Abstract
The DtxR protein is a global iron-dependent repressor in Corynebacterium diphtheriae that regulates transcription from multiple promoters. A search of the partially completed C. diphtheriae genome identified a gene, mntR, whose predicted product has significant homology with the DtxR repressor protein. The mntR gene is the terminal gene in a five-gene operon that also carries the mntABCD genes, whose predicted products are homologous to ABC metal transporters. Transcription of this genetic system, as measured by expression of an mntA-lacZ reporter fusion, is strongly repressed by Mn2+. The divalent metals Fe2+, Cu2+, and Zn2+ did not repress expression of the mntA-lacZ construct. A mutation in the mntR gene abolished Mn2+-dependent repression of the mntA-lacZ fusion, demonstrating that MntR is essential for the Mn2+-dependent regulation of this promoter. Footprinting experiments showed that MntR protects from DNase I digestion an approximately 73-bp AT-rich region that includes the entire mntA promoter. This large region protected from DNase I suggests that as many as three MntR dimer pairs may bind to this region. Binding studies also revealed that DtxR failed to bind to the MntR binding site and that MntR exhibited weak and diffuse binding at the DtxR binding site at the tox promoter. A C. diphtheriae mntA mutant grew as well as the wild type in a low-Mn2+ medium, which suggests that the mntABCD metal transporter is not required for growth in a low-Mn2+ medium and that additional Mn2+ transport systems may be present in C. diphtheriae. This study reports the characterization of MntR, a Mn2+-dependent repressor, and the second member of the family of DtxR-like metalloregulatory proteins to be identified in C. diphtheriae.
Corynebacterium diphtheriae, the causative agent of the severe respiratory disease diphtheria, colonizes the upper respiratory tract of the human host, where it secretes the potent exotoxin, diphtheria toxin (DT) (14, 25). Expression of DT is regulated by iron availability, with maximum production occurring in low-iron conditions (14, 25). The tox gene, the structural gene for DT, is regulated in an iron-dependent manner by the diphtheria toxin repressor protein, DtxR (3, 38, 49). DtxR is the prototype of a family of metal-dependent regulatory proteins that have been identified in numerous bacteria, including both gram-positive (3, 7, 11, 17, 18, 32, 38, 42) and gram-negative (20, 26, 29) species. While DtxR is functionally similar to the ferric uptake regulator, Fur, the two proteins share little if any amino acid homology (3, 12, 38). DtxR, a global iron-dependent repressor in C. diphtheriae, regulates the phage-encoded tox gene, and at least seven chromosomally encoded promoters, including the irp1 to irp6 promoters (23, 30, 41) and the promoter for the hmuO gene (35, 36, 55). DtxR also controls the production of the C. diphtheriae siderophore (38, 46).
DNase I footprinting studies at various C. diphtheriae DtxR-regulated promoters have demonstrated that DtxR binds in a metal-dependent manner to an approximately 30-bp region that includes portions of the −10 or −35 promoter elements (23, 41, 43, 47). It is predicted that DtxR inhibits transcription from these promoters by interfering with the ability of the RNA polymerase to bind to the promoter region. In vitro studies demonstrated that the divalent metals Fe2+, Co2+, Ni2+, Zn2+, Mn2+, and Cd2+ were able to activate the DNA binding function of DtxR (40, 43, 47). In vivo, ferrous iron (Fe2+) is believed to be the physiologically relevant metal that activates DtxR; however, other transition metals, including Mn2+, are known to weakly repress DT production (10). A 19-bp DtxR consensus-binding site was derived by aligning the sequences of several DtxR-responsive promoters (23) and by in vitro genetic methods (48). The 19-bp consensus-binding site contains a 9-bp inverted repeat sequence separated by a single base pair. The crystal structure of DtxR revealed an N-terminal helix-turn-helix DNA binding region, two distinct metal binding sites, a dimerization interface region, and a C-terminal domain that contains an SH3-like fold (27, 28, 31, 53, 54). The metal-bound form of DtxR binds to its cognate operator region as a dimer pair with each dimer interacting on opposite faces of the DNA helix (54).
The in vivo repressor activity of DtxR-like proteins is activated either by Fe2+, as reported for C. diphtheriae and Mycobacterium tuberculosis (9, 38, 40), or by Mn2+, as shown for various gram-negative and gram-positive bacteria (5, 17, 18, 21, 26, 29, 32). Recent analyses of the genomes of Staphylococcus aureus (16) and Bacillus subtilis (4) reveal that these bacteria have three Fur homologs, termed PerR, Zur, and Fur, and a single DtxR homolog, MntR. The MntR protein functions as an Mn2+-responsive regulator of genes involved in manganese homeostasis (17, 32). Other Mn2+-responsive DtxR-like proteins are present in various species of streptococci, where they control the expression of ATP-binding cassette (ABC)-type Mn2+ transporters that are required for virulence (5, 18, 21, 51). MntR-like repressors also control the expression of non-ABC Mn2+ transporters such as the NRAMP-like proteins found in Escherichia coli and Salmonella (20, 26).
In this study, the mntR gene, a homolog of dtxR, was identified from the recently completed genome sequence of C. diphtheriae. MntR was shown to function as an Mn2+-dependent repressor and controlled transcription from a promoter located upstream of a putative ABC metal transport operon. Footprinting experiments indicated that MntR binds to an unusually large 73-bp AT-rich sequence, suggesting that MntR interacts at its primary binding site at the mntA promoter in a manner distinct from that previously reported for other metalloregulatory proteins.
MATERIALS AND METHODS
Bacterial strains and media.
The wild-type C. diphtheriae strain C7(−) has been described (15). Strain 1716 is a clinical isolate from the recent diphtheria epidemic in the former Soviet Union (52) and was provided by Tanya Popovich (Centers for Disease Control and Prevention, Atlanta, Ga.). C7(β)hm723 is derived from the C7 strain and carries a point mutation in the dtxR gene, which results in a defective DtxR protein (39, 40). E. coli DH5α, Bethesda Research Laboratories, was used for routine plasmid isolation and plasmid maintenance. The wild-type B. subtilis strain CU1065 was obtained from the Bacillus Genetic Stock Center and has been previously described (32). The E. coli TOP10 strain (Invitrogen) was used for the analysis and propagation of PCR-derived DNA fragments that were cloned into the pCRII-Blunt-TOPO vector (pCRII-TOPO) (Invitrogen). E. coli BL21(DE3) (45) was used for the overexpression of the mntR gene cloned into the pET expression vectors (Novagen). E. coli and B. subtilis were grown in Luria-Bertani (LB) media (Difco), and C. diphtheriae strains were cultured in heart infusion broth (Difco) containing 0.2% Tween 80 (HIBTW) (Sigma Chemical Co.).
Modified PGT (mPGT) medium has been described (46) and was depleted of metals by Chelex-100 treatment of Casamino Acids and maltose. Fe2+ and Mn2+ were added to the medium as needed (46). The addition of at least 1 μM Fe2+ (ferrous sulfate) to the PGT medium was required for maximum growth of C. diphtheriae strains.
Plasmid construction and DNA manipulations.
All PCR-generated DNA fragments used in this study were initially cloned into the pCRII-TOPO vector. The chromosomal DNA inserts present in the six mntA-lacZ reporter fusion constructs pPO1 through pPO6 were generated by PCR. These PCR-derived DNA fragments were initially ligated into the pCRII-TOPO vector and then excised with BamHI and SalI sites that were incorporated into the 5′ end of the primers, and the resulting fragments were ligated into the promoter probe vector, pCM502 (36).
Derivatives of the pET vectors were used to overexpress the mntR gene. Plasmids p24-R34 (pET24a derivative) and p28-R34H (pET28a derivative), which express either the native or an N-terminal histidine tagged form of MntR, respectively, were produced by PCR amplification with primers R3, 5′GGCTACTGTCATATGCATGTCTCTGAGC-3′, and R4, 5′GCACAAACCTAAGAATCTATT-3′. Genomic C7 DNA was used as a template for PCR. The PCR products were excised from the PCRII-TOPO vector with NdeI (indicated in bold in the R3 primer) and EcoRI (a site located in the vector) and then ligated into the corresponding sites in the appropriate pET vectors. The ATG sequence within the NdeI site serves as the start codon for the native mntR construct. The DNA sequences of all PCR-derived DNA fragments were determined to ensure that the PCR process did not create any sequence errors (34).
The Sanger Institute recently completed the genome sequence of the C. diphtheriae strain NCTC13129 (http://www.sanger.gc.uk/Projects/). Two nucleotide differences between the mntR genes from C7 and NCTC13129 were found. The nucleotide differences did not result in any predicted amino acid changes in MntR. The sequence differences detected between C7 and NCTC13129 were not the result of PCR errors, since duplicate PCR products gave identical sequences for the C7 mntR gene. The DNA sequence of all PCR-generated fragments derived from the clinical strain, 1716, were identical to the sequence reported for strain NCTC13129. Both 1716 and NCTC13129 are clinical isolates obtained from the recent diphtheria epidemic in the former Soviet Union (52).
Mutagenesis techniques.
The procedure for generating site-directed mutations in the C. diphtheriae chromosome by plasmid integration has been described (37). The plasmids used for disruption of the mntR and mntA genes were generated by first ligating PCR-derived DNA fragments, which contain internal portions of the 5′ coding region of either mntA (320 bp) or mntR (300 bp), into the pCRII-TOPO vector. The primers used for PCR had 5′ tail regions that contained either a BglII site or an EcoRV site and NcoI sites. Stop codons were also incorporated into the primers to prevent the formation of fusion proteins that might be generated after recombination into the chromosome. The BglII and EcoRV sites were used to excise the PCR product from the pCRII-TOPO vector, and the resulting DNA fragment was then ligated into the pKN2.6Z vector (8, 38). The resulting plasmids, pMutA and pMutR, were transformed into C7 by electroporation (13). Plasmid DNA was isolated from the C7 transformants and then digested with NcoI, which excised sequences carrying the plasmid origin of DNA replication (37). A 2.6-kb NcoI fragment carrying the Knr gene and the internal fragment of either mntR or mntA was self-ligated under conditions that favor the formation of circular molecules (37). The products of this ligation, which are replication defective since they lack an origin of plasmid replication, were transformed into C7, where they recombined into the chromosome by homologous recombination between the portion of the mnt gene present on the plasmid and the chromosomal copy of the mnt gene. Passage of pMutA and pMutR through C7 was required in order to avoid restriction barriers in this strain (37, 44). Knr recombinants were recovered for both the mntR and mntA constructs. The C7 mntR (C7R) and mntA (C7A) strains were characterized by PCR and Southern blotting, which confirmed that the plasmids had stably integrated into the chromosome at the predicted mnt locus (not shown).
β-Galactosidase assays.
β-Galactosidase assays with C. diphtheriae strains were done as described previously (24, 38). Briefly, strains were grown overnight with shaking at 37°C in HIBTW. Cultures were diluted 1:100 into fresh HIBTW media or into mPGT medium containing the indicated supplements and grown for 18 h. Assays were performed with these 18-h cultures.
Gel mobility shift assays and DNase I protection experiments.
The 202-bp DNA insert present in plasmid pPO2 was used for the DNA binding studies with MntR. A DNA fragment containing the tox promoter region was derived from plasmid pPOB (43). A PCR-derived DNA fragment used as a negative control for the DNA binding studies contained sequences located between the 5′ termini of the inserts present in pPO1 and pPO3 (Fig. 1B). DNA fragments at 0.5 nM were end labeled on one strand at their 3′ termini with [α-32P]dCTP by using the Klenow fragment (33). Protein preparations of DtxR or MntR and various divalent cations were used at the indicated concentrations. The binding buffer for the gel shift experiments contained 20 mM NaPO4 (pH 7.0), 50 mM NaCl, 20 μg of bovine serum albumin/ml, 1 μg of salmon sperm DNA/ml, 0.7% 2-mercaptoethanol, and 10% glycerol. FeSO4 solutions used for the DNA binding studies were prepared immediately before use to limit oxidation. The gel mobility shift experiments and DNase I footprinting studies were done as described previously (40, 43).
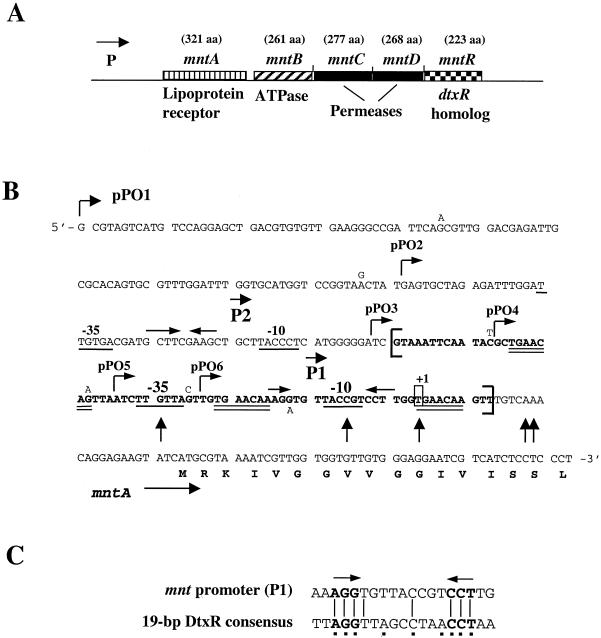
FIG. 1.
(A) Genetic map of the mnt locus. The predicted size of the gene product is indicated in amino acids (aa) above each gene designation. P, location of the mntA promoter. (B) Nucleotide sequence of the mntA promoter region and 5′ portion of the mntA coding region. The sequence shown is from C. diphtheriae strain NCTC13129, the strain used for the genome sequencing project by the Sanger Institute. Nucleotide differences in the C7 strain are indicated adjacent to the nucleotide sequence for NCTC13129. The 5′ ends for the inserts in the mnt-lacZ fusion plasmids, pPO1-pPO6, are indicated. The −10 and −35 sequences for the P1 and P2 promoters are underlined. Inverted arrows within the P1 and P2 promoter regions indicate inverted repeat sequences. Transcription initiates at a T residue (boxed) 7 bp downstream from the −10 sequence of the P1 promoter (+1). The 73-bp nucleotide sequence protected from DNase I digestion by MntR is indicated in bold and flanked by brackets, and the TGAACAA repeat is double underlined. Vertical arrows identify hypercleavable sites from the MntR footprinting experiments. (C) Sequence alignment of the 19-bp consensus DtxR binding site with the inverted repeat region within the mnt P1 promoter. Squares beneath the DtxR consensus sequence indicate the most highly conserved residues (23).
Purification of MntR and DtxR.
DtxR was overexpressed from BL21(DE3)/pDtxR-7 by using the T7 expression system and was purified by Ni2+ affinity chromatography followed by dialysis in the presence of Chelex-100 to remove contaminating metals (40). The MntR protein was overexpressed also by using the T7 system in BL21(DE3) from pET vectors (Novagen). MntR was expressed from plasmid p24-R34 in its native form and from plasmid p28-R34H, where it contained an N-terminal six-histidine tag, MntR-His. Bacterial cells grown to mid-log phase were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and allowed to grow for an additional 2 h. Cells that expressed the native MntR protein were washed in 20 mM NaPO4 buffer (pH 7.0) containing 50 mM NaCl and 3 mM imidazole. Cells expressing MntR-His were washed in 20 mM Tris-HCl (pH 7.5) containing 300 mM NaCl and 20 mM imidazole. Cells were lysed with a French pressure cell, and bacterial extracts were recovered after centrifugation at 10,000 × g for 10 min to remove cell debris. Both forms of the MntR protein were purified by Ni2+ affinity chromatography followed by Chelex-100 treatment as described earlier (40). The MntR-His protein was purified to apparent homogeneity as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. The native MntR protein was greater than 85% pure as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. There was no detectable difference in the DNA binding activity between the native MntR protein and MntR-His in the gel mobility shift or DNase I footprinting experiments (not shown). All of the data presented for gel mobility shift experiments and DNase I footprinting were obtained with the native MntR protein.
Primer extension studies.
C. diphtheriae cells were grown overnight at 37°C in HIBTW and then passaged into fresh media and grown for an additional 6 to 8 h, at which time total bacterial RNA was isolated (39). Primer extension was done using a 30-base oligonucleotide, MntPE1, 5′-ACCAACGAGGAGGGAGGAGATGACGATTCC, which was complementary to the mRNA sequence and located approximately 75 bp downstream from the putative −10 element of promoter P1. Ten micrograms of total bacterial RNA was used for the primer extension experiments. Primer MntPE1 was end labeled with [γ-32P]ATP by T4 kinase (33) and used for primer extension studies as described previously (33).
Growth assays of C. diphtheriae strains in mPGT medium.
Overnight cultures of C. diphtheriae grown in HIBTW or B. subtilis grown in LB were washed in mPGT medium and then inoculated 1:100 into fresh mPGT medium containing various supplements as indicated. Cultures were grown for 18 to 24 h with shaking at 37°C, and then measurements of optical density at 600 nm were performed.
Computer analysis.
Nucleic acid and amino acid sequences were analyzed by DNA analysis software provided by the Genetics Computer Group Wisconsin Package version 10.3. Amino acid sequence similarity searches were done by using the BLAST program (1) at the National Center for Biotechnology Information and also by using the BLAST servers provided at the online sites for The Institute for Genomic Research (http://www.tigr.org/) and at the Sanger Institute (http://www.sanger.ac.uk/). The unpublished genomic DNA sequences for the C. diphtheriae mnt locus were produced by the C. diphtheriae Sequencing Group at the Sanger Institute and can be obtained from the following website: http://www.sanger.ac.uk/Projects/C_diphtheriae.
RESULTS
Identification of the mnt operon on the C. diphtheriae chromosome.
A BLAST search of the recently completed C. diphtheriae genome sequence was done to determine if additional DtxR-like proteins are present in C. diphtheriae. The results of this analysis revealed the presence of a gene whose predicted product displays significant similarity to the family of DtxR-like metal-dependent repressors but is distinct from DtxR. The gene has been designated mntR, for manganese transport regulator, and is the terminal gene in a putative operon that also carries four genes whose predicted products are homologous to ABC metal transport systems (Fig. 1A). The genes carried by the putative ABC transporter, which are named mntA, mntB, mntC, and mntD, show the highest similarity to genes involved in the transport of Mn2+ (5, 22, 29, 32), which suggests that this system encodes an Mn2+ transporter. There are no gaps between the coding regions of the mntB, mntC, mntD, and mntR genes; however, a 68-bp gap exists between the mntA and mntB genes. Inspection of the mntA-mntB intergenic region did not reveal any σ70-like promoter sequences.
Analysis of mnt promoter activity.
The presence of the mntR gene in an operon that also encodes a putative ABC metal transporter suggests that MntR may have a role in the regulation of this operon. To determine if mntR is able to control expression of the mnt operon, promoter-lacZ fusions were constructed in the promoter-probe vector pCM502 (36). Plasmid pCM502 contains a promoterless lacZ gene and replicates in C. diphtheriae at one to three copies per cell (36, 44). Analysis of the sequence upstream of the mntA gene revealed the presence of two putative promoter sequences, designated P1 and P2 (Fig. 1B). Partially overlapping each of these promoter regions were inverted repeat sequences that may serve as binding sites for regulatory proteins. The inverted repeat at the P1 promoter shared some homology with the consensus DtxR binding site, matching 7 of 9 of the most conserved residues but only 8 of 19 overall (Fig. 1C). A 304-bp DNA fragment (the complete sequence is shown in Fig. 1B), which contains the P1 and P2 promoter regions, was placed into pCM502 to generate plasmid pPO1.
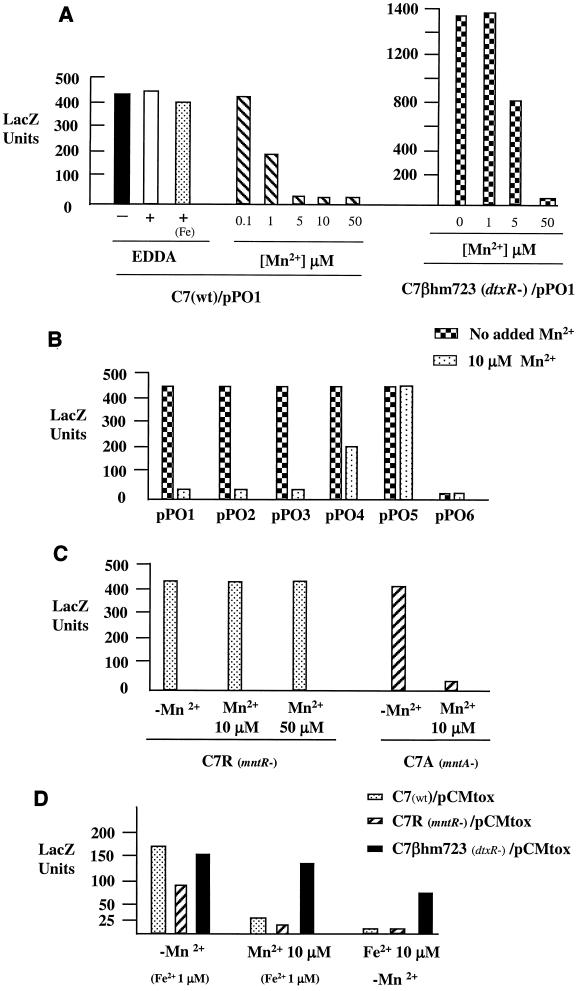
β-Galactosidase assays were used to quantitate expression from pPO1 in C. diphtheriae C7 that was grown either in HIBTW medium or in a metal-depleted mPGT medium. Figure 2A shows that the addition of EDDA [ethylenediamine di(o-hydroxyphenylacetic acid)], an iron chelator, to the HIBTW medium (an iron-replete medium) had no effect on expression of the promoter activity from pPO1. The addition of 50 μM Fe2+ to the HIBTW-EDDA medium also had no effect on expression (Fig. 2A). These observations strongly suggest that iron has no role in the regulation of the promoter activity in this region. The addition of Mn2+ to the HIBTW medium or the mPGT medium (not shown) repressed transcription from the mntA promoter (Fig. 2A). Mn2+ concentrations as low as 1 μM resulted in partial repression (2-fold), and concentrations at 5 μM or higher resulted in 20- to 25-fold repression. Almost identical results were seen when expression from pPO1 was measured in the metal-depleted mPGT medium (data not shown). In mPGT medium, concentrations of Fe2+ up to 50 μM and Cu2+ and Zn2+ at 5 μM did not repress expression of the mntA promoter on pPO1, while the presence of Mn2+ resulted in a level of repression of the mntA promoter similar to that seen in HIBTW medium (Fig. 2A and data not shown).
FIG. 2.
β-Galactosidase assays. (A) C. diphtheriae C7 (wild type) and C7(β)hm723 (dtxR) carrying the mntA-lacZ fusion on plasmid pPO1 were grown overnight in HIBTW medium with the indicated supplements. +, EDDA, an iron chelator, was added at 30 μM to produce low-iron medium; Fe, 50 μM Fe2+ was added to the HIBTW medium containing EDDA; −, no supplement was added to the HIBTW medium (an iron-replete medium). (B) C. diphtheriae C7 carrying mnt-lacZ fusions on plasmids pPO1-pPO6 was grown in HIBTW medium in the presence and absence of 10 μM Mn2+. (C) C. diphtheriae C7R (mntR-) and C7A (mntA-) were grown in HIBTW medium containing the indicated supplements. (D) C. diphtheriae strains C7, C7R, and C7(β)hm723 carrying pCMtox (tox-lacZ) were grown overnight in mPGT medium containing the indicated supplements. The addition of 1 μM Fe2+ (Fe2+ 1 μM) was required for maximum growth of C. diphtheriae strains, and this is considered a low-iron concentration, since maximum derepression of the tox promoter is observed at this iron concentration. LacZ units were determined as described previously (36). Values for all assays are the averages of at least three independent experiments, and results from each experiment did not vary by >15% from the average.
The addition of EDTA, which chelates divalent cations, such as Mn2+, to HIBTW medium did not result in increased levels of activity from pPO1 (data not shown). This result indicates that expression from the mnt promoter region could not be further derepressed by the removal of the existing Mn2+ present in HIBTW medium. The similar results observed with HIBTW and mPGT with regard to Mn2+-dependent repression suggest that the Mn2+ concentration in HIBTW is less than 1 μM, which is the lowest Mn2+ concentration that provided repression of the mntA-lacZ fusion.
Various mntA-lacZ fusions were also constructed by using DNA derived from the clinical strain 1716. No significant differences were observed in the expression or in the level of Mn2+-dependent repression between the 1716 constructs and the plasmids containing C7 DNA (data not shown).
To determine if the dtxR gene affected expression from the mntA promoter, the promoter activity from pPO1 in a dtxR mutant of C7 (C7βhm723) was examined. β-Galactosidase assays revealed that promoter activity in HIBTW medium (+Fe) without any supplements was at least threefold higher than that detected in C7 (Fig. 2A). Higher concentrations of Mn2+ were required to repress expression of the mntA promoter in the dtxR mutant background than in the wild-type C7 strain (Fig. 2A). At 5 μM Mn2+ only a 40% reduction in promoter activity was seen in the dtxR mutant C7βhm723, while in the wild-type C7 strain, 5 μM Mn2+ resulted in full repression. Fifty micromolar Mn2+ was required to fully repress (20-fold reduction) the promoter activity from pPO1 in C7βhm723. The reason for the increased expression and altered Mn2+ repression at the mntA promoter in C7βhm723 is not known.
Identification of sequences within the mntA promoter region that are required for transcription and regulation.
To identify the essential sequence elements required for the transcription and the Mn2+-dependent repression of the mntA promoter region, five additional mnt-promoter-lacZ fusions were constructed. The fusions are designated pPO2-pPO6 and were constructed in pCM502. All of the fusions have the same 3′ terminus as that of pPO1 but contain various amounts of sequence upstream of the mntA gene (Fig. 1B). The promoter fusion plasmids were assessed for promoter activity in C. diphtheriae C7 that was grown in HIBTW medium in the presence or absence of 10 μM Mn2+ (Fig. 2B). There was no significant difference in the promoter activity or the level of Mn2+-dependent repression from pPO1, pPO2, or pPO3. While the inserts in plasmids pPO1 and pPO2 both contain the P1 and P2 promoters, the P2 promoter is deleted in pPO3, which suggests that the P2 promoter is neither required for expression nor involved in the Mn2+-dependent repression seen in this region (Fig. 1B and 2B). Plasmid pPO4 showed approximately 50% repression in the presence of Mn2+, while expression from plasmid pPO5 was not repressed by Mn2+. The promoter activity from pPO4 and pPO5 in the absence of added Mn2+ was equivalent to that seen from plasmids pPO1-pPO3, which suggests that the pPO4 and pPO5 constructs contain all of the sequences required for full promoter activity. However, these promoter-lacZ constructs showed either partial (pPO4) or no (pPO5) repression in the presence of Mn2+, which suggests that these fusions are lacking sequences essential for Mn2+-dependent repression. Plasmid pPO6, which lacks the putative −35 sequence for the P1 promoter, showed only basal levels of promoter activity and no Mn2+ repression, which suggests that the P1 promoter is the only promoter required for the expression of the mnt genes.
Site-directed mutations, using a vector integration method (37) (see Materials and Methods), were made in the C7 chromosomal mntR (C7R) and mntA (C7A) genes to determine what effect mutations in these genes would have on the expression and regulation of the mntA promoter. As shown in Fig. 2C, expression from the mntA-lacZ fusion construct in C7R was no longer sensitive to Mn2+ repression, even at 50 μM Mn2+. This finding confirms that MntR is required for the Mn2+-dependent repression at the mntA promoter. The mntA promoter in strain C7A was regulated in a manner similar to that of the wild-type strain. The vector insertion in the mntA gene did not appear to have a polar effect on the downstream mntR gene, which is likely due to promoter activity present on the integrated vector sequences (M. P. Schmitt, unpublished observation).
To determine if the mntR gene affects transcription of the dtxR-regulated tox promoter, expression from the tox-lacZ fusion construct, pCMtox (36), was examined in various C. diphtheriae strains that were grown in the iron-depleted mPGT medium. One micromolar Fe2+ was added to the mPGT medium to allow growth of C. diphtheriae. This concentration of Fe2+ allows maximum expression of the tox promoter and is considered low iron. As shown in Fig. 2D, expression from the tox promoter is repressed at similar levels in both wild-type C7 and C7R (mntR) in the presence of Fe2+ (>100-fold repression) and Mn2+ (6- to 8-fold repression). This finding suggests that MntR does not cause the Mn2+-dependent repression at the tox promoter. In the dtxR mutant, C7(β)hm723, the tox promoter is only weakly repressed by Mn2+ (20% reduction) and Fe2+ (two- to threefold repression) (Fig. 2D). The product of the dtxR allele from C7(β)hm723 is known to have low-level repressor activity (39, 40), and it is likely that the weak repression seen with Mn2+ and Fe2+ at the tox promoter in C7(β)hm723 is due to low-level DtxR activity. These findings strongly suggest that the Mn2+-dependent repression seen at the tox promoter is largely due to the activity of DtxR and that MntR has little if any role in the regulation of the tox promoter.
Primer extension analysis.
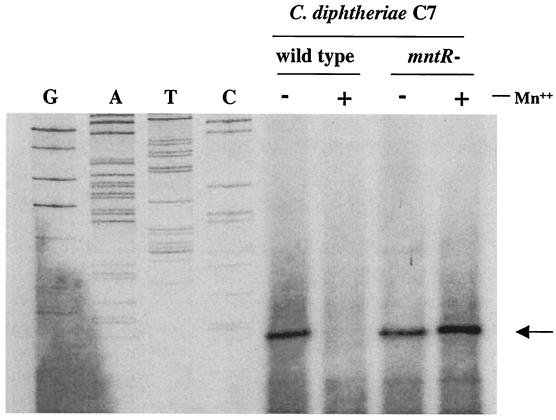
Whole-cell RNA was isolated from both wild-type C. diphtheriae C7 and the mntR mutant C7R that were grown in HIBTW medium in the presence and absence of 20 μM Mn2+. Primer extension studies, which used 10 μg of the RNA, showed that transcription in both strains initiated from a T residue located 7 bp downstream from the −10 sequence of the P1 promoter (Fig. 3 and 1B). No transcriptional start site was seen downstream from the P2 promoter. Consistent with the mnt-lacZ fusion studies, the results of the primer extension experiments confirmed that mRNA levels expressed from the mntA promoter were repressed by Mn2+ in the wild-type strain but constitutively expressed in C7R.
FIG. 3.
Primer extension analysis of C. diphtheriae C7 (wild type) and C7 mntR mutant strain (C7R). Whole-cell RNA was isolated from strains grown to mid-log phase in HIBTW medium containing either 20 μM Mn2+ (+) or no added supplements (−). The arrow indicates the location of RNA transcripts. G, A, T, or C indicates the DNA sequencing ladder used for the identification of the transcriptional start site. Primer extension analysis was done as described in Materials and Methods.
DNA binding studies with MntR.
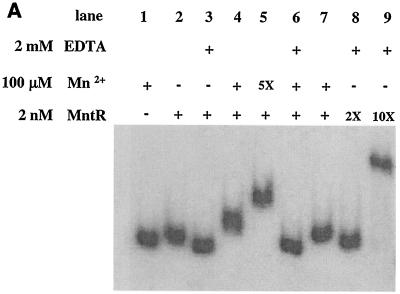
A mutation of the mntR gene results in loss of Mn2+-dependent repression at the mntA promoter. To determine if MntR is capable of binding to the mntA promoter region, a partially purified preparation of native MntR protein and a highly purified MntR-His fusion protein were used in DNA binding studies. The DNA fragment used in the binding experiments is the 202-bp insert present in pPO2 (Fig. 1B). The gel mobility shift assay shown in Fig. 4A demonstrates that a 2 nM concentration of the native MntR protein in the presence of 100 μM Mn2+ results in reduced mobility of a DNA fragment that carries the mntA promoter region (compare lane 4 to lane 2). The purified MntR-His protein showed results almost identical to those of the native MntR protein in all DNA binding studies described in this report (not shown). The MntR protein caused a slight reduction in mobility of the mnt promoter fragment in the absence of added Mn2+ (compare lane 1 with lane 2), but when EDTA was added to the binding buffer, no mobility shift was detected (lane 3). This finding suggests that despite dialysis of the MntR protein preparations in the presence of Chelex-100, there may be some contamination of MntR with an activating metal (such as the Ni2+ used in the purification). If 500 μM Mn2+ was used in the binding assays, the migration of the DNA was further reduced (lane 5); however, this reduced mobility was reversed in the presence of EDTA (lane 6), indicating the importance of the divalent metal in the activation of the DNA binding ability of MntR. It was also observed that 10-fold-higher concentrations of MntR (20 nM) in the absence of added Mn2+, but in the presence of EDTA, resulted in a sharp reduction in the mobility of the DNA fragment (lane 9), suggesting that MntR, when present at high concentrations, has the ability to bind DNA in the absence of added metal.
FIG. 4.
Gel mobility shift assays. A 202-bp 32P-labeled DNA fragment (DNA insert in plasmid pPO2) was incubated at room temperature for 10 min in the presence or absence of MntR with the indicated supplements. The sample was then applied to a nondenaturing 5% acrylamide gel and electrophoresed in a 20 mM NaPO4 buffer at 50 V for approximately 1 h. Gels were dried and analyzed by autoradiography. In panel A, 5X, 2X, and 10X indicate multiples of the concentration indicated to the left.
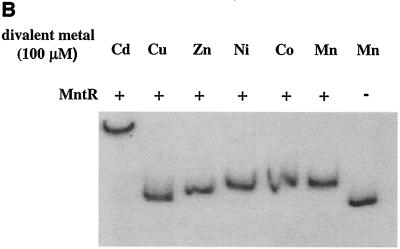
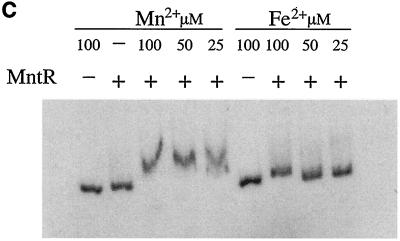
The DNA binding ability of certain metal-dependent repressors, including DtxR, can be activated in vitro by several different transition metals (32, 40, 47). In the gel mobility shift assay in Fig. 4B, several divalent metals including Mn2+, Co2+, Ni2+, Zn2+, and Cd2+, are capable of activating the DNA binding ability of MntR. Cu2+ had no effect on the mobility of the DNA fragment, and the reason for the strong reduction in mobility of the DNA fragment in the presence of Cd2+ is not known. Cd2+ had no effect on the mobility of the DNA fragment in the absence of MntR (data not shown). In Fig. 4C, Mn2+ at 100, 50, and 25 μM concentrations was able to activate MntR, whereas Fe2+ only weakly activated MntR at 100 μM and failed to activate MntR at lower concentrations. These findings are consistent with the in vivo data from Fig. 2A that suggest that Mn2+ is the physiologically relevant metal and that Fe2+ does not repress the mntA promoter. Mn2+ was also shown to activate MntR binding at 10 μM (data not shown).
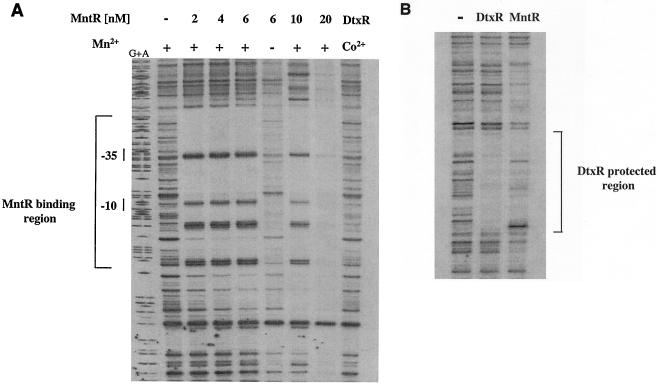
To better define the region bound by the MntR protein, DNase I footprinting experiments were done by using a DNA fragment that carried the mnt promoter region. As seen in Fig. 5A, the MntR protein, at concentrations as low as 2 nM, was able to protect an approximately 73-bp region from DNase I digestion in an Mn2+-dependent manner. This region was relatively AT rich (65% AT) and included the entire P1 promoter (Fig. 5A and 1B). Located within the protected region were at least five hypercleavable sites. At higher concentrations of MntR, sequences flanking the primary 73-bp binding site were also protected from DNase I digestion (Fig. 5A). This binding to sequences outside the primary 73-bp binding site likely represents polymerization of MntR along the DNA, a phenomenon observed with other DNA binding proteins (43). MntR failed to bind to a DNA fragment that carried sequences upstream of the 73-bp region (data not shown) (see Materials and Methods), and MntR did not bind to the mnt promoter region at concentrations below 1 nM (data not shown).
FIG. 5.
DNase I footprinting experiments. (A) Footprinting studies were done by using the DNA fragment used in the gel mobility shift assays. Mn2+ and Co2+ were used at 100 μM concentration, and native MntR was used at the concentrations indicated. The use of Co2+ to activate DtxR has been described previously (36). The bracket identifies the region protected by MntR from DNase I digestion, and −10 and −35 show the locations of the P1 promoter elements. G+A indicates the DNA sequencing ladder. (B) Footprinting experiments were done with a DNA fragment carrying the tox promoter region (43). MntR was used at 6 nM, and DtxR was present at 100 nM. The bracket indicates a 30-bp region protected by DtxR from DNase I digestion (43, 47). Co2+ and Mn2+ were used at 100 μM to activate DtxR and MntR, respectively.
The region protected by MntR contains the area of dyad symmetry that shares limited identity to the DtxR binding site (Fig. 1B and C). It is unclear what role if any this sequence has in the binding of MntR to this region. However, the inability of DtxR to bind to the mnt promoter region (Fig. 5A) indicates that this sequence does not function as a binding site in vitro for DtxR. While no extensive areas of dyad symmetry were identified within the 73-bp MntR protected region at the mnt promoter, the 7-bp sequence TGAACAA was repeated three times within the MntR binding site (Fig. 1B). The significance of this repeated sequence within the MntR binding site has not been determined. Surprisingly, the DtxR binding site at the tox promoter does serve as a weak binding site for MntR, although the binding of MntR to this region is poorly defined and includes the protection of sequences outside the area protected by DtxR (Fig. 5B).
The results of the DNase I protection experiments, which defined the MntR binding region, are consistent with the LacZ data shown in Fig. 2B. The DNA inserts in the mnt-lacZ fusion constructs pPO4 and pPO5 contain only a portion of the MntR binding site, and LacZ assays with these constructs showed either partial or no Mn2+ repression (Fig. 2B and 1B). The insert in pPO3 contains the entire MntR protected region (Fig. 1B), and transcription from pPO3 showed full Mn2+-dependent repression, which is consistent with this construct carrying the complete MntR binding site.
Growth of C. diphtheriae strains in Mn2+-depleted medium.
Since the mntABCD gene products show significant similarity to systems involved in the transport of Mn2+, it is likely that this system in C. diphtheriae transports Mn2+. To determine if the mntA or mntR genes are required for growth in a low-Mn2+ medium, C7, C7R, and C7A were grown in metal-depleted mPGT medium, which was supplemented with various divalent metals. A wild-type strain of B. subtilis, CU1065, was included as a control in these studies, since its Mn2+ requirement had been previously defined (32). As shown in Table 1, all of the strains grew poorly in the mPGT medium in the absence of added metal. However, all of the C. diphtheriae strains grew to high levels in the presence of added Fe2+. No further growth was seen with the addition of Mn2+ (even when Mn2+ was supplemented at 10 μM; data not shown). C. diphtheriae strains showed no growth stimulation when Mn2+ alone was added to mPGT medium, although B. subtilis CU1065 showed some growth enhancement in the presence of Mn2+. B. subtilis CU1065 grew to only about 20% of maximum growth in the presence of Fe2+ alone, but the addition of both Mn2+ and Fe2+ resulted in full growth (maximum growth for all strains was considered mPGT medium containing Fe2+ and Mn2+). This finding with CU1065 is consistent with an earlier report (32) and suggests that the Mn2+ concentration in the mPGT medium is approximately 10 to 20 nM, since that was the concentration of Mn2+ reported in the previous study that allowed 20% of the maximum growth of CU1065 in a minimal medium supplemented with Fe2+. The findings in this experiment clearly demonstrate that the Mn2+ requirements for C. diphtheriae and B. subtilis are markedly different and that the mutations in the mntA and mntR genes have no effect on the growth characteristics of C. diphtheriae in mPGT medium.
TABLE 1.
Growth in mPGT medium
| Strain | Avg OD600 in mPGT medium with indicated supplement(s)a
|
|||
|---|---|---|---|---|
| None | Mn | Fe | Fe + Mn | |
| C. diphtheriae C7 wt | 0.3 | 0.3 | 4.0 | 3.6 |
| C. diphtheriae C7 mntR | 0.2 | 0.4 | 4.2 | 4.0 |
| C. diphtheriae C7 mntA | 0.2 | 0.2 | 3.4 | 4.0 |
| B. subtilis CU1065 wt | 0.2 | 1.4 | 0.8 | 3.6 |
Ferrous sulfate (Fe) was added to mPGT medium at 5 μM, and MnCl2 (Mn) was added at 0.1 μM. Measurements were taken on 18- to 24-h cultures. Values are averages of three independent experiments, and no experiment varied by more than 15% from the average. OD600, optical density at 600 nm; wt, wild type.
DISCUSSION
It has been over a decade since the identification and characterization of the DtxR protein in C. diphtheriae (3, 38). Subsequent studies confirmed the role of DtxR as a global iron-dependent repressor and the prototype of a new family of bacterial metalloregulatory proteins (49). In recent years, there has been a significant increase in the identification of new members within the family of DtxR-like repressors, which is largely the result of the completion or partial completion of numerous bacterial genome sequences. These new members of the DtxR family have been found in both gram-negative and gram-positive species and vary in size and metal selectivity (Table 2) (5).
TABLE 2.
Characteristics of DtxR-like repressors
| Organism | Protein (aa) | % Identity (% similarity)a | C-terminal SH3 domain | Metalb | Amino acid residues in primary metal binding sitec |
|---|---|---|---|---|---|
| C. diphtheriae | MntR (223) | Yes | Mn | D12E105E108H109 | |
| DtxR (226) | 27 (41) | Yes | Fe | M10C102E105H106 | |
| M. tuberculosis | IdeR (230) | 32 (46) | Yes | Fe | M..C.EH |
| S. aureus | MntR (214) | 33 (52) | Yes | Mn | D..E.EH |
| B. subtilis | MntR (142) | 23 (39) | No | Mn | D..E.EH |
| T. pallidum | TroR (153) | 39 (58) | No | Mn | N..E.EH |
Compared to MntR of C. diphtheriae.
Divalent metals known to activate repressor activity in vivo.
Data from reference 6. Residue positions are given for C. diphtheriae MntR and DtxR. Residues for other proteins are at the location corresponding to that found in DtxR.
Database searches of the recently completed or partially completed genomes within the closely related genera Mycobacterium, Streptomyces, and Corynebacterium revealed that these bacteria harbor two DtxR-like proteins. Various species in Mycobacterium (7) (M. tuberculosis, M. bovis, M. leprae, and M. smegmatis) and in Corynebacterium (C. diphtheriae and C. glutamicum) encode a DtxR-like repressor that is predicted to be Fe2+ responsive. These species also encode a second member of the DtxR family that is predicted to be an MntR homolog and responsive to Mn2+. The MntR class of repressors are predicted to be Mn2+ responsive based solely on the sequence of the primary metal binding site (with the exception of the C. diphtheriae MntR protein described in this study).
The ability of C. diphtheriae to grow in metal-depleted mPGT medium supplemented only with Fe2+ suggests that this organism has a low requirement for Mn2+. Manganese concentrations vary widely in humans (from 0.05 μM on skin to over 100 μM in the central nervous system [19, 50]), and the precise Mn2+ concentration at the site of colonization by C. diphtheriae in the human host is not known. The ability of the mntA mutant strain, C7A, to grow as well as the wild type in a low-Mn2+ medium suggests that the MntABCD transport system is not required for growth in this medium. The C. diphtheriae wild-type and C7A strains showed no growth defect after multiple passages in this medium (not shown), indicating that growth in this medium is not due to intracellular stores of Mn2+. The observation that C7A was able to grow in mPGT medium containing only added Fe2+ has several possible explanations. (i) C. diphtheriae contains an alternate mechanism for the transport of Mn2+; redundancy in metal transport systems in bacteria is quite common. B. subtilis (32) and other bacteria (2, 22) contain multiple Mn2+ transporters, and it was observed that mutations in the mntABCD system in B. subtilis, the transport system homologous to the mntABCD genes in C. diphtheriae, had no growth defect compared to the wild-type strain in a low-Mn2+ minimal medium (32). It was proposed in that study that alternate systems could substitute for the mntABCD system for the transport of Mn2+. (ii) The MntABCD transporter is not involved in the transport of Mn2+ but transports another metal. This possibility seems unlikely since the mnt genes are regulated by Mn2+ availability and show the highest similarity to systems involved in Mn2+ transport. (iii) C. diphtheriae does not require Mn2+ for growth. Almost nothing is known about the Mn2+ requirements in this species or the existence of Mn2+-containing enzymes.
Footprinting studies demonstrated that MntR protected an approximately 73-bp region from DNase I digestion. The 73-bp region appears to be the minimal protected region, since smaller areas of protection could not be identified even at the lowest MntR concentrations that provided any detectable protection (data not shown). The size of the region protected by MntR is quite large relative to what has been observed in footprinting studies with other metalloregulators. DtxR and IdeR protect an approximately 30-bp region and are thought to recognize a 19-bp core palindromic operator sequence (42, 43, 47). The DtxR-like Mn-responsive repressors from Treponema pallidum (29) and E. coli (26), TroR and MntR, respectively, protected regions of 22 bp (TroR) and 25 bp (MntR) that included inverted repeat sequences of either 8 bp (TroR) or 9 bp (MntR). In Streptococcus gordonii, the ScaR protein, a Mn-responsive DtxR-like repressor, protected a 46-bp region from DNase I digestion (18). This protected region included the −10 and −35 sequences of the Mn-regulated scaC promoter and two distinct 8-bp regions of dyad symmetry, suggesting that this promoter may contain two separate repressor binding sites. The larger protected region observed with ScaR compared to the smaller areas protected by TroR, E. coli MntR, and DtxR, is consistent with two repressor binding sites at the scaC operator.
The amino acid sequence of MntR suggests that it shares significant structural similarities to other DtxR-like proteins, and therefore it likely binds DNA in a manner similar to that of other metalloregulatory proteins. The crystal structure of DtxR and IdeR revealed that these proteins bind to their cognate operator regions as dimer pairs, with each dimer contacting opposite faces of the DNA helix. Previous footprinting experiments with DtxR-like repressors suggested that a dimer pair protected a region in the range of 22 to 30 bp (26, 29, 42, 47). If MntR binds DNA like other DtxR-like proteins, this suggests that as many as three dimer pairs may bind to the 73-bp region protected by MntR.
A sequence that has limited homology to the DtxR consensus binding site was identified within the region protected by MntR (Fig. 1B and C). The relevance of this weak DtxR consensus site with regards to MntR binding is not known. The DtxR protein failed to bind to this region, and MntR binding to this relatively small region alone would not account for the very large 73-bp footprint. Therefore, it is almost certain that additional sequences are important for MntR binding. The 73-bp sequence bound by C. diphtheriae MntR shares no obvious homology with the binding sites for any other MntR-like proteins. However, the 7-bp sequence TGAACAA is present three times within the protected region, and several (imperfect) inverted repeat sequences that contain portions of the TGAACAA sequence can be identified (Fig. 1B). Whether this 7-bp sequence constitutes a portion of the MntR recognition sequence will require additional studies; however, the presence of three TGAACAA repeats within the binding region suggests that this sequence may be important in MntR recognition, especially since the size of the MntR footprint suggests that as many as three dimer pairs may bind to this region.
REFERENCES
- 1.Altschul, S. F., G. Warren, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, J. M., O. N. Manish, and J. R. Murphy. 1990. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. USA 87:5968-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 5.Claverys, J.-P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152:231-243. [DOI] [PubMed] [Google Scholar]
- 6.Ding, X., H. Zeng, N. Schiering, D. Ringe, and J. R. Murphy. 1996. Identification of the primary metal ion-activation sites of the diphtheria tox repressor by X-ray crystallography and site-directed mutational analysis. Nat. Struct. Biol. 3:382-387. [DOI] [PubMed] [Google Scholar]
- 7.Doukhan, L., M. Predich, G. Nair, O. Dussurget, I. Mandic-Mulec, and S. T. Cole. 1995. Genomic organization of the mycobacterial sigma gene cluster. Gene 165:67-70. [DOI] [PubMed] [Google Scholar]
- 8.Drazek, E. S., C. A. Hammack, and M. P. Schmitt. 2000. Corynebacterium diphtheriae genes required for acquisition of iron from hemin and hemoglobin are homologous to ABC hemin transporters. Mol. Microbiol. 36:68-84. [DOI] [PubMed] [Google Scholar]
- 9.Gold, B., G. M. Rodriguez, S. A. Marras, M. Pentecost, and I. Smith. 2001. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol. Microbiol. 42:851-865. [DOI] [PubMed] [Google Scholar]
- 10.Groman, N., and K. Judge. 1979. Effect of metal ions on diphtheria toxin production. Infect. Immun. 26:1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunter-Seeboth, K., and T. Schupp. 1995. Cloning and sequence analysis of the Corynebacterium diphtheriae dtxR homologue from Streptomyces lividans and S. pilosus encoding a putative iron repressor protein. Gene 166:117-119. [DOI] [PubMed] [Google Scholar]
- 12.Hantke, K. 1984. Cloning of the repressor protein gene of iron regulated systems in Escherichia coli K-12. Mol. Gen. Genet. 197:337-341. [DOI] [PubMed] [Google Scholar]
- 13.Haynes, J. A., and M. L. Britz. 1989. Electrotransformation of Brevibacterium lactofermentum and Corynebacterium glutamicum: growth in Tween 80 increases transformation frequencies. FEMS Microbiol. Lett. 61:329-334. [Google Scholar]
- 14.Holmes, R. K. 2000. Biology and molecular epidemiology of diphtheria toxin and the tox gene. 2000. J. Infect. Dis. 181:56-67. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, R. K., and L. Barksdale. 1969. Genetic analysis of tox+ and tox bacteriophages of Corynebacterium diphtheriae. J. Virol. 3:586-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsburgh, M. J., S. J. Wharton, A. G. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1286. [DOI] [PubMed] [Google Scholar]
- 18.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 19.Keen, C. L., B. Lonnerdal, and L. S. Hurley. 1984. Manganese, p. 89-132. In I. Frieden (ed.), Biochemistry of the essential ultratrace elements. Plenum Press, New York, N.Y.
- 20.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. Regulation of Salmonella enterica serovar typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J. Bacteriol. 184:3151-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolenbrander, P. E., R. N. Anderson, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. H., T. Wang, K. Ault, J. Liu, M. P. Schmitt, and R. K. Holmes. 1997. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Pappenheimer, A. M., Jr. 1977. Diphtheria toxin. Annu. Rev. Biochem. 46:69-94. [DOI] [PubMed] [Google Scholar]
- 26.Patzer, S. I., and K. Hantke. 2002. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 183:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl, E., R. K. Holmes, and W. G. Hol. 1999. Crystal structure of the iron-dependent regulator (IdeR) from Mycobacterium tuberculosis shows both metal binding sites fully occupied. J. Mol. Biol. 285:1145-1156. [DOI] [PubMed] [Google Scholar]
- 28.Pohl, E., R. K. Holmes, and W. G. Hol. 1999. Crystal structure of a cobalt-activated diphtheria toxin repressor-DNA complex reveals a metal-binding SH3-like domain. J. Mol. Biol. 292:653-667. [DOI] [PubMed] [Google Scholar]
- 29.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian, Y., J. H. Lee, and R. K. Holmes. 2002. Identification of a DtxR-regulated operon that is essential for siderophore-dependent iron uptake in Corynebacterium diphtheriae. J. Bacteriol. 184:4846-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu, X., L. M. Christophe, S. Zhang, M. P. Schmitt, R. K. Holmes, and W. G. Hol. 1995. Three-dimensional structure of the diphtheria repressor in complex with divalent cation co-repressor. Structure 3:87-100. [DOI] [PubMed] [Google Scholar]
- 32.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt, M. P. 1997. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenase and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt, M. P. 1997. Transcription of the Corynebacterium diphtheriae hmu O gene is regulated by iron and heme. Infect. Immun. 65:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitt, M. P., and E. S. Drazek. 2001. Construction and consequences of directed mutations affecting the hemin receptor in pathogenic Corynebacterium species. J. Bacteriol. 183:1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt, M. P., and R. K. Holmes. 1991. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect. Immun. 59:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt, M. P., and R. K. Holmes. 1991. Characterization of a defective diphtheria toxin repressor (dtxR) allele and analysis of dtxR transcription in wild-type and mutant strains of Corynebacterium diphtheriae. Infect. Immun. 59:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt, M. P., and R. K. Holmes. 1993. Analysis of diphtheria toxin repressor-operator interactions and characterization of a mutant repressor with decreased binding activity for divalent metals. Mol. Microbiol. 9:173-181. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt, M. P., and R. K. Holmes. 1994. Cloning, sequence and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor and iron. J. Bacteriol. 176:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt, M. P., M. Predich, L. Doukhan, I. Smith, and R. K. Holmes. 1995. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect. Immun. 63:42844.289. [DOI] [PMC free article] [PubMed]
- 43.Schmitt, M. P., E. M. Twiddy, and R. K. Holmes. 1992. Purification and characterization of the diphtheria toxin repressor. Proc. Natl. Acad. Sci. USA 89:7576-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serwold-Davis, T. M., N. B. Groman, and M. Rabin. 1987. Transformation of Corynebacterium diphtheriae, Corynebacterium ulcerans, Corynebacterium glutamicum, and Escherichia coli with the C. diphtheriae plasmid pNG2. Proc. Natl. Acad. Sci. USA 84:4964-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Expression in E. coli: use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 46.Tai, S.-P. S., A. E. Krafft, P. Nootheti, and R. K. Holmes. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267-273. [DOI] [PubMed] [Google Scholar]
- 47.Tao, X., and J. R. Murphy. 1992. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J. Biol. Chem. 267:21761-21764. [PubMed] [Google Scholar]
- 48.Tao, X., and J. R. Murphy. 1994. Determination of the minimal essential nucleotide sequence for diphtheria tox repressor binding by in vitro affinity selection. Proc. Natl. Acad. Sci. USA 91:9646-9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao, X., S. Nikolaus, H. Zeng, D. Ringe, and J. R. Murphy. 1994. Iron, DtxR and the regulation of diphtheria toxin expression. Mol. Microbiol. 14:191-197. [DOI] [PubMed] [Google Scholar]
- 50.Tayarani, I., I. Cloez, M. Clement, and J. M. Bourre. 1989. Antioxidant enzymes and related trace elements in aging brain capillaries and choroids plexus. J. Neurochem. 53:817-824. [DOI] [PubMed] [Google Scholar]
- 51.Tseng, H.-J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitek, R. C., and M. Wharton. 1998. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg. Infect. Dis. 4:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, G., G. P. Wylie, P. D. Twigg, D. L. Caspar, J. R. Murphy, and T. M. Logan. 1999. Solution structure and peptide binding studies of the C-terminal Src homology 3-like domain of the diphtheria toxin repressor protein. Proc. Natl. Acad. Sci. USA 96:6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White, A., X. Ding, J. C. Vanderspek, J. R. Murphy, and D. Ringe. 1998. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature 394:502-506. [DOI] [PubMed] [Google Scholar]
- 55.Wilks, A., and M. P. Schmitt. 1998. Expression and characterization of a heme oxygenase (HmuO) from Corynebacterium diphtheriae. J. Biol. Chem. 273:837-841. [DOI] [PubMed] [Google Scholar]