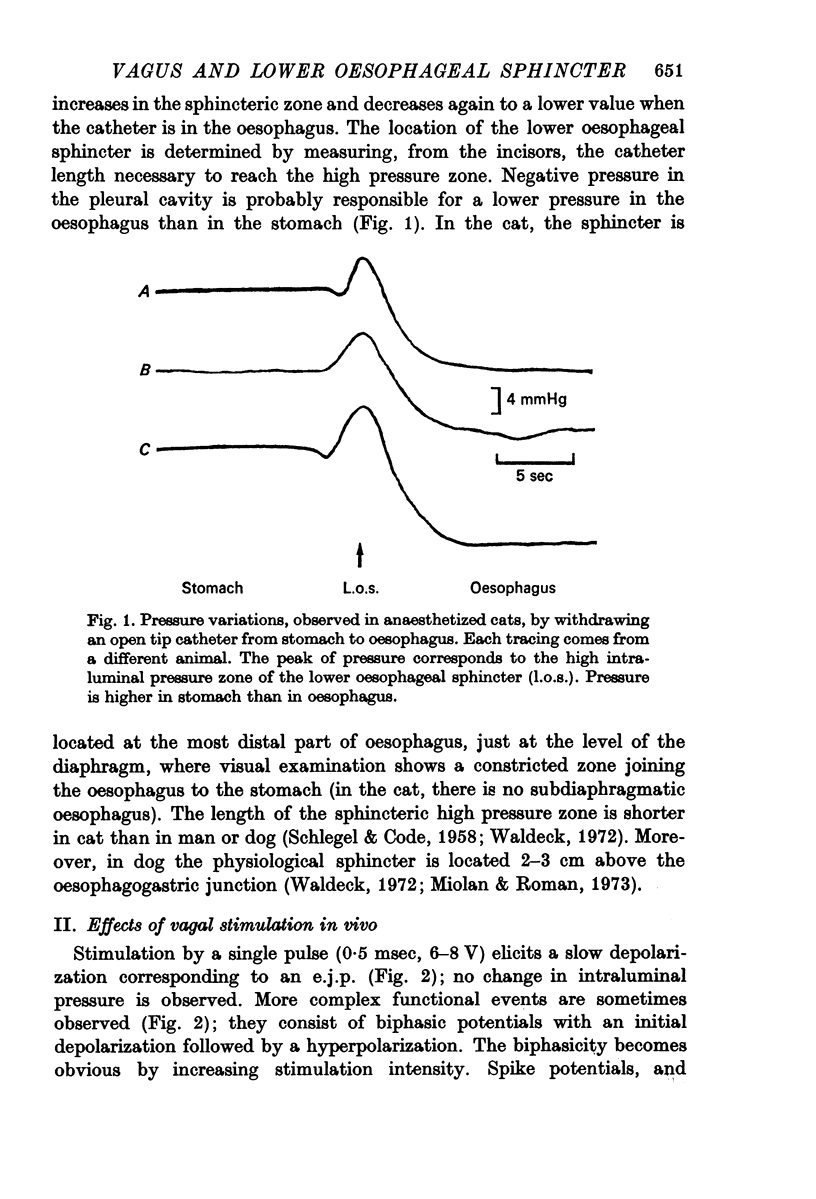
Abstract
1. The effects of vagal efferent fibre stimulation on the smooth muscle of the lower oesophageal sphincter have been studied on the anaesthetized animal and on the isolated and perfused organ.
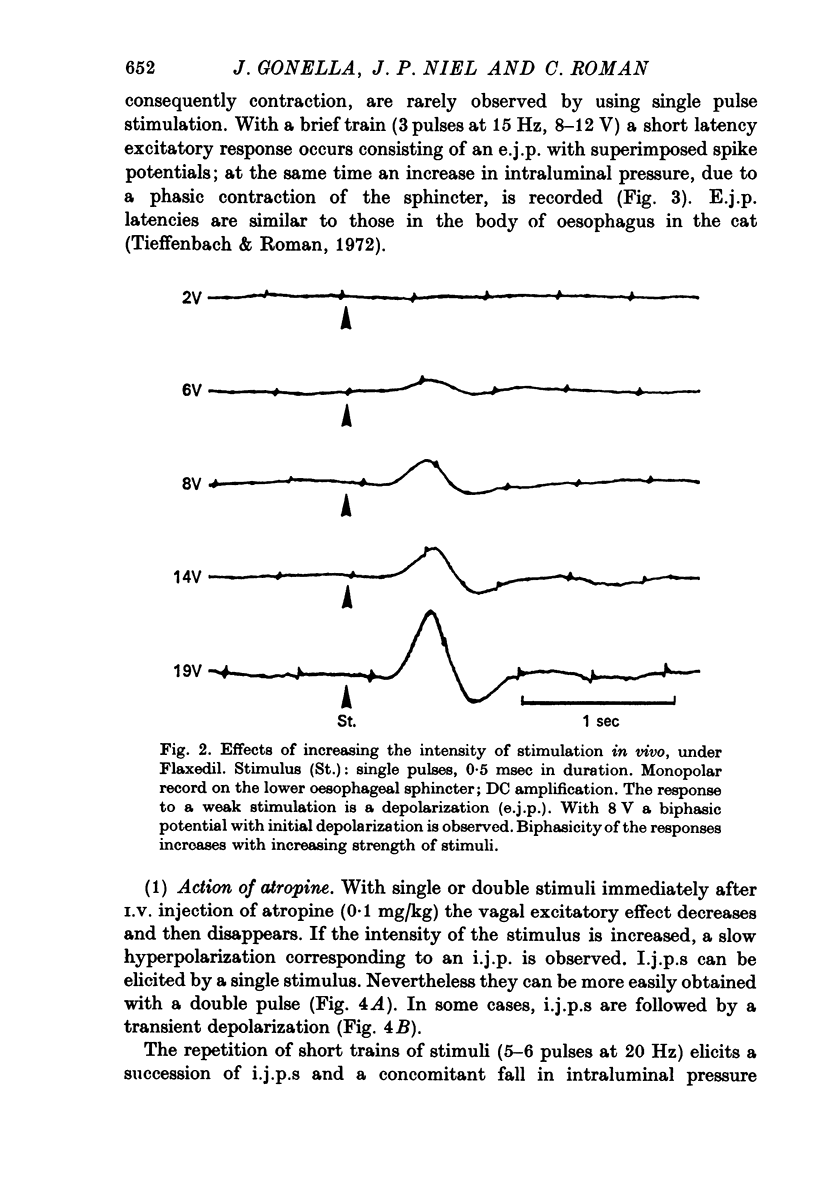
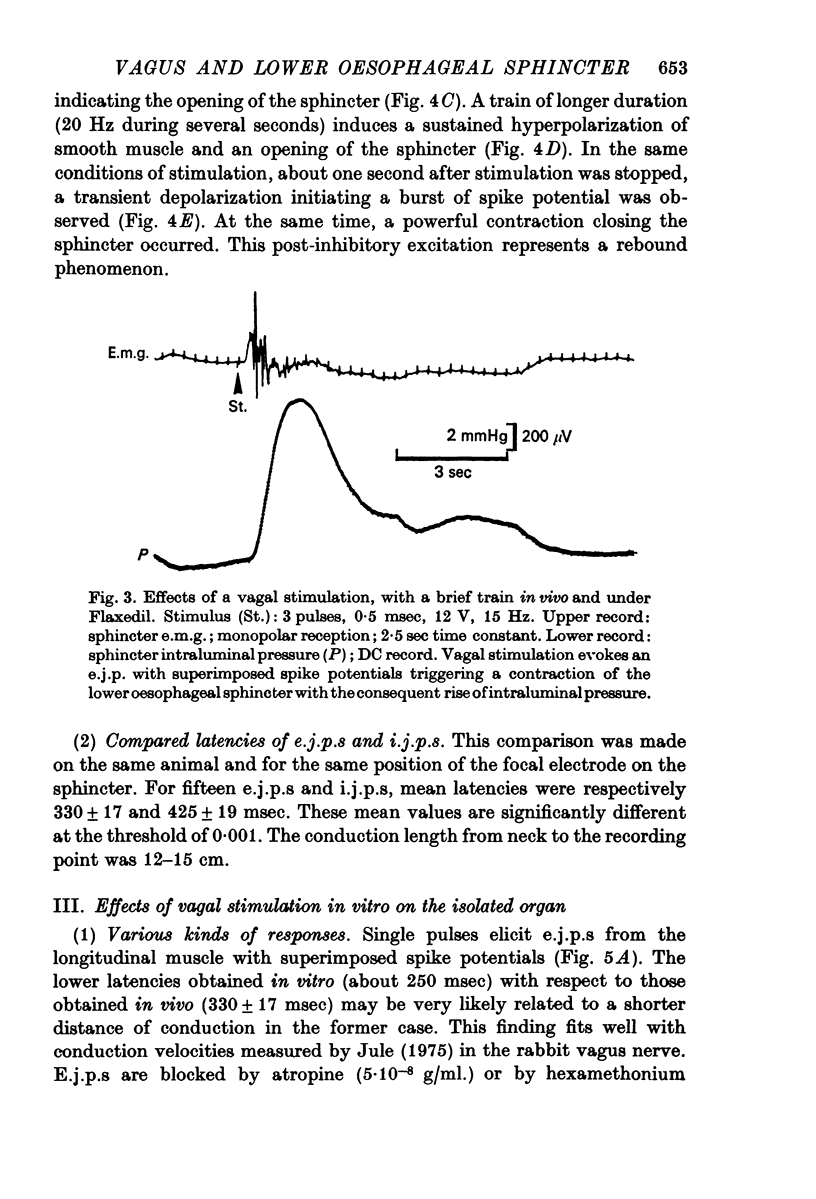
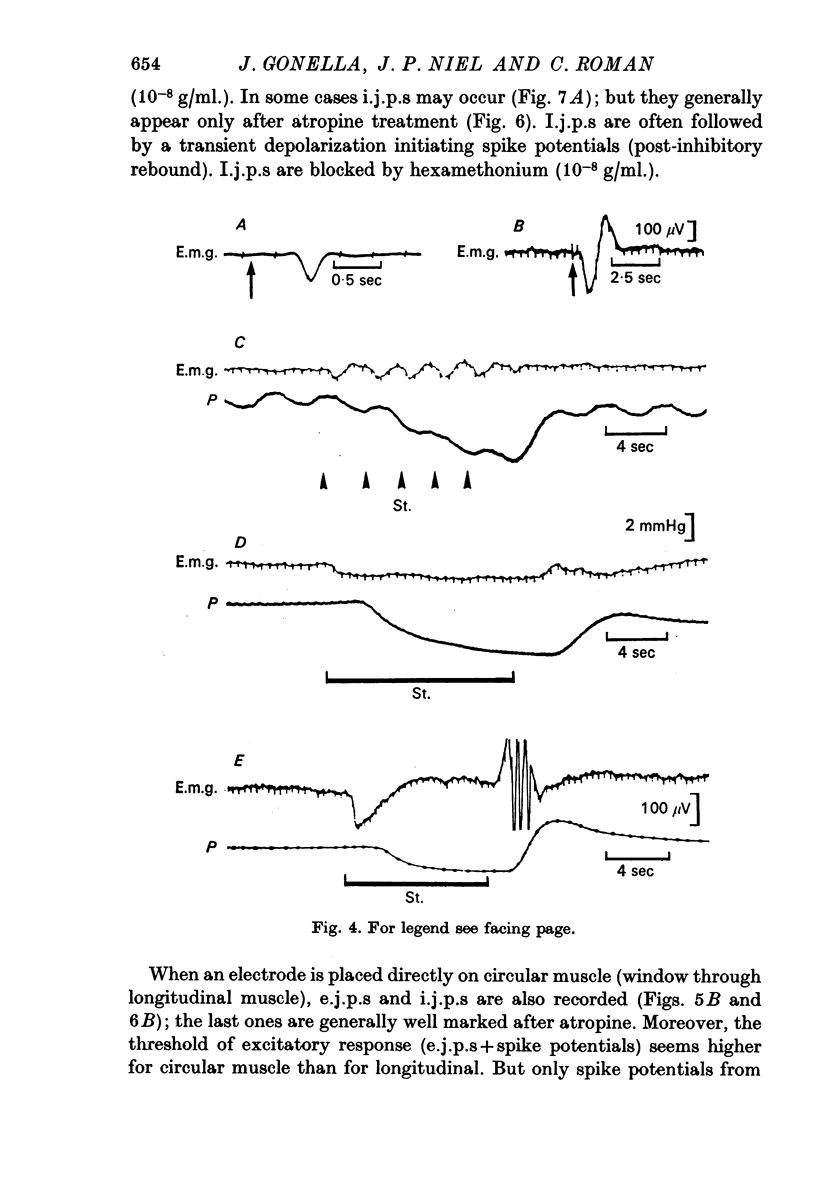
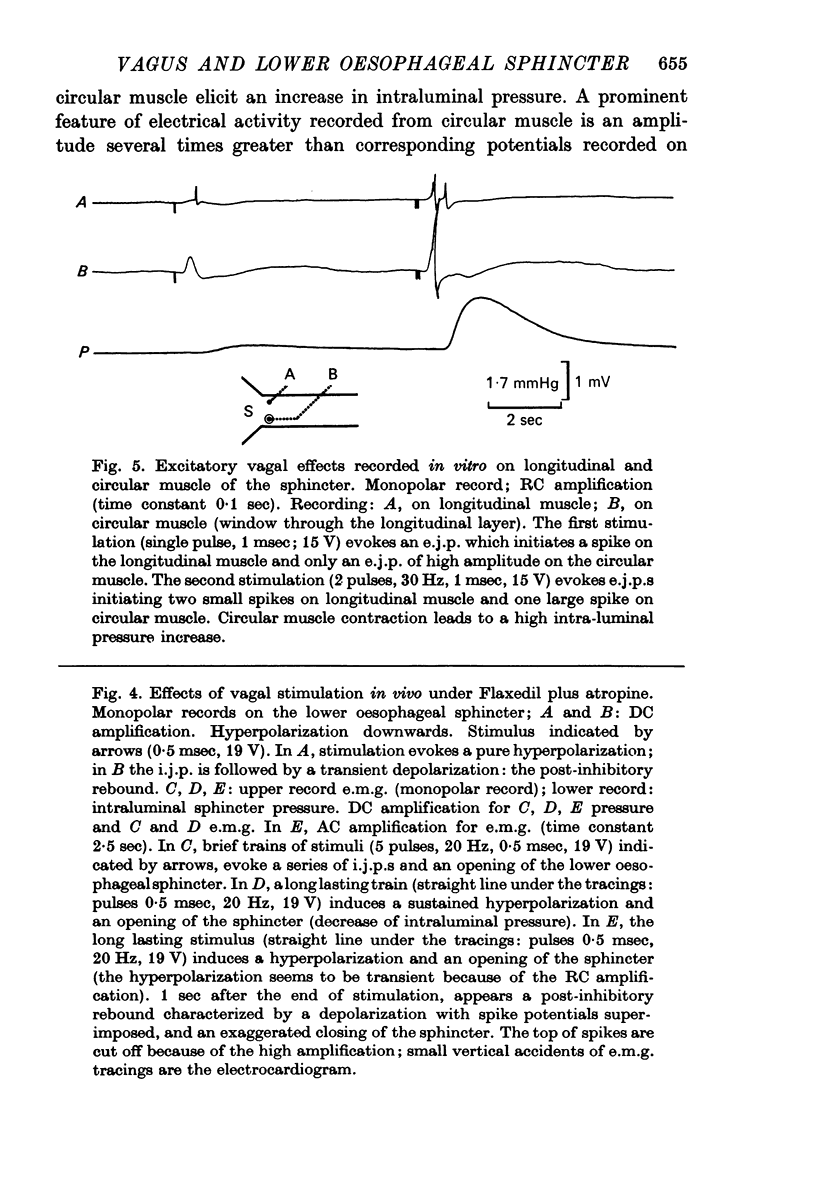
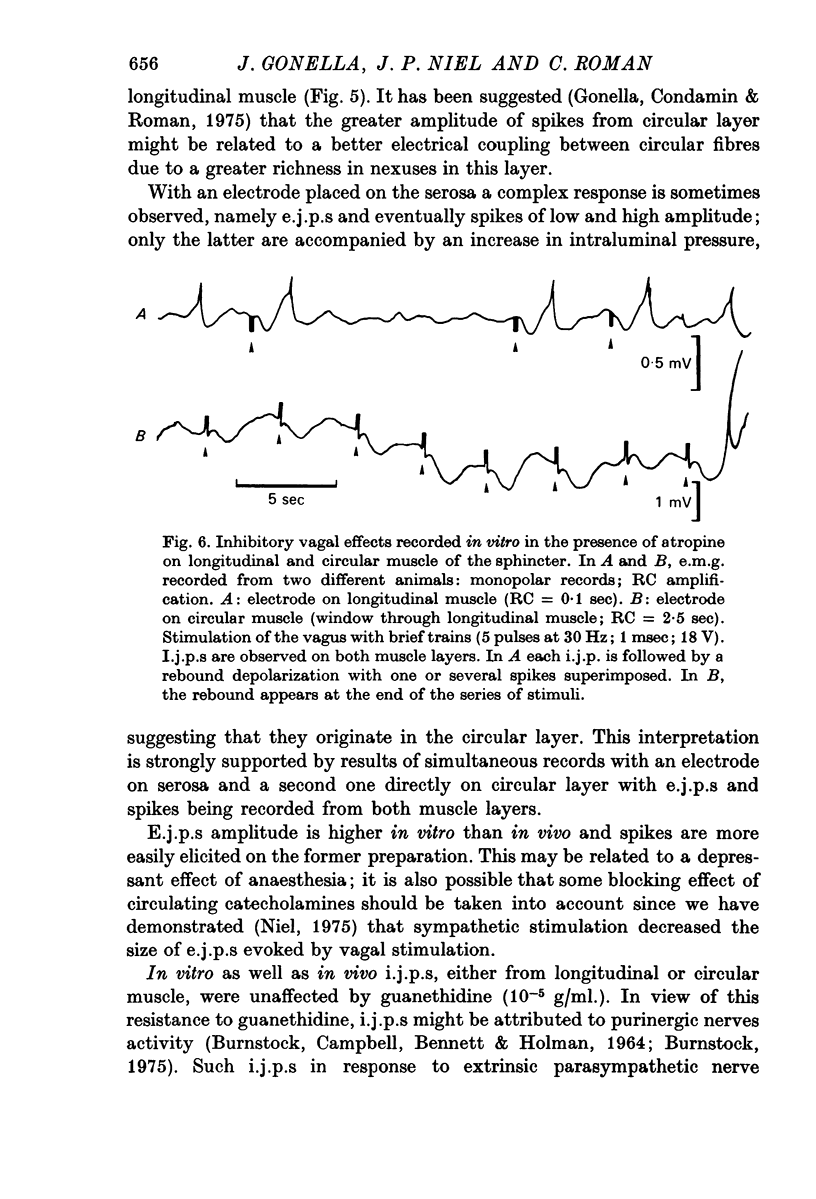
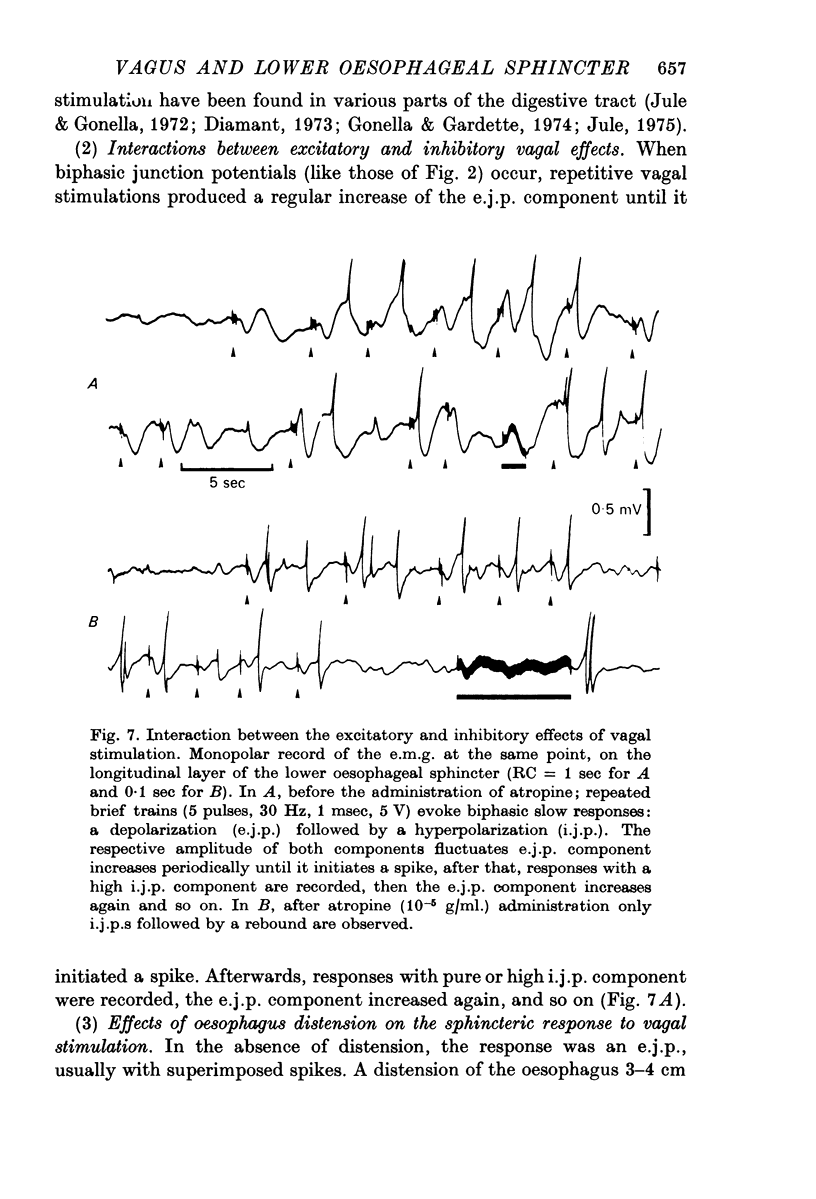
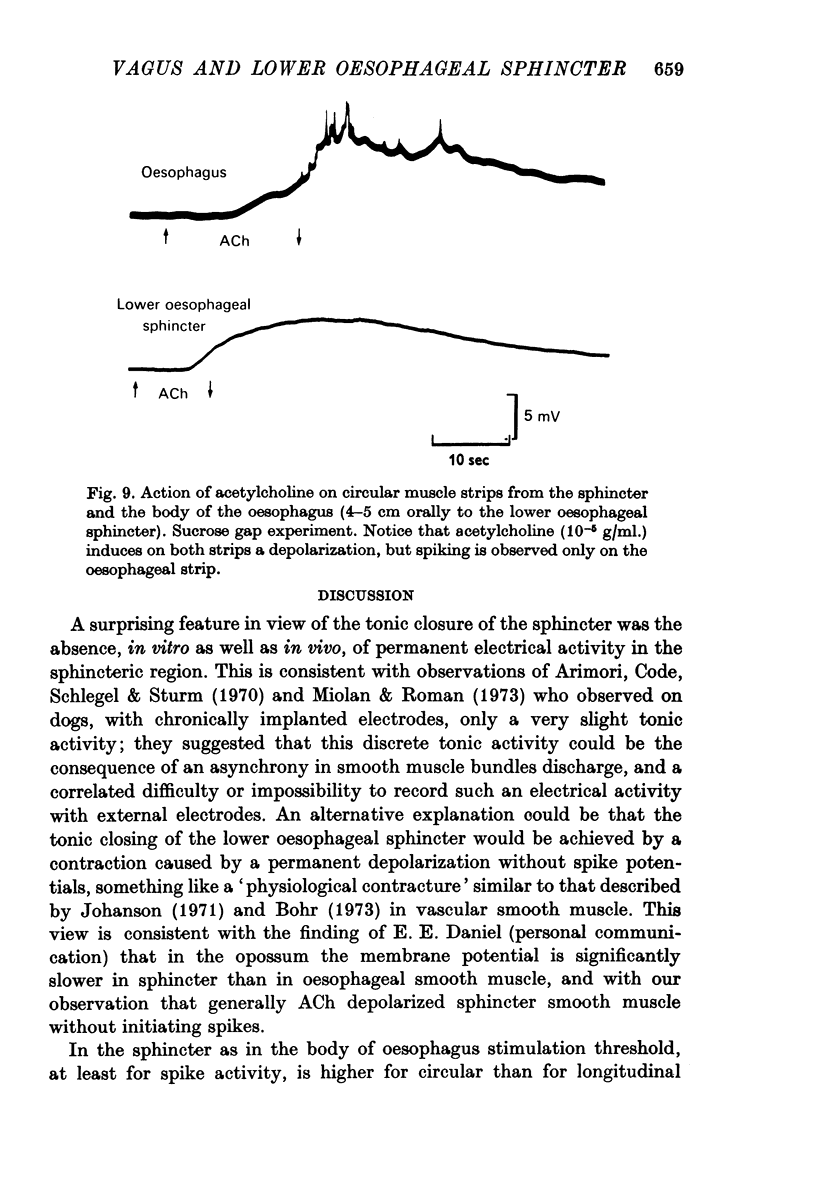
2. In both muscle layers (longitudinal and circular) vagal stimulation elicits two types of electromyographic (e.m.g.) potentials: (a) excitatory junction potentials (e.j.p.s) where there is a depolarization of the smooth muscle fibres. E.j.p.s can give rise to spike potentials inducing a contraction of the sphincter; (b) inhibitory junction potentials (i.j.p.s) where there is hyperpolarization of the smooth muscle fibres, often followed by a transient depolarization which may initiate spikes (post-inhibitory rebound).
3. Pure i.j.p.s are observed after atropine treatment which suppresses e.j.p.s. Under these conditions, a long lasting vagal stimulation induces a long duration hyperpolarization concomitant with an opening of the lower oesophageal sphincter followed after the cessation of stimulation by a powerful rebound leading to a strong contraction which closes the sphincter.
4. Several arguments, pharmacological (action of acetylcholine (ACh), atropine and hexamethonium) and physiological (threshold and latency of responses) lead to the following conclusions.
Preganglionic vagal fibres are cholinergic and they activate (a) intramural excitatory cholinergic neurones; (b) intramural non-adrenergic inhibitory neurones (purinergic neurones). Preganglionic fibres leading to inhibition have a higher threshold than those leading to excitation.
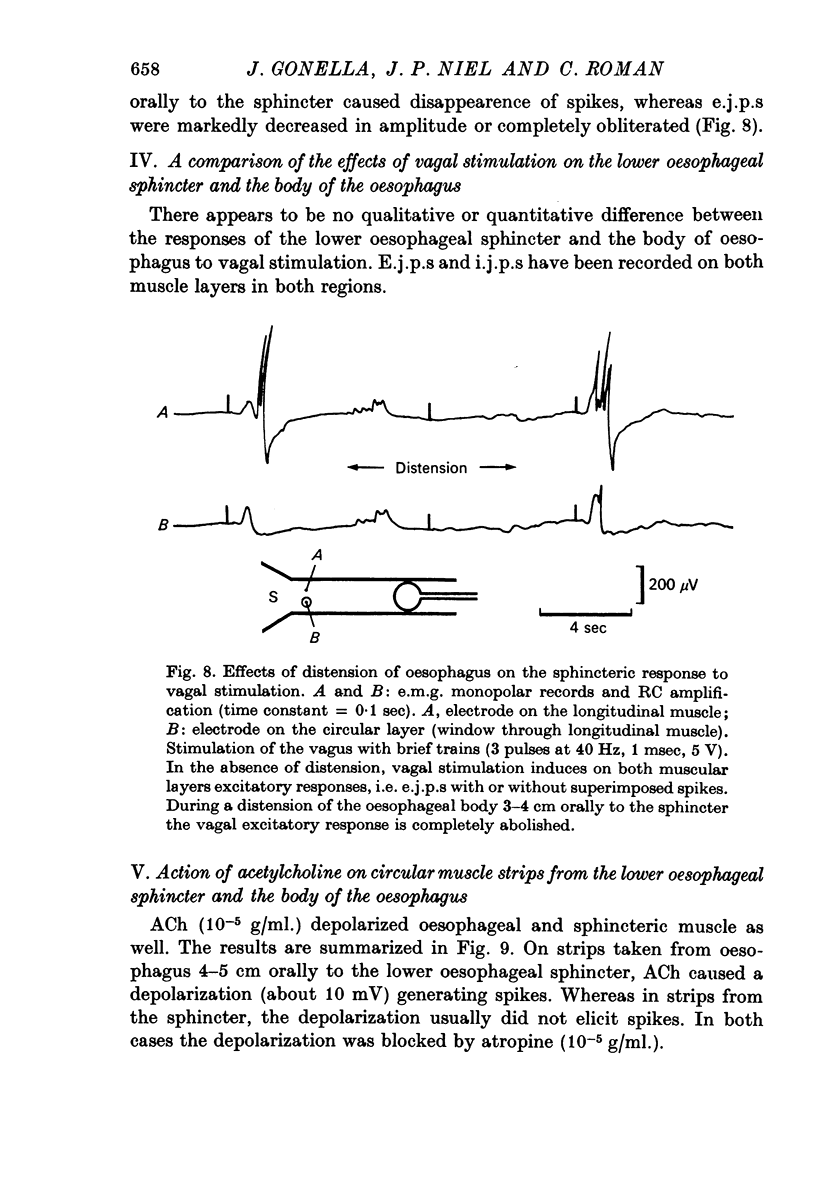
Both excitatory and inhibitory pathways are interconnected inside the intramural network. In particular, activation of intramural inhibitory neurones, by relaxing the oesophagus orally to the lower oesophageal sphincter, inhibits intramural excitatory neurones and subsequently blocks vagal excitatory responses.
5. Two functions may be attributed to the vagal extrinsic innervation: (a) closure of the lower oesophageal sphincter by maintaining the basal tone of the sphincter; this would imply that at rest the inhibitory control is supplanted by the excitatory one; (b) sphincter opening during swallowing by suppressing the excitatory stimulus and reinforcing the inhibitory one (it may be recalled that after bilateral vagotomy, swallowing is no longer followed by a relaxation of the sphincter).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimori M., Code C. F., Schlegel J. F., Sturm R. E. Electrical activity of the canine esophagus and gastroesophageal sphincter: its relation to intraluminal pressure and movement of material. Am J Dig Dis. 1970 Mar;15(3):191–208. doi: 10.1007/BF02233449. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INNERVATION OF THE GUINEA-PIG TAENIA COLI: ARE THERE INTRINSIC INHIBITORY NERVES WHICH ARE DISTINCT FROM SYMPATHETIC NERVES? Int J Neuropharmacol. 1964 May;3:163–166. doi: 10.1016/0028-3908(64)90003-6. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. J Physiol. 1899 May 11;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr D. F. Vascular smooth muscle updated. Circ Res. 1973 Jun;32(6):665–672. doi: 10.1161/01.res.32.6.665. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Paddle B., Staszewska-Barczak J. Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol. 1975 Apr;31(2):360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Gonella J. Modifications of electrical activity of the rabbit duodenum longitudinal muscle after contractions of the circular muscle. Am J Dig Dis. 1972 Apr;17(4):327–332. doi: 10.1007/BF02231733. [DOI] [PubMed] [Google Scholar]
- Higgs B., Ellis F. H., Jr The effect of bilateral supranodosal vagotomy on canine esophageal function. Surgery. 1965 Nov;58(5):828–834. [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., McKirdy H. C. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975 Jan;244(1):113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURDAN F., HUTET G., SAGOLS L., FAUCON G. L'innervation du cardia. C R Seances Soc Biol Fil. 1955 Aug-Sep;149(15-18):1571–1573. [PubMed] [Google Scholar]
- JOURDAN F. L'innervation du sphincter cardiaque et sa mise en jeu. J Physiol (Paris) 1957 Jan-Mar;49(1):227–227. [PubMed] [Google Scholar]
- Johansson B. Electromechanical and mechanoelectrical coupling in vascular smooth muscle. Angiologica. 1971;8(3-5):129–143. doi: 10.1159/000157888. [DOI] [PubMed] [Google Scholar]
- Julé Y., Gonella J. Modifications de l'activité électrique du côlon terminal de lapin par stimulation des fibres nerveuses pelviennes et sympathiques. J Physiol (Paris) 1972;64(6):599–621. [PubMed] [Google Scholar]
- Julé Y. Modifications de l'activité électrique du côlon proximal de lapin in vivo, par stimulation des nerfs vagues et splanchniques. J Physiol (Paris) 1975 May;70(1):5–26. [PubMed] [Google Scholar]
- Kuriyama H., Mishima K., Suzuki H. Some differences in contractile responses of isolated longitudinal and circular muscle from the guinea-pig stomach. J Physiol. 1975 Oct;251(2):317–331. doi: 10.1113/jphysiol.1975.sp011095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On Inhibitory Fibres in the Vagus for the end of the OEsophagus and the Stomach. J Physiol. 1898 Dec 30;23(5):407–414. doi: 10.1113/jphysiol.1898.sp000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTTA G. Contributo sperimentale allo studio del tono muscolare dell'esofago e del cardias nel cane. Arch Fisiol. 1955 Jun 30;55(1-2):1–38. [PubMed] [Google Scholar]
- Mann C. V., Code C. F., Schlegel J. F., Ellis F. H., Jr Intrinsic mechanisms controlling the mammalian gastro-oesophageal sphincter deprived of extrinsic nerve supply. Thorax. 1968 Nov;23(6):634–639. doi: 10.1136/thx.23.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miolan J. P., Roman C. Décharge des fibres vagales efférentes destinées au cardia du chien. J Physiol (Paris) 1973;66(2):171–198. [PubMed] [Google Scholar]
- Miolan J. P., Roman C. Modification de l'électromyogramme gastrique du chien par stimulation des nerfs extrinséques. J Physiol (Paris) 1971;63(5):561–576. [PubMed] [Google Scholar]
- Roman C., Tieffenbach L. Motricité de l'oesophage à musculeuse lisse après bivagotomie. Etude électromyographique (E.M.G. J Physiol (Paris) 1971;63(8):733–762. [PubMed] [Google Scholar]
- SCHLEGEL J. F., CODE C. F. Pressure characteristics of the esophagus and its sphincters in dogs. Am J Physiol. 1958 Apr;193(1):9–14. doi: 10.1152/ajplegacy.1958.193.1.9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Electrical and mechanical properties of longitudinal and circular muscles of the guinea-pig ileum. Jpn J Physiol. 1975;25(6):759–773. doi: 10.2170/jjphysiol.25.759. [DOI] [PubMed] [Google Scholar]
- Tieffenbach L., Roman C. Rle de l'innervation extrinsèque vagale dans la motricité de l'oesophage à musculeuse lisse: Etude électromyographique chez le chat et le babouin. J Physiol (Paris) 1972;64(3):193–226. [PubMed] [Google Scholar]
- Waldeck F. A new procedure for functional analysis of the lower esophageal sphincter (LES). Pflugers Arch. 1972;335(1):74–84. doi: 10.1007/BF00586936. [DOI] [PubMed] [Google Scholar]


