Abstract
In a Rhodobacter sphaeroides ribulose 1,5-bisphosphate carboxylase-oxygenase deletion strain that requires an exogenous electron donor for photoheterotrophic growth, transcription of the genes of the Calvin-Benson-Bassham (CBB) cycle was increased. This finding pointed to a potential physiological effector that enhances the capability of the positive transcriptional activator CbbR to mediate cbb transcription. This effector is most likely ribulose 1,5-bisphosphate or a metabolite derived from this CBB pathway intermediate.
Rhodobacter sphaeroides is a nonsulfur purple bacterium that assimilates CO2 via the Calvin-Benson-Bassham (CBB) reductive pentose phosphate cycle. The enzyme which catalyzes CO2 fixation, ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO), is found in two distinct structural forms in R. sphaeroides, form I and form II, which are synthesized from genes of the cbbI and cbbII operons, respectively (7-10). The other enzyme unique to the CBB cycle, phosphoribulokinase (PRK), is also encoded by distinct, but highly homologous, gene copies within each operon. CbbR, a LysR-type transcriptional activator, is encoded by a gene (cbbR) divergently transcribed from the cbbI operon. It positively regulates the expression of both operons and is responsible for increased levels of gene products from one operon when the other is inactivated upon insertion (4, 9, 10). In addition to CbbR, the PrrA/PrrB (RegA/RegB) two-component global regulatory system is necessary to fully activate transcription, via unidentified signals or effectors (2, 13, 18, 23).
Differential regulation of the cbbI and cbbII operons is well documented (9, 14). The cbbI operon has a more significant role than the cbbII operon under photoautotrophic growth conditions, where CO2 is the sole carbon source (i.e., under an atmosphere of 1.5% CO2 and 98.5% H2). The addition of organic carbon to the medium (for photoheterotrophic growth) results in lowered expression of both cbbI and cbbII, but the relative ratio of cbbII gene expression to cbbI gene expression is enhanced under these conditions. Photoheterotrophic growth on malate results in the production of CO2 upon malate oxidation. This CO2 is used primarily as an electron acceptor via cbbII-encoded enzymes of the CBB cycle. Thus, the CBB cycle, using genes primarily within the cbbII operon, is more important for maintenance of the redox poise of the cell under conditions of photoheterotrophic growth on malate since the amount of CO2 used for carbon is minimal (11, 14, 15, 24, 25).
In the R. sphaeroides wild-type strain HR, deletions of genes that encode form I and form II RubisCO (cbbLS and cbbM, respectively) resulted in a mutant, strain 16, that could not grow photoheterotrophically on malate. The exogenous electron acceptor dimethyl sulfoxide (DMSO) was required to grow strain 16 photoheterotrophically on malate. Strains 16PHC (24) and 16PHG (18, 19) were independently isolated from strain 16 as adaptive mutants that grew without DMSO. Strain 16PHC derepressed the synthesis of the nitrogenase enzyme complex, allowing excess electrons to be scavenged via its hydrogenase activity, even in the presence of ammonia in the medium (13). The basis for the photoheterotrophic competence of strain 16PHG has not been explained but does not appear to involve the same mechanism employed by 16PHC (18, 19). It was of interest to determine how the transcription of the cbb operons was affected in strains 16, 16PHC, and 16PHG relative to that in the wild-type strain HR, since such information may have implications about signaling patterns that affect the transcription of both cbbI and cbbII operons and may provide insights into the interdependence of the CBB enzymes, the nitrogenase enzyme complex, and unidentified processes (such as those in strain 16PHG) that are used to maintain the redox poise of the cell.
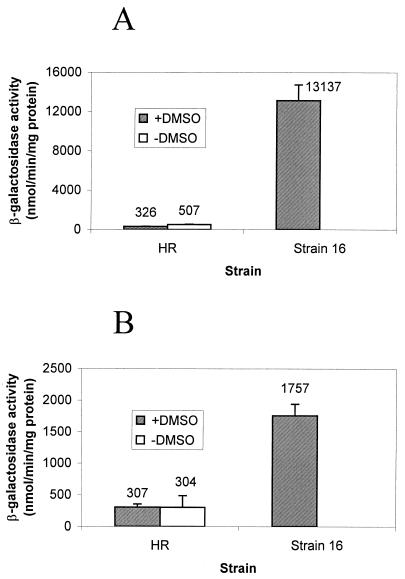
The promoter and upstream regulatory regions of the cbbI and cbbII operons were translationally fused to lacZYA. Plasmids pVKCI (1) and pVKCII (J. Dubbs, unpublished data) contained 636 and 1,017 bp, respectively, of the upstream regulatory regions required for maximal expression of the cbbI and cbbII operons. Plasmid pVK1403-6 lacked the promoter region insertion in front of lacZYA and was used to detect background levels of activity. Each plasmid was introduced into strains HR, 16, 16PHC, and 16PHG by triparental matings (5). Cultures of strains HR, 16PHC, and 16PHG were supplemented with 30 mM DMSO for comparison with strain 16, which requires DMSO to grow under these conditions. Cells were sonicated in Tris-EDTA supplemented with 5 mM β-mercaptoethanol, and the soluble fraction from a low-speed centrifugation (16,000 × g) was assayed for β-galactosidase activity. β-Galactosidase activity was calculated by using the molar extinction coefficient for the product ρ-nitrophenol as previously described (1). In all cases, the background activity from pVK1403-6 was negligible. Strain 16, containing plasmid pVKC1 (the cbbI promoter fusion), possessed greatly elevated β-galactosidase levels relative to those of the wild-type strain HR containing the same plasmid and grown under the same conditions (Fig. 1). Strains 16PHC and 16PHG had negligible cbbI promoter activity when they were grown either with or without DMSO (data not shown). Strain 16 also had higher β-galactosidase activity than the wild type with the cbbII promoter fusion, plasmid pVKCII. This difference was usually considerably smaller, however, than that seen between strain 16 and the wild type with pVKCI, the cbbI promoter fusion (Fig. 1). Strains 16PHC and 16PHG possessed no detectable cbbII promoter activity (data not shown).
FIG. 1.
β-Galactosidase activities for R. sphaeroides strains containing the cbbI translational fusion plasmid pVKC1 (A) or the cbbII translational fusion plasmid pVKCII (B). Cultures were grown photoheterotrophically in the absence or presence of 30 mM DMSO, except strain 16, which does not grow in the absence of DMSO. Error bars indicate standard errors; each bar and the value above it represent the averages of results for several independent cultures. Control plasmid pVK1403-6 yielded no β-galactosidase activity for any strain. Strains 16PHG and 16PHC had activities ranging from 5 to 8 and 4 to 10 nmol/min/mg of protein, respectively, from plasmid pVKC1 and no detectable activity from plasmid pVKCII.
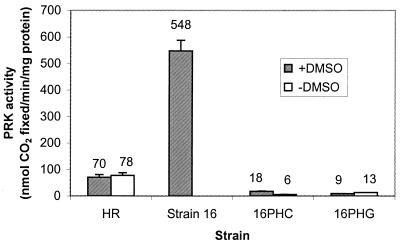
PRK activity was measured as an additional indicator of cbb operon activity. The cbbP genes encode PRK and are located upstream from the RubisCO genes of each operon. Cells were grown and harvested as described above, with the addition of 10 mM ATP to the sonication buffer to stabilize PRK. PRK activity was coupled to RubisCO activity and measured by the incorporation of 14CO2 into acid-stable products (3-phosphoglycerate) (17, 21). Strain 16 had about eightfold higher PRK activity than strain HR, while strains 16PHC and 16PHG possessed extremely low activity (Fig. 2). Note that it was not possible to distinguish with this assay how much activity was attributable to form I PRK versus that attributable to form II PRK. Thus, Western immunoblotting was performed as previously described (6) to qualitatively examine the levels of the 32- and 34-kDa monomers of form I and form II PRK, respectively. PRK standards were prepared from recombinant Escherichia coli (Fig. 3, lanes 1 and 2) (17). It appeared that strain 16 had more form I PRK than form II PRK and also more of both PRK isozymes than the wild-type strain HR (Fig. 3). This is consistent with the fusion data presented above, which indicated higher expression from both cbb promoters in strain 16 and higher expression from the cbbI promoter than from the cbbII promoter (Fig. 1A). Neither PRK isozyme could be detected by using immunoblots prepared from extracts of strain 16PHC or strain 16PHG (data not shown).
FIG. 2.
PRK activities for photoheterotrophically grown R. sphaeroides strains in the presence or absence of 30 mM DMSO. Strain 16 does not grow in the absence of DMSO under these conditions. Error bars indicate standard errors; each bar and the value above it represent the averages of results for several independent cultures.
FIG. 3.
Western immunoblot of crude extracts from photoheterotrophically grown R. sphaeroides strains using form I PRK antiserum, which cross-reacts with both form I and II PRKs. Shown here is a scanned image of the original colorimetric blot. Form I PRK is ∼32 kDa, and form II PRK is ∼34 kDa. Lane 1, E. coli JM109 containing pJN940A (form I PRK); lane 2, E. coli JM109 containing pJN1050BS (form II PRK); lane 3, wild-type strain HR; lane 4, strain 16; lane 5, strain 16PHC; lane 6, strain 16PHG. These extracts were from strains grown with 30 mM DMSO in the medium. Ten micrograms of protein was loaded in each lane.
Conclusions.
These data indicated that the loss of a functional CBB cycle affected transcriptional regulation of the cbb operons in strain 16 compared to that in the wild-type strain HR during photoheterotrophic growth with DMSO. An effect of some type was not unexpected, since previous results with mutations in the genes encoding fructose 1,6-bisphosphatase (cbbFI and cbbFII), aldolase (cbbAI and cbbAII), or phosphoribulokinase (cbbPI and cbbPII) from each operon were also shown to affect both cbbLS and cbbM transcript levels or steady-state levels of both form I and form II RubisCO proteins (9-12). Effects on downstream gene expression are due to polar effects on cbbLS and cbbM transcription. Northern blots would be impossible to interpret for mutants with double deletions of any the above-mentioned genes, since the different sizes of the transcripts would not be directly comparable for quantitation of expression. It has therefore never been clearly shown that complete and direct elimination of RubisCO function would affect the expression of the cbb operons in R. sphaeroides. The finding that a block at the CO2 fixation step of the CBB cycle resulted in increased expression of both cbb operons is thus deemed significant. From these results, it is quite conceivable that the accumulation either of CBB pathway intermediates or of molecules involved in redox balance (the primary function of the CBB cycle during this growth condition) triggered the regulatory changes noted here.
The alternative redox routes taken by strains 16PHC and 16PHG were manifested by additional differences in the levels of transcriptional regulation of the cbb operons. Apparently the ability of strains 16PHC and 16PHG to maintain redox poise completely independently of the presence of either DMSO or the CBB cycle has led to a situation where there is virtually no cbb expression. Strain 16PHC presents an interesting case for the global regulatory role of the PrrA/PrrB (RegA/RegB) system. In both R. sphaeroides and the closely related bacterium R. capsulatus, the response regulator PrrA (RegA) is at least partially responsible for transcriptional activation of photosynthetic reaction center and light-harvesting operons (3, 16). There is evidence that this is also the case for the cbbI operon of R. sphaeroides (2). Inactivation of sensor kinase PrrB (RegB) has already been shown to relieve strain 16PHC of its photoheterotrophic competence by rendering it incapable of derepressing synthesis of the nitrogenase complex (13, 18). It may be interesting in the future to test the promoter fusions used in the present study in strain 16PHCΩ (a prrB knockout mutant of strain 16PHC) or in a strain that is unable to derepress nitrogenase synthesis for some other reason, such as a mutation in the RegA or NifA binding site upstream of the nitrogenase promoter. One might predict that in the absence of nitrogenase derepression and in the presence of DMSO, conditions that might mimic the redox imbalance in strain 16, cbb expression in strain 16PHCΩ would match that seen for strain 16.
The results presented here and in previous experiments demonstrated that the very complex system for transcriptional regulation of the cbb operons may involve gene product-specific, carbon- and nitrogen-linked signals, as well as redox-specific signals, any of which may be mediated locally (through CbbR) as well as globally (through PrrA/PrrB). Like most LysR-type regulators (20), effectors or coinducer metabolites may be important. These are usually derived from a unique product of one of the genes whose transcription is enhanced, which in the R. capsulatus CBB system appears to be a metabolite either identical to or derived from the product of the PRK reaction (22). Thus, in R. sphaeroides, a similar situation might be operable, since ribulose-1,5-bisphosphate (RuBP), the product of the PRK reaction and the substrate for CO2 fixation by RubisCO, is a unique metabolite of the CBB pathway. We are currently engaged in measuring the intracellular pools of RuBP in strains HR, 16, 16PHC, and 16PHG. However, merely demonstrating alterations in RuBP levels would not allow one to conclude that RuBP is responsible for the changes in regulation observed with strain 16PHC. Thus, the binding of CbbR and RegA to upstream cbb promoter elements in vitro in the presence and absence of potential metabolic and redox-specific effectors is also being studied (J. Dubbs, unpublished results). These experiments, in conjunction with in vitro transcription studies, should help elucidate further details of the regulatory mechanism.
REFERENCES
- 1.Dubbs, J. M., and F. R. Tabita. 1998. Two functionally distinct regions upstream of the cbbI operon of Rhodobacter sphaeroides regulate gene expression. J. Bacteriol. 180:4903-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubbs, J. M., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Interaction of CbbR and Reg A∗ transcription regulators with the Rhodobacter sphaeroides cbbI promoter-operator region. J. Biol. Chem. 275:19224-19230. [DOI] [PubMed] [Google Scholar]
- 3.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcone, D. L., R. G. Quivey, Jr., and F. R. Tabita. 1988. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J. Bacteriol. 170:5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figurski, D., and D. R. Helinski. 1979. Replication of an origin-containing derivative of the plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson, J. L., and F. R. Tabita. 1987. Organization of phosphoribulokinase and ribulose bisphosphate carboxylase/oxygenase genes in Rhodopseudomonas (Rhodobacter) sphaeroides. J. Bacteriol. 169:3685-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson, J. L., and F. R. Tabita. 1988. Localization and mapping of CO2 fixation genes within two gene clusters in Rhodobacter sphaeroides. J. Bacteriol. 170:2153-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson, J. L., J.-H. Chen, P. A. Tower, and F. R. Tabita. 1990. The form II fructose 1,6-bisphosphatase and phosphoribulokinase genes form part of a large operon in Rhodobacter sphaeroides: primary structure and insertional mutagenesis analysis. Biochemistry 29:8085-8093. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, J. L., and F. R. Tabita. 1991. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J. Biol. Chem. 266:14646-14653. [PubMed] [Google Scholar]
- 10.Gibson, J. L., and F. R. Tabita. 1993. Nucleotide sequence and functional analysis of CbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J. Bacteriol. 175:5778-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallenbeck, P. L., R. Lerchen, P. Hessler, and S. Kaplan. 1990. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase in Rhodobacter sphaeroides. J. Bacteriol. 172:1736-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallenbeck, P. L., R. Lerchen, P. Hessler, and S. Kaplan. 1990. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J. Bacteriol. 172:1749-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi, H. M., and F. R. Tabita. 1996. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc. Natl. Acad. Sci. USA 93:14515-14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jouanneau, Y., and F. R. Tabita. 1986. Independent regulation of synthesis of form I and form II ribulose bisophosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J. Bacteriol. 165:620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouanneau, Y., and F. R. Tabita. 1987. In vivo regulation of form I ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. Arch. Biochem. Biophys. 254:290-303. [DOI] [PubMed] [Google Scholar]
- 16.Mosley, C. S., J. Y. Suzuki, and C. E. Bauer. 1994. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J. Bacteriol. 176:7566-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak, J. N., and F. R. Tabita. 1999. Molecular approaches to probe differential NADH activation of phosphoribulokinase isozymes from Rhodobacter sphaeroides. Arch. Biochem. Biophys. 363:273-282. [DOI] [PubMed] [Google Scholar]
- 18.Qian, Y. 1997. Ph.D. dissertation. The Ohio State University, Columbus.
- 19.Qian, Y., and F. R. Tabita. 1996. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J. Bacteriol. 178:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 21.Tabita, F. R., P. Caruso, and W. Whitman. 1978. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal. Biochem. 84:462-472. [DOI] [PubMed] [Google Scholar]
- 22.Tichi, M., and F. R. Tabita. 2002. Metabolic signals that lead to control of cbb gene expression in Rhodobacter capsulatus. J. Bacteriol. 184:1905-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vichivanives, P., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol. 300:1079-1099. [DOI] [PubMed] [Google Scholar]
- 24.Wang, X., D. L. Falcone, and F. R. Tabita. 1993. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J. Bacteriol. 175:3372-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, H. H., and F. R. Tabita. 1994. Positive and negative regulation of sequences upstream of the form II cbb CO2 fixation operon of Rhodobacter sphaeroides. J. Bacteriol. 176:7299-7308. [DOI] [PMC free article] [PubMed] [Google Scholar]