Abstract
Profilin (PRF) is a low-molecular-weight actin binding protein encoded by a diverse gene family in plants. Arabidopsis PRF1 transcripts are moderately well expressed in all vegetative organs. A regulatory mutant in PRF1, prf1-1, was isolated from a library of T-DNA insertions. The insertion disrupted the promoter region of PRF1 100 bp upstream from the transcriptional start site. Although steady state levels of PRF1 transcripts appeared normal in mature prf1-1 plants, the levels in young seedlings were only one-half those observed in wild type. Reactions with a PRF1 isovariant–specific monoclonal antiserum and general anti-profilin antisera demonstrated that PRF1 protein levels also were one-half those found in wild-type seedlings, although total profilin levels were unaffected. Mutant seedlings no longer could downregulate PRF1 levels in the light, as did wild type. Consistent with their molecular phenotypes, young mutant seedlings displayed several morphological phenotypes but developed into apparently normal adult plants. Their initial germination rate and development were slow, and they produced excessive numbers of root hairs. Mutant seedlings had abnormally raised cotyledons, elongated hypocotyls, and elongated cells in the hypocotyl, typical of phenotypes associated with some defects in light and circadian responses. A wild-type PRF1 transgene fully complements the hypocotyl phenotypes in the prf1-1 mutant. The ability of profilin to regulate actin polymerization and participate directly in signal transduction pathways is discussed in light of the prf1-1 phenotypes.
INTRODUCTION
The actin cytoskeleton contributes to many of the dynamic processes directing plant development: cell polarity, division plane determination, cell elongation, and cell wall deposition (Meagher et al., 1999b). As seedlings emerge from the soil and respond to light, significant changes occur in plant morphology that must be directed at the cellular level by alterations in the cytoskeleton (Meagher et al., 2000). Because the actin binding protein profilin is particularly important to the dynamics of actin polymerization and sequestration, the regulation of plant profilin expression in cells and organs responding to changes in light levels should affect plant development. This article provides initial data demonstrating the connection between profilin regulation and plant morphology.
Profilin binds monomeric G-actin and participates in several cytoskeletal functions (Gibbon and Staiger, 2000) with the potential to alter cellular and organismal development. Profilin accelerates assembly at the barbed ends of actin filaments and sequesters actin monomers, which decreases the pool of polymerizable actin once barbed ends are capped (Carlier et al., 1993; Pantaloni and Carlier, 1993; Perelroizen et al., 1996). Profilin activates actin for assembly by catalyzing ADP-to-ATP exchange on actin monomers. Profilin binds tightly to poly-l-proline, a motif present in some proteins involved in regulating the actin cytoskeleton (Ahern-Djamali et al., 1999). In addition, profilin responds to calcium and phosphoinositide signaling with resulting changes in actin cytoarchitecture (Ostrander et al., 1995; Singh et al., 1996). Finally, profilin appears to participate directly in classical signal transduction, because its binding of phosphatidylinositol 4,5-bisphosphate inhibits hydrolysis by unphosphorylated phospholipase C (Goldschmidt-Clermont et al., 1990, 1991). Dictyostelium profilin mutants show impaired cytokinesis and arrested development (Karakesisoglou et al., 1999). Yeast profilin mutants are altered in viability, size, and shape (Haarer et al., 1990) and in actin stability after heat shock (Yeh and Haarer, 1996). Plant profilins have been shown to rescue profilin mutants in Dictyostelium (Karakesisoglou et al., 1996) and yeast (Christensen et al., 1996), suggesting that they share many of these mechanistic capabilities with their counterparts in other kingdoms. Some of these properties have been characterized experimentally for a few plant profilins (Staiger et al., 1994; Gibbon et al., 1997, 1998; Clarke et al., 1998; von Witsch et al., 1998).
Profilin is encoded by only one or two genes in most fungi, animals, and protists in which they have been characterized, including profilins from yeast (Haarer et al., 1993), humans (Kwiatkowski and Bruns, 1988), and Dictyostelium (Haugwitz et al., 1991). In contrast, plants have several genes encoding highly divergent profilin isovariants, based on the best characterized families in two very distant angiosperms, Arabidopsis (Christensen et al., 1996; Huang et al., 1996) and maize (Staiger et al., 1993; Gibbon et al., 1997). The most divergent isovariants in Arabidopsis, the vegetative and reproductive profilins, share only 77% amino acid sequence identity with each other and 26% identity with the vertebrate proteins (Huang et al., 1996). In maize, the diversity in profilin isovariants manifests itself as differences in affinity for ATP, actin, and poly-l-proline (Gibbon et al., 1997, 1998), suggesting that isovariant dynamics among coexpressed plant profilin proteins may expand the responses of the actin cytoskeleton or buffer it against stress (Meagher et al., 1999a). But the in vivo cellular or organismal roles of any one plant profilin isovariant have yet to be demonstrated.
One of the Arabidopsis profilin genes, PRF1, expresses moderate levels of mRNA in the vegetative tissues and organs examined (Christensen et al., 1996; Huang et al., 1996). The PRF1 protein sequence differs in only 10 of its 131 amino acid residues from that of its closest relative in the profilin family, PRF2. It has not been shown that the PRF1 isovariant makes any distinct contribution to the plant cytoskeleton or to plant development.
In this report, we characterize an Arabidopsis profilin mutant, prf1-1, which contains a large insertion in the PRF1 promoter region. In mutant seedlings, PRF1 mRNA and PRF1 protein were both reduced to approximately half of the normal levels, and the downregulation of PRF1 levels observed in the light was lost. Although homozygous mutant plants were quite viable, they showed a number of seedling defects consistent with PRF1 playing roles in intracellular signaling and/or in the regulation of cell architecture. Mutant seedlings displayed altered light responsiveness, delayed germination, elongated hypocotyls, and excessive numbers of root hairs.
RESULTS
A Mutation in the Profilin Gene PRF1
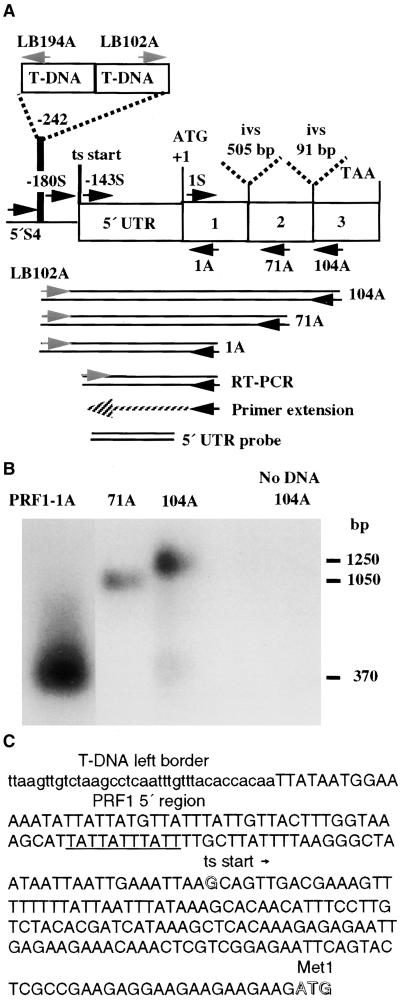
To investigate the roles of various plant profilin isovariants in actin dynamics and plant development, we screened for Arabidopsis profilin mutants in a T-DNA insertion library. As shown in Figure 1A, polymerase chain reaction (PCR) was used to amplify junction sequences between the T-DNA left border (LB102A) and any disrupted or adjacent profilin gene using a highly degenerate profilin primer (PRF-104A; see details in Methods and Table 1). A disrupted profilin gene was corroborated in one pool of mutants when a ladder of profilin-hybridizing fragments of 1250, 1050, and 370 bp were generated that terminated on the nested profilin primers PRF-104A, PRF-71A, and PRF1-1A, respectively (Figures 1A and 1B, Table 1). DNA sequence analysis of the smallest of these junction fragments confirmed the identity of an insertion in the previously characterized profilin gene, PRF1 (Huang et al., 1996).
Figure 1.
Characterization of the prf1-1 Mutation and PRF1 Transcripts.
(A) The location of the T-DNA insertion in the prf1-1 mutant is shown on a map of the Arabidopsis profilin gene PRF1. PRF1 encodes a 131–amino acid protein. The locations of primers used in this study are shown, with their 3′ ends indicated by arrows. The PCR products used to map the insertion, the primer extension product and RT-PCR products that were used to map the 5′ end of the PRF1 transcript (ts start), and the 5′ untranslated region (5′ UTR) used as a gene-specific probe are shown below the gene. Two introns (ivs) interrupt the coding region.
(B) prf1-1 mutant plants were identified by a sequence-based screening strategy using a primer (see Table 1) located in the T-DNA left border (LB202) and a degenerate profilin primer, PRF104A, which produced a 1.25-kb product. A ladder of prf1-1/left border junction products is produced by a set of nested antisense profilin primers (PRF104A, 71A, and 1A).
(C) Sequence of the 5′ region of the PRF1 gene (uppercase letters) in the prf1-1 mutant and location of the proximal left border of the T-DNA insertion (lowercase letters). The putative promoter TATA region (underlined), the G residue mapped as the start of transcription (open letter), and the ATG initiation codon (open letters) are indicated.
Table 1.
Oligonucleotide Primers
| Name | Gene(s) | Location | Length (nucleotide) Degeneracy |
Sequencea |
|---|---|---|---|---|
| PRF-1S | Profilin family | Codons 1–11 | 33/2 × 106 | ATG(A/T)(G/C)NTGGCARNNNTAYGTNGAYGANCAYYTNATGT |
| PRF1-5′S4 | PRF1 | −476 bp from ATG | 24 mer | GAAAGTATTATGATAAAGAAGGAT |
| PRF1-1A | PRF1 | Codons 1–6 | 38 mer | ACCCGGGGATCCGTCGACACGTATGATTGCCAAGACAT |
| PRF-71A | Profilin family | Codons 71–78 | 24/2 × 103 | YTCNCCYTGNAYNACCATRTAYTT |
| PRF-104A | Profilin family | Codons 104–113 | 32/2 × 105 | TTRCAYTGNCCNC/GC/GNGTNAYNGGYTCNTCRTA |
| PRF1-180S | PRF1 | −177 to −148 bp from ATG | 29 mer | GCTTATTTTAAGGGCTAATTAATTGA |
| PRF1-143S | PRF1 | −119 to −142 bp from ATG | 26 mer | GCAGTTGACGAAAGTTTTTTTTATTA |
| LB102A | T-DNA | 102 bp from end of left TDNA border | 29 mer | GATGCAATCGATATCAGCCAATTTTAGAC |
| LB194A | T-DNA | 194 bp from end of left TDNA border | 30 mer | AACTGTAATGACTCCGCGCAATATTTACAC |
R = purine, Y1 = pyrimidine, N = all four bases. Most primers can be found on prf1-1 map in Figure 1.
Figures 1A and 1C show the location of the T-DNA insertion in the prf1-1 mutant. The element was inserted 242 bp upstream of the translational initiation codon, ATG. Six base pairs were deleted from the PRF1 sequence during the insertion event (the upstream end of the insertion sequence is not shown). Primer extension analysis of the wild-type transcript was used to map the start of transcription to −143 bp upstream from the ATG, as shown in Figures 1A and 1C. Using a sense primer immediately downstream of this transcriptional start site (PRF1-143S) in conjunction with an antisense profilin primer (PRF1-1A) located at the start of translation, reverse transcription–mediated PCR (RT-PCR) produced PRF1 products from Arabidopsis leaf cDNAs (Senecoff and Meagher, 1993) (Figure 1A). In contrast, no RT-PCR product was detected when a primer upstream of position −PRF1-143S, such as −PRF1-180S, was used as the sense primer (Figure 1A). This confirmed that the 5′ ends of the majority of PRF1 transcripts are positioned very close to PRF1-143. Thus, the T-DNA insertion in prf1-1 disrupts the presumptive promoter region of PRF1 and is positioned 100 bp upstream of the transcription start site.
Further mapping of the insertion demonstrated that left border sequences faced outward from the upstream and downstream ends of the insertion, with 40 and 12 bp of T-DNA sequence lost from the two ends, respectively. Therefore, the insertion contains DNA from two or more T-DNA elements and is relatively large.
The prf1-1 Mutant Expresses Decreased mRNA and Protein Levels in Seedlings
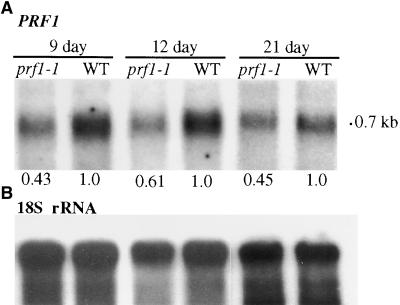
The prf1-1 mutation was backcrossed twice to the wild-type Wassilewskija (Ws) strain into a clean genetic background for subsequent analysis (see Methods). PRF1 is normally expressed in vegetative tissues with moderate levels of transcripts in all organs (Huang et al., 1996). RNA gel blot analysis of transcripts from homozygous prf1-1 plants was performed using a PRF1-specific probe from the 5′ untranslated region (Huang et al., 1996). Seven- to 12-day-old mutant seedlings reproducibly contained one-half to one-third the levels of the 700-nucleotide PRF1 mRNA relative to wild-type seedlings, as shown in Figure 2A and quantified at the bottom of the RNA gel blot image. Mutant seedlings and leaves harvested more than 12 days after germination contained either reduced PRF1 mRNA levels relative to wild type, as shown for 21-day-old plants in Figure 2A, or approximately the same levels of mRNA. Thus, the prf1-1 mutation resulted in reduced steady state levels of PRF1 transcripts in young seedlings and plants.
Figure 2.
Transcript Levels in prf1-1 Mutant Seedlings.
(A) RNA gel blot hybridized with a PRF1-specific DNA fragment from the 5′ untranslated region (see Figure 1). Total RNA samples from 9-, 12-, and 21-day-old wild-type (WT) and mutant (prf1-1) plants are shown. The level of PRF1 mRNA in the prf1-1 mutant is quantified relative to the level in the wild type after correcting for the loading of the 18S rRNA shown in (B).
(B) The 18S rRNA is identified in the same blot shown in (A) to demonstrate equal loading and transfer of total RNA samples.
Densitometric scanning was used to quantify the bands in (A) and (B).
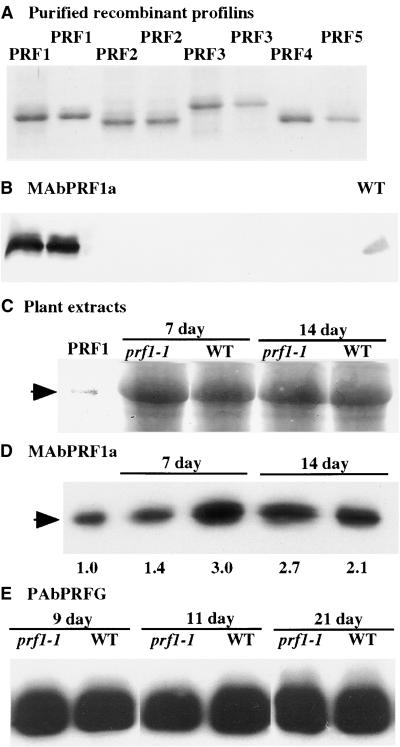
A PRF1-specific mouse monoclonal antibody, MAbPRF1a, was isolated to distinguish the PRF1 isovariant from other Arabidopsis profilin proteins that are coexpressed, such as PRF2 and PRF3. The five known Arabidopsis profilins, PRF1, PRF2, PRF3, PRF4, and PRF5, were expressed in Escherichia coli, purified, and resolved by SDS-PAGE, as shown by the Coomassie blue–stained gel in Figure 3A. Duplicate gels were imprinted to membranes and reacted with the antiserum. The specificity of MAbPRF1a reacting with PRF1 and not with the other Arabidopsis profilin proteins is shown in Figure 3B. This antiserum also reacts with PRF1 protein in leaf extracts from wild-type plants (Figure 3B). Similar gels and protein gel blots were used to resolve equal amounts of total protein extracted from mutant and wild-type seedlings (Figures 3C and 3D).
Figure 3.
Analysis of PRF1 Protein Levels in Seedlings.
(A) Coomassie blue–stained 12.5% polyacrylamide gel resolves 2-μg samples of the recombinant profilins PRF1, PRF2, PRF3, PRF4, and PRF5. Membrane imprints of duplicate gels are examined by protein gel blotting in (B).
(B) A duplicate of the same blot shown in (A) reacted with the PRF1-specific mouse monoclonal antibody MAbPRF1a. This antibody does not react with the other four recombinant Arabidopsis profilin proteins on a protein gel blot. A sample of 15 μg of total Arabidopsis leaf protein was run in the far right lane as a control (WT).
(C) A Coomassie blue–stained SDS-PAGE gel in the region of profilin migration shows equal loading of plant protein extracts. Samples are as follows: lane 1, 0.1 μg of PRF1 standard protein; lanes 2 and 4, 30-μg total protein extracts from prf1-1 mutants; lanes 3 and 5, Ws wild type (WT). The image of the stained profilin standard, recombinant PRF1, is enhanced to show its position of migration.
(D) Membrane imprints of a duplicate of the gel shown in (C) reacted with antibody MAbPRF1a to determine the levels of PRF1 protein in 7- and 14-day-old seedlings. Densitometric quantification of protein levels was performed relative to the signal obtained with the PRF1 loading control (left lane) that contained 0.1 μg of total PRF1 protein.
(E) A rabbit polyclonal antibody (PAbPRFG) that reacts with all five Arabidopsis profilins was used to detect total profilin in extracts from wild type (WT) and prf1-1 mutant plants that were germinated and grown for 9, 11, and 21 days.
On the basis of the reactions with MAbPRF1, PRF1 levels were indistinguishable between prf1-1 and wild type in 14-day-old and older plants but were consistently twofold to threefold lower in 7-day-old mutant seedlings than in wild type (Figure 3D). The quantification of this experiment is shown below the protein gel blot image. Additional experiments showed that PRF1 levels were significantly lower in 7- to 11-day-old mutant seedlings than in wild type, but this difference from wild type was not detected in more mature mutant plants. The approximately twofold reduced PRF1 protein levels measured in the mutant are highly consistent with the results showing reduced PRF1 mRNA levels in young mutant seedlings. Decreased PRF1 expression was observed repeatedly in independent RNA and protein preparations from young seedlings. When the levels of all five possible profilin proteins were examined with a general plant profilin antibody (PAbPRFG), no consistent difference was detected in total profilin levels between the mutant and wild type, as shown in Figure 3E. Thus, the levels of the PRF1 isovariant that are altered by the prf1-1 mutation appear to account for only a small fraction of the total profilin in these seedlings and plants.
The prf1-1 Mutation Results in Altered Seedling Development
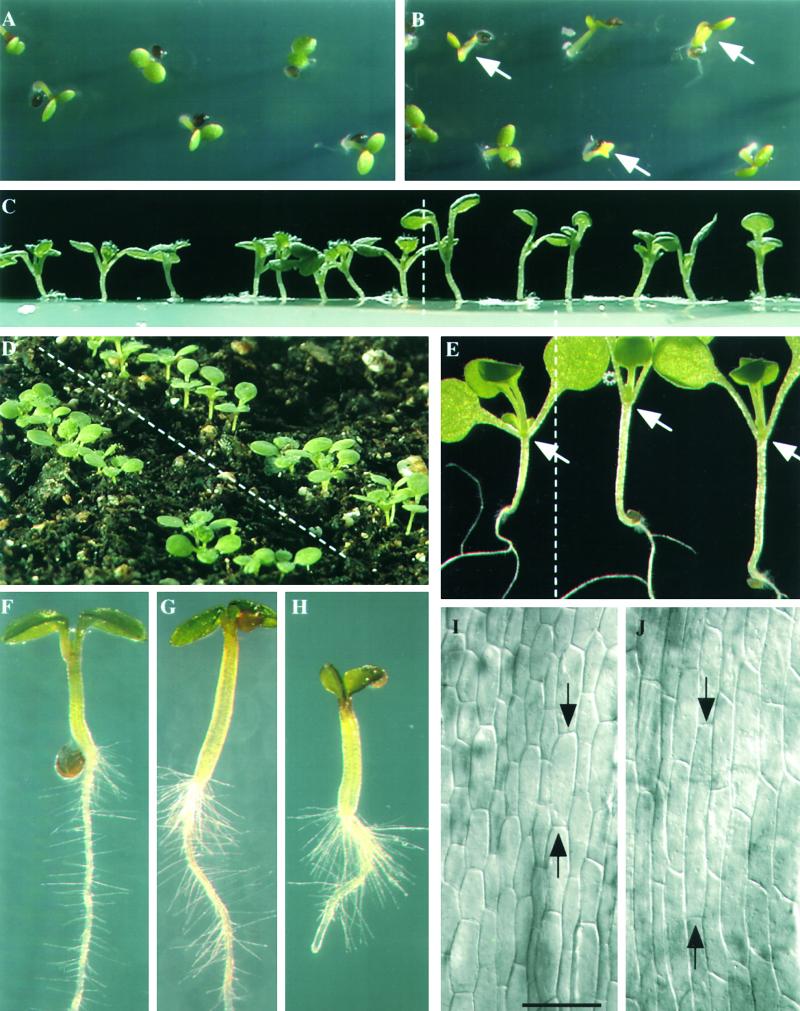
Greenhouse-grown mutant and wild-type plants were indistinguishable after 2 or more weeks on soil. However, when homozygous prf1-1/prf1-1 mutant seed were germinated for only 3 to 10 days, the mutant seedlings were distinguished relatively easily from Ws parental wild-type seedlings, as shown in Figure 4. Mutants (Figure 4B; arrows indicate delayed individuals) had a more variable and slightly delayed rate of seed germination relative to the wild type (Figure 4A). In addition, the mutants displayed slower expansion of cotyledons and late emergence of secondary leaves (Figure 4C, right) relative to the wild type (Figure 4C, left). One of the most notable characteristics of the mutant seedlings was that the cotyledons remained raised after subjective dawn (Figures 4C and 4E, right), whereas wild-type cotyledons were lowered just before or after dawn (Figures 4C and 4E, left). Typical mutant cotyledons formed acute angles of ∼30°, indicated by arrows in Figure 4E (right), compared with wild-type cotyledons (Figure 4E, left), which emerged with angles approaching 90°. Mutant cotyledons may remain raised all day or lower in the late afternoon. All wild-type and mutant cotyledons were raised again at subjective dusk. This pattern of raising and lowering cotyledons with the light/dark cycle can be entrained in Arabidopsis and is under circadian clock control (Dowson-Day and Millar, 1999). Mutant seedlings follow an abnormal cycle of movement.
Figure 4.
Morphology of prf1-1 Mutant Seedlings.
(A) and (B) Many mutant prf1-1 seed are delayed in their germination, and the resulting seedlings are chlorotic after 2 to 3 days ([B], arrows) compared with wild type (A).
(C) to (E) Seven- to 10-day-old mutant seedlings have raised cotyledons and elongated hypocotyls (right of dashed lines) compared with wild type (left of dashed lines). In (E), mutant cotyledons form an acute angle, whereas most wild-type cotyledons form an angle greater than 90° (arrows). These seedlings were harvested at 1 pm, 8 hr after subjective dawn, but mutants still show raised cotyledons.
(F) to (H) Mutant prf1-1 seedlings ([G] and [H]) have increased root hair density and elongated root hairs compared with wild type (F).
(I) and (J) Differential interference contrast microscopy of epidermal cells of 7-day-old hypocotyls from mutant (J) and wild-type (I) seedlings. Arrows indicate the ends of the longest cell in each frame. (I) and (J) represent same magnification. Bar in (I) = 85 μm.
The prf1-1 Mutation Results in an Elongated Hypocotyl Phenotype
Maximum hypocotyl elongation coincides with the period in which the cotyledons are raised, usually from dusk until dawn (Dowson-Day and Millar, 1999). Mutations in genes controlling light perception or the clock itself often affect both cotyledon position and hypocotyl length (Hicks et al., 1996; Reed et al., 1998; Schaffer et al., 1998; Wang and Tobin, 1998; Fankhauser and Chory, 1999). Thus, cotyledon position and hypocotyl length appear linked in their regulation. One of the most striking phenotypes associated with mutant prf1-1 seedlings in addition to their raised cotyledons are their elongated hypocotyls, ∼1.5 to 2 times normal length (Figures 4C to 4E). The long mutant hypocotyls were characterized by longer than normal cell lengths (Figure 4J) compared with the wild type (Figure 4I). Although the relationship was not quantified, it appears that increased cell length could account entirely for the increased length of hypocotyls.
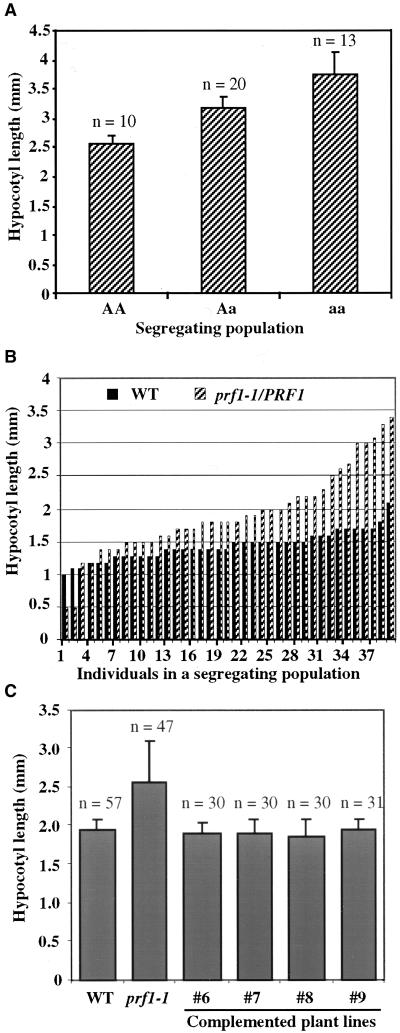
The mean hypocotyl lengths were measured for a population of 9-day-old seedlings segregating from a prf1-1/PRF1 heterozygous parent. A piece of tissue from each seedling was used to identify homozygous mutant, heterozygous, and wild-type genotypes by PCR (see Methods). The relationship between genotype and hypocotyl length is shown in Figure 5A. The majority of homozygous mutant seedlings (aa) have significantly longer hypocotyls than wild-type seedlings (AA). This difference was statistically significant (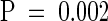 ). Seedlings were transferred to kanamycin-containing medium to assay for the neomycin phosphotransferase II resistance locus on the T-DNA. One hundred percent of those seedlings classified as containing the prf1-1 mutant allele were kanamycin resistant, whereas none of the wild-type seedlings were resistant. This further confirms that the prf1-1 allele alone results in the long hypocotyl phenotype.
). Seedlings were transferred to kanamycin-containing medium to assay for the neomycin phosphotransferase II resistance locus on the T-DNA. One hundred percent of those seedlings classified as containing the prf1-1 mutant allele were kanamycin resistant, whereas none of the wild-type seedlings were resistant. This further confirms that the prf1-1 allele alone results in the long hypocotyl phenotype.
Figure 5.
Hypocotyl Lengths in prf1-1 Mutants.
(A) Mean hypocotyl lengths in a population of 9-day-old seedlings segregating for the prf1-1 mutation from a heterozygous parent plant. Hypocotyl lengths were measured, and then the genotype of each plant was determined by PCR amplification of the wild-type PRF1 promoter region, the mutant junction sequences, or both sequences (heterozygotes) from each seedling. The mean length values from homozygous wild-type (AA), heterozygous mutant (Aa), and homozygous mutant (aa) seedlings are indicated. Standard errors from the mean and number of plants (n) are indicated for each genotype. The P value of 0.008 determined by analysis of variance supports the dependence of hypocotyl length on genotype.
(B) Distribution of individual hypocotyl lengths from a 9-day-old wild-type population (WT) and a mutant population segregating from prf1-1/PRF1 parents. Each population contained 39 plants.
(C) Mean hypocotyl lengths of 9-day-old homozygous prf1-1 mutant seedlings that were complemented with the wild-type PRF1 transgene. Populations derived from four different independent complemented transgenic lines (#6, #7, #8, and #9) were compared with wild-type (WT) and homozygous prf1-1 mutant populations. The number of individuals scored (n) and the standard errors from the mean are indicated for each population. Analysis of variance confirmed that the wild-type and complemented transgenic lines were all significantly different from the prf1-1 mutant (P < 0.0001) and indistinguishable from each other (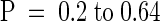 ).
).
In Figure 5B, the wide variation in individual hypocotyl lengths in a separate segregating population derived from a prf1-1/PRF1 heterozygous parent is compared with the variation in a wild-type population. Delayed germination for some individual prf1-1 seedlings caused them to develop days later than others in the population, whereas wild-type populations showed much more uniform germination and growth (Figures 4C, 4D, and 5B). Although the long hypocotyl phenotype is principally a recessive trait, the phenotype appears to be expressed in a gene dosage–dependent manner because (1) the heterozygous individuals are significantly longer than wild-type individuals (Figure 5A), and (2) the majority of individual mutant hypocotyls are longer than all but the longest wild-type hypocotyl (Figure 5B). These results were observed in several repetitions of this experiment performed on separate populations of seedlings between 5 and 11 days of age.
Complementation of the prf1-1 Mutation
Although the mutant hypocotyl phenotype segregated with the prf1-1 mutation (Figure 5A), we could not exclude the possibility of a closely linked mutation that was not part of the PRF1 locus giving rise to the observed phenotypes. Therefore, the wild-type PRF1 gene was transformed into a prf1-1 mutant background. The PRF1 gene fully complemented the phenotypes of the prf1-1 mutation in all 10 of the transformed lines examined. Complemented lines showed a normal germination rate, normal hypocotyl lengths, and normal root hair densities. Quantification of the elongated hypocotyl phenotype was performed for four complemented lines segregating for one or two copies of the PRF1 transgene. The results are shown in Figure 5C. Each complemented line showed an essentially normal distribution of hypocotyl lengths compared with wild-type and mutant (prf1-1) populations. Again, the homozygous prf1-1 mutant population showed the widest variation in phenotype and hence the largest standard error, due to the delayed germination of some individuals and the exceptionally long hypocotyls of others.
Loss of Light Regulation in prf1-1 Mutants
The elongated hypocotyl phenotype was similar to that observed in Hy mutants disrupted in phytochrome perception or other light-regulated developmental responses (Parks and Quail, 1991; Somers et al., 1991; Dehesh et al., 1993). The elongated hypocotyl phenotype might result simply from reduced PRF1 levels, disruption of the actin-based cytoskeleton, and an inability of seedling tissues to respond to light signals. Alternatively, there could be a more direct connection to this phenotype if the actin cytoskeletal system itself is light regulated. For example, the expression of plant tubulin genes is light regulated (Jacobshagen and Johnson, 1994; Tonoike et al., 1994; Vassilevskaia et al., 1996), and several Arabidopsis α- and β-tubulin gene family members and one actin (ACT11) are repressed by light during seedling development (Leu et al., 1995; Huang et al., 1997). Microtubule dynamics in some cell types are entrained as part of the circadian clock (Fukuda et al., 1998). The actin-related protein ARP2 binds profilin and may be involved in filament nucleation and branching. The mRNA for the Arabidopsis homolog AtARP2 is negatively regulated by light (Klahre and Chua, 1999). In contrast, light regulation of other plant actins or actin binding proteins such as the profilins has not been demonstrated.
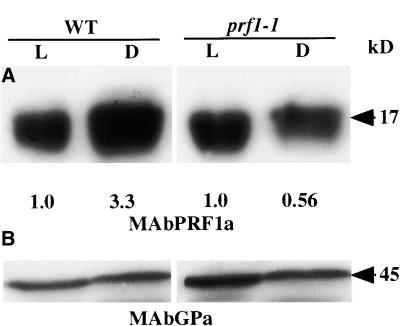
An analysis of the light regulation of PRF1 levels is shown in Figure 6. We consistently observed threefold to fourfold higher PRF1 levels for wild-type seedlings germinated in darkness compared with those germinated in the light (Figure 6A, right). In contrast, mutant seedlings reproducibly showed the same or lower levels of PRF1 in darkness relative to levels in the light (Figure 6A, left). A general monoclonal antiserum that reacts with all plant actin isovariants (MAbGPa) (Kandasamy et al., 1999) demonstrated equal protein loading and transfer to the membrane for a higher-molecular-weight region of the same gel (Figure 6B). Mutant prf1-1 seedlings appear to have lost their ability to express more PRF1 protein in darkness. When prf1-1 seedlings were germinated in total darkness, no reproducible phenotype was observed relative to wild type (unpublished results).
Figure 6.
PRF1 Levels Are Light Regulated, and prf1-1 Mutants Are Defective in This Regulation.
(A) Wild-type (WT) Arabidopsis seedlings show increased levels of PRF1 protein when grown in darkness (D) relative to being grown in the light (L), as measured by the PRF1-specific antibody, MAbPRF1a, on protein gel blots (left). Mutant prf1-1 seedlings have essentially the same low levels of protein when grown in light or darkness (right). Exposure intensities were adjusted to normalize the light-grown levels of PRF1 in wild-type and mutant plants. Densitometric quantification of each dark lane relative to the corresponding light lane is shown below the image.
(B) The top half of the same membrane blot was reacted with an anti-actin monoclonal antibody (MAbGPa) that binds all eight Arabidopsis actins. This blot shows that similar levels of protein were loaded and transferred to the membrane from the light- and dark-grown samples that were compared.
prf1-1 Mutant Seedlings Develop a High Density of Long Root Hairs
Figures 4F to 4H compare root hairs among wild-type seedlings (Figure 4F) and prf1-1/prf1-1 mutant seedlings (Figures 4G and 4H) germinated in agar medium. All of the mutant seedlings had an increased number of root hairs and longer root hairs than were observed in wild type. Furthermore, mutant seedlings often developed roots hairs earlier than Ws wild-type seedlings (data not shown). The dense root hair phenotype was observed for mutants regardless of whether seedlings were germinated on nutrient salts medium or distilled water and on solid or liquid medium.
DISCUSSION
In this study, we determined PRF1 function by examining a mutant allele. The T-DNA insertion in prf1-1 lies in the promoter region upstream of the transcriptional start site and thus should not alter the quality of the PRF1 protein product. Homozygous prf1-1 mutants showed reduced gene expression in young seedlings but not in older plants. In the mutant seedlings, both PRF1 mRNA and PRF1 protein levels were reduced at least twofold compared with the levels observed in wild type. PRF1 protein levels were threefold to fourfold higher when wild-type seedlings were germinated in darkness compared with light. How higher PRF1 levels in the darkness might be linked to increased hypocotyl elongation in etiolated wild-type seedlings is not clear. However, PRF1 levels are not light-regulated in prf1-1 mutant seedlings. One simple interpretation of the molecular genetic phenotype is that PRF1 levels are normally induced by darkness, and the necessary regulatory elements for this response have been separated from the PRF1 gene by the T-DNA insertion in the 5′ flanking region in the prf1-1 mutant allele.
Plant profilins are encoded by members of a diverse gene family with overlapping patterns of expression (Christensen et al., 1996; Huang et al., 1996). The expression of PRF2, for example, overlaps considerably with that of PRF1 in vegetative organs. In addition, the level of total profilin appears unaffected in the prf1-1 mutant relative to the wild type (Figure 3E). This redundancy in profilin expression might be expected to buffer the levels of profilin so that the loss of just one isovariant would not affect development. However, even small changes in the levels of cytoskeletal protein expression result in changes in fission yeast cell morphology. When variations in fission yeast morphology are used to screen clones in cDNA expression libraries from Drosophila (Edwards et al., 1994) and Arabidopsis (Xia et al., 1996), primarily cytoskeletal and cell cycle–related genes are identified. Thus, our results showing that a twofold to threefold decrease in PRF1 expression altered Arabidopsis seedling development are consistent with the effects of altering gene expression levels for many cytoskeletal genes. Further evidence that minor changes in profilin levels affect plant development comes from the intermediate hypocotyl length phenotype observed for heterozygous prf1-1/PRF1 seedlings (Figure 5A).
In agreement with the molecular phenotype of altered gene expression in seedlings, the morphological and developmental phenotypes were observed in prf1-1 seedlings but not in older plants. Visible defects included delays in germination, in cotyledon development, and in first leaf development and elongated hypocotyls. It is possible that the same molecular phenomena that result in the cotyledons remaining raised throughout most of the daily growth cycle also allow for continuous hypocotyl elongation (Dowson-Day and Millar, 1999). Perhaps the decreased PRF1 levels in seedlings and the inability to increase PRF1 protein levels in darkness release too many actin monomers that would otherwise be sequestered in profilactin complexes. This in turn could lead to more rapid cell elongation and hypocotyl expansion and, similarly, expansion of cells on the underside of the petioles, resulting in raised cotyledons. It also could account for the increased length of root hairs in the mutants. This would agree with the observation in Thyone sperm that a rapid release of actin monomers from profilactin complexes resulted in rapid F-actin polymerization and cell extension (Tilney and Inoue, 1985).
One interpretation of the raised cotyledons and the associated increase in hypocotyl length observed in prf1-1 mutants is that they indicate a simple cytoarchitectural defect with developmental consequences. In animals, changes in actin cytoarchitecture associated with diurnal cycles (Iovanna et al., 1990; Calman and Chamberlain, 1992) generally are interpreted as part of the elaboration of a signal from the circadian clock, with the clock directing these changes. In other words, profilin and actin are responding at the extreme phenotypic end of an information pathway to direct a physical change in cell structure. Rhythmic leaf movements and hypocotyl elongation appear to be controlled by circadian regulation of cell expansion on different sides of the leaf in likely homologs of flexor and extensor cells (Kim et al., 1993). Because actin filaments are arranged longitudinally in elongating cells, actin is thought to positively direct cell elongation, acting in opposition to transversely arranged microtubules (Kandasamy and Meagher, 1999). Profilin is in a distinct position as both a signaling molecule and the principal actin binding protein to respond quickly to changes in intracellular calcium and phosphoinositides and orchestrate immediate changes in the actin cytoskeleton that would guide cell elongation. Future work on prf1-1 mutant plant cells will need to examine associated changes in the actin cytoskeleton itself.
An alternative interpretation of the raised cotyledon/elongated hypocotyl phenotype is that prf1-1 mutants are defective in some property affecting circadian cycling itself and the prf1-1 mutation is acting at the top of an information pathway. As mentioned above, the elongated hypocotyl phenotype also is observed in Hy mutants defective in light perception or in the clock itself, and through its role in the cytoskeleton profilin it could affect these systems indirectly. For example, loss of profilin in yeast and Dictyostelium results in increases in actin filament concentrations (Haugwitz et al., 1994; Yeh and Haarer, 1996); similar F-actin increases may occur in prf1-1 mutant seedlings. Changes in seedling cytoarchitecture could alter the positioning of light receptors or signal molecules within cells, thereby resulting in defects in clock function. In addition, profilin also can participate in phosphorylation/dephosphorylation signal transduction cascades (Goldschmidt-Clermont et al., 1990, 1991). Decreased PRF1 levels might directly affect the phosphorylation state and the activation/deactivation of those clock proteins whose phosphorylation is under circadian control (Garceau et al., 1997; Kloss et al., 1998). By combining Arabidopsis circadian mutants with the prf1-1 mutation, it might be possible to determine the position at which prf1-1 acts in these signaling pathways.
Although PRF1 is just one of five members in the Arabidopsis profilin gene family, it appears to play a significant role in normal seedling development. The prf1-1 mutation affects gene regulation and results in lower PRF1 protein expression levels in seedlings. Light regulation of PRF1 levels appears to be lost in the mutant. Although it is difficult to determine where in the signaling pathway PRF1 acts, the simplest mechanistic interpretation of our data is that decreased PRF1 levels free more actin monomers to form F-actin filaments in seedlings. This should lead to more rapid cell elongation, directly resulting in raised cotyledons, increased hypocotyl elongation, and longer than normal root hairs. Future work will examine F-actin architecture and profilin's role in signaling in these cells.
METHODS
Seed Germination and Plant Growth
Seed were sterilized by soaking for 2 min in 70% ethanol, followed by 30 min in 30% Clorox bleach with 0.02% Triton X-100, and then washing three times in sterile distilled water. Seed were then placed on agar plates for 48 hr at 4°C and transferred to growth chambers. Plants were grown with 12 hr of light and 12 hr of darkness in growth chambers with fluorescent lights at 50 to 150 μE intensity and 22 to 24°C. Media contained half-strength Murashige and Skoog (1962) salts with trace nutrients (Life Technologies, Inc., Rockville, MD), 1% sucrose, and 0.8% Phytagar (Life Technologies, Inc.). Dark-grown plants had the same nutrient and temperature conditions, were kept in a box wrapped in three layers of aluminum foil, and were harvested with the aid of a green safelight.
Sequence-Based Isolation of a Profilin Mutant
An Arabidopsis library of 7000 T-DNA insertion lines containing ∼1.5 insertions per line was screened in pools of 100 lines for disruptions of profilin sequences (Feldmann, 1991). One intact T-DNA element is ∼16 kb from left to right border. On the basis of the alignment of diverse plant, fungus, and protist profilins (Huang et al., 1996), degenerate profilin sense (PRF-1S) and antisense (PRF-104A) primers were synthesized. They contained codons 1 and 104, respectively, of presumptive profilin sequences (Table 1). The location of these primers relative to a plant profilin gene sequence is shown in Figure 1A. These profilin primers were used in conjunction with T-DNA border primers (Table 1) to amplify profilin/T-DNA junction sequences using the polymerase chain reaction (PCR). The products were transferred to membrane (Biotrans Plus; ICN, Costa Mesa, CA) and probed with an 800-bp profilin cDNA amplified with the same two profilin primers from an Arabidopsis cDNA library. The sequence of the PCR product produced from the unique primer pairs LB102A/PRF1-1A and LB102A/PRF1-5′N1 identified the boundaries and the extent of the mutation in PRF1 (Figure 1 and Table 1). Subsequent rounds of screening seed pools were used to identify the particular seed line containing this insertion.
Genetic Isolation of the prf1-1 Mutation
The prf1-1 mutant identified from the T2 generation of the T-DNA library was backcrossed twice into a clean Arabidopsis var Wassilewskija (Ws) parental genetic background and subsequently selfed twice. Two heterozygous prf1-1/PRF1 lines were chosen for the studies reported in this article. Three-fourths of their progeny segregated for the linked kanamycin resistance marker, and 100% of these plants also contained the prf1-1 T-DNA insertion.
RNA gel blot analysis of transcripts and reverse transcriptase–mediated polymerase chain reaction (RT-PCR) analysis of relative transcript levels were performed as described previously (Tanzer and Meagher, 1994; An et al., 1996).
Transformation of prf1-1 Mutants with Wild-Type PRF1
A 2.4-kb SalI–SacI DNA fragment containing the wild-type PRF1 gene was amplified by PCR from Arabidopsis var Ws genomic DNA and cloned into the replacement region of vector pCambia (Hajdukiewicz et al., 1994) to make pCamPRF1. The confirmed sequence of this cloned fragment contains 1.0 kb of promoter and 5′ flanking sequence upstream of the PRF1 start codon and 0.45 kb of sequence downstream of the stop codon. The pCamPRF1 construct was transformed into a homozygous clean prf1-1 kanamycin-resistant mutant background by vacuum infiltration (Bariola et al., 1999), selecting for hygromycin resistance on the vector. A dozen independent transformed lines (T1 generation) were selected. T2 generation seed from these lines were germinated, and seedlings were examined for phenotype.
Preparation of Mouse Monoclonal Antisera and Protein Gel Blots
PRF1, PRF2, PRF3, PRF4, and PRF5 cDNAs were amplified by PCR and cloned into a PET15b expression vector (Novagen, Madison, WI). Escherichia coli extracts were prepared according to published protocols (PET-His.Tag system protocols; Novagen). All five profilin isovariants were purified on poly-l-proline affinity columns (Kaiser et al., 1989). Pure PRF1 (200 μg) was injected into each of five female Balb/c mice. These mice were boosted 4 and 8 weeks later with an additional 100 μg of protein. Splenocytes from the two mice with the highest anti-PRF1 titers in whole serum as determined by ELISA were fused with myeloma (SP2/0) cells to make hybridoma cell lines. The cell line producing the monoclonal antibody MAbPRF1 was identified, and antiserum was prepared as described previously (Kandasamy et al., 1999). Protein samples were prepared, resolved by SDS-PAGE on 12.5% gels, and analyzed by protein gel blotting as described previously (Kandasamy et al., 1999).
Microscopy
Seedlings were photographed directly with an Olympus digital D-400 Zoom camera (Tokyo, Japan) or on a Leica MZFLIII dissecting microscope (Wetzlar, Germany) or a Zeiss compound microscope (Jena, Germany) using a Hamamatsu color-chilled charge-coupled device camera (Bridgewater, NJ).
Acknowledgments
We thank Kenneth Feldmann for generously supplying the T-DNA lines, Scott Bizily for discussion of the presentation of genetic data, and Yolanda Lay and the University of Georgia Monoclonal Facility for technical help with the isolation of a PRF1-specific monoclonal antibody. Steve Almo (Albert Einstein College of Medicine, Bronx, NY) gave helpful advice with the profilin purification. Gay Gragson, Marcus Fechheimer, Laura Gilliland, and Kelly Dawe provided helpful discussion and criticism of the manuscript. This research was supported by a grant from the National Institutes of Health (GM 36397).
References
- Ahern-Djamali, S.M., Bachmann, C., Hua, P., Reddy, S.K., Kastenmeier, A.S., Walter, U., and Hoffmann, F.M. (1999). Identification of profilin and src homology 3 domains as binding partners for Drosophila enabled. Proc. Natl. Acad. Sci. USA 96 4977–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y.-Q., Huang, S., McDowell, J.M., McKinney, E.C., and Meagher, R.B. (1996). Conserved expression of the Arabidopsis ACT1 and ACT3 actin subclass in organ primordia and mature pollen. Plant Cell 8 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola, P.A., MacIntosh, G.C., and Green, P.J. (1999). Regulation of S-like ribonuclease levels in Arabidopsis: Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol. 119 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calman, B.G., and Chamberlain, S.C. (1992). Localization of actin filaments and microtubules in the cells of the Limulus lateral and ventral eyes. Vis. Neurosci. 9 365–375. [DOI] [PubMed] [Google Scholar]
- Carlier, M.F., Jean, C., Rieger, K.J., Lenfant, M., and Pantaloni, D. (1993). Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: Possible implication in the regulation of actin dynamics. Proc. Natl. Acad. Sci. USA 90 5034–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, H.E., Ramachandran, S., Tan, C.T., Surana, U., Dong, C.H., and Chua, N.H. (1996). Arabidopsis profilins are functionally similar to yeast profilins: Identification of a vascular bundle–specific profilin and a pollen-specific profilin. Plant J. 10 269–279. [DOI] [PubMed] [Google Scholar]
- Clarke, S.R., Staiger, C.J., Gibbon, B.C., and Franklin-Tong, V.E. (1998). A potential signaling role for profilin in pollen of Papaver rhoeas. Plant Cell 10 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh, K., Franci, C., Parks, B.M., Seeley, K.A., Short, T.W., Tepperman, J.M., and Quail, P.H. (1993). Arabidopsis HY8 locus encodes phytochrome A. Plant Cell 5 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson-Day, M.J., and Millar, A.J. (1999). Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J. 17 63–71. [DOI] [PubMed] [Google Scholar]
- Edwards, K.A., Montague, R.A., Shepard, S., Edgar, B.A., Erikson, R.L., and Kiehart, D.P. (1994). Identification of Drosophila cytoskeletal proteins by induction of abnormal cell shape in fission yeast. Proc. Natl. Acad. Sci. USA 91 4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., and Chory, J. (1999). Light receptor kinases in plants! Curr. Biol. 9 R123–R126. [DOI] [PubMed] [Google Scholar]
- Feldmann, K.A. (1991). T-DNA insertion mutagenesis in Arabidopsis: Mutational spectrum. Plant J. 1 71–82. [Google Scholar]
- Fukuda, M., Hasezawa, S., Asai, N., Nakajima, N., and Kondo, N. (1998). Dynamic organization of microtubules in guard cells of Vicia faba L. with diurnal cycle. Plant Cell Physiol. 39 80–86. [DOI] [PubMed] [Google Scholar]
- Garceau, N.Y., Liu, Y., Loros, J.J., and Dunlap, J.C. (1997). Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell 89 469–476. [DOI] [PubMed] [Google Scholar]
- Gibbon, B.C., and Staiger, C.J. (2000). Profilin. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 45–65.
- Gibbon, B.C., Ren, H., and Staiger, C.J. (1997). Characterization of maize (Zea mays) pollen profilin function in vitro and in live cells. Biochem. J. 327 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon, B.C., Zonia, L.E., Kovar, D.R., Hussey, P.J., and Staiger, C.J. (1998). Pollen profilin function depends on interaction with proline-rich motifs. Plant Cell 10 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, P.J., Machesky, L.M., Baldassare, J.J., and Pollard, T.D. (1990). The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science 247 1575–1578. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, P.J., Kim, J.W., Machesky, L.M., Rhee, S.G., and Pollard, T.D. (1991). Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science 251 1231–1233. [DOI] [PubMed] [Google Scholar]
- Haarer, B.K., Lillie, S.H., Adams, A.E., Magdolen, V., Bandlow, W., and Brown, S.S. (1990). Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J. Cell Biol. 110 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer, B.K., Petzold, A.S., and Brown, S.S. (1993). Mutational analysis of yeast profilin. Mol. Cell. Biol. 13 7864–7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25 989–994. [DOI] [PubMed] [Google Scholar]
- Haugwitz, M., Noegel, A.A., Rieger, D., Lottspeich, F., and Schleicher, M. (1991). Dictyostelium discoideum contains two profilin isoforms that differ in structure and function. J. Cell Sci. 100 481–489. [DOI] [PubMed] [Google Scholar]
- Haugwitz, M., Noegel, A.A., Karakesisoglou, J., and Schleicher, M. (1994). Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell 79 303–314. [DOI] [PubMed] [Google Scholar]
- Hicks, K.A., Millar, A.J., Carre, I.A., Somers, D.E., Straume, M., Meeks-Wagner, D.R., and Kay, S.A. (1996). Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274 790–792. [DOI] [PubMed] [Google Scholar]
- Huang, S., McDowell, J.M., Weise, M.J., and Meagher, R.B. (1996). The Arabidopsis profilin gene family: Evidence for an ancient split between constitutive and pollen-specific profilin genes. Plant Physiol. 111 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., An, Y.-Q., McDowell, J.M., McKinney, E.C., and Meagher, R.B. (1997). The Arabidopsis ACT11 actin gene is strongly expressed in tissues of the emerging inflorescence, pollen, and developing ovules. Plant Mol. Biol. 33 125–139. [DOI] [PubMed] [Google Scholar]
- Iovanna, J., Dusetti, N., Calvo, E., and Cardinali, D.P. (1990). Diurnal changes in actin mRNA levels and incorporation of 35S-methionine into actin in the rat hypothalamus. Cell. Mol. Neurobiol. 10 207–216. [DOI] [PubMed] [Google Scholar]
- Jacobshagen, S., and Johnson, C.H. (1994). Circadian rhythms of gene expression in Chlamydomonas reinhardtii: Circadian cycling of mRNA abundances of cab II, and possibly of beta-tubulin and cytochrome c. Eur. J. Cell Biol. 64 142–152. [PubMed] [Google Scholar]
- Kaiser, D.A., Goldschmidt-Clermont, P.J., Levine, B.A., and Pollard, T.D. (1989). Characterization of renatured profilin purified by urea elution from poly-l-proline agarose columns. Cell Motil. Cytoskeleton 14 251–262. [DOI] [PubMed] [Google Scholar]
- Kandasamy, M.K., and Meagher, R.B. (1999). Actin-organelle interactions: Association with chloroplast in Arabidopsis leaf me-sophyll cells. Cell Motil. Cytoskeleton 44 110–118. [DOI] [PubMed] [Google Scholar]
- Kandasamy, M.K., McKinney, E.C., and Meagher, R.B. (1999). The late pollen specific actins in angiosperms. Plant J. 18 681–691. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou, I., Schleicher, M., Gibbon, B.C., and Staiger, C.J. (1996). Plant profilins rescue the aberrant phenotype of profilin-deficient Dictyostelium cells. Cell Motil. Cytoskeleton 34 36–47. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou, I., Janssen, K.P., Eichinger, L., Noegel, A.A., and Schleicher, M. (1999). Identification of a suppressor of the Dictyostelium profilin-minus phenotype as a CD36/LIMP-II homologue. J. Cell Biol. 145 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.Y., Cote, G.G., and Crain, R.C. (1993). Potassium channels in Samanea saman protoplasts controlled by phytochrome and the biological clock. Science 260 960–962. [DOI] [PubMed] [Google Scholar]
- Klahre, U., and Chua, N.H. (1999). The Arabidopsis actin-related protein 2 (AtARP2) promoter directs expression in xylem precursor cells and pollen. Plant Mol. Biol. 41 65–73. [DOI] [PubMed] [Google Scholar]
- Kloss, B., Price, J.L., Saez, L., Blau, J., Rothenfluh, A., Wesley, C.S., and Young, M.W. (1998). The Drosophila clock gene double-time encodes a protein closely related to human casein kinase I. Cell 94 97–107. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski, D.J., and Bruns, G.A. (1988). Human profilin: Molecular cloning, sequence comparison, and chromosomal analysis. J. Biol. Chem. 263 5910–5915. [PubMed] [Google Scholar]
- Leu, W.-M., Hao, X.-L., Wilson, T.J., Snustad, D.P., and Chua, N.-H. (1995). Phytochrome A and phytochrome B mediate the hypocotyl-specific downregulation of TUB1 by light in Arabidopsis. Plant Cell 7 2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher, R.B., McKinney, E.C., and Kandasamy, M.K. (1999. a). Isovariant dynamics expands and buffers the responses of complex systems: The diverse plant actin gene family. Plant Cell 11 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher, R.B., Vitale, A., and McKinney, E.C. (1999. b). The evolution of new structures: Clues from plant cytoskeletal genes. Trends Genet. 15 278–284. [DOI] [PubMed] [Google Scholar]
- Meagher, R.B., McKinney, E.C., and Kandasamy, M.K. (2000). The significance of diversity in the plant actin gene family: Studies in Arabidopsis. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 3–27.
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Ostrander, D.B., Gorman, J.A., and Carman, G.M. (1995). Regulation of profilin localization in Saccharomyces cerevisiae by phosphoinositide metabolism. J. Biol. Chem. 270 27045–27050. [DOI] [PubMed] [Google Scholar]
- Pantaloni, D., and Carlier, M.F. (1993). How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell 75 1007–1014. [DOI] [PubMed] [Google Scholar]
- Parks, B.M., and Quail, P.H. (1991). Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in chromophore biosynthesis. Plant Cell 3 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelroizen, I., Didry, D., Christensen, H., Chua, N.H., and Carlier, M.F. (1996). Role of nucleotide exchange and hydrolysis in the function of profilin in action assembly. J. Biol. Chem. 271 12302–12309. [DOI] [PubMed] [Google Scholar]
- Reed, J.W., Elumalai, R.P., and Chory, J. (1998). Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics 148 1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer, R., Ramsay, N., Samach, A., Corden, S., Putterill, J., Carre, I.A., and Coupland, G. (1998). The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93 1219–1229. [DOI] [PubMed] [Google Scholar]
- Senecoff, J., and Meagher, R.B. (1993). Isolating the Arabidopsis thaliana genes for de novo purine synthesis by suppression of Escherichia coli mutants: 5-Phosphoribosyl-5-aminoimadazole synthetase. Plant Physiol. 103 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.S., Chauhan, A., Murakami, N., and Chauhan, V.P. (1996). Profilin and gelsolin stimulate phosphatidylinositol 3-kinase activity. Biochemistry 35 16544–16549. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Sharrock, R.A., Tepperman, J.M., and Quail, P.H. (1991). The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger, C.J., Goodbody, K.C., Hussey, P.J., Valenta, R., Drøbak, B.K., and Lloyd, C.W. (1993). The profilin multigene family of maize: Differential expression of three isoforms. Plant J. 4 631–641. [DOI] [PubMed] [Google Scholar]
- Staiger, C.J., Yuan, M., Valenta, R., Shaw, P.J., Warn, R.M., and Lloyd, C.W. (1994). Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr. Biol. 4 215–219. [DOI] [PubMed] [Google Scholar]
- Tanzer, M.M., and Meagher, R.B. (1994). Faithful degradation of soybean rbcS mRNA in vitro. Mol. Cell. Biol. 14 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L.G., and Inoue, S. (1985). Acrosomal reaction of the Thyone sperm. III. The relationship between actin assembly and water influx during the extension of the acrosomal process. J. Cell Biol. 100 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoike, H., Han, I.S., Jongewaard, I., Doyle, M., Guiltinan, M., and Fosket, D.E. (1994). Hypocotyl expression and light downregulation of the soybean tubulin gene, tubB1. Plant J. 5 343–351. [DOI] [PubMed] [Google Scholar]
- Vassilevskaia, T.D., Bekman, E., Jackson, P., Pinto Ricardo, C., and Rodrigues-Pousada, C. (1996). Developmental expression and regulation by light of two closely related beta-tubulin genes in Lupinus albus. Plant Mol. Biol. 32 1185–1189. [DOI] [PubMed] [Google Scholar]
- von Witsch, M., Baluska, F., Staiger, C.J., and Volkmann, D. (1998). Profilin is associated with the plasma membrane in microspores and pollen. Eur. J. Cell Biol. 77 303–312. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y., and Tobin, E.M. (1998). Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93 1207–1217. [DOI] [PubMed] [Google Scholar]
- Xia, G., Ramachandran, S., Hong, Y., Chan, Y.S., Simanis, V., and Chua, N.H. (1996). Identification of plant cytoskeletal, cell cycle–related and polarity-related proteins using Schizosaccharomyces pombe. Plant J. 10 761–769. [DOI] [PubMed] [Google Scholar]
- Yeh, J., and Haarer, B.K. (1996). Profilin is required for the normal timing of actin polymerization in response to thermal stress. FEBS Lett. 398 303–307. [DOI] [PubMed] [Google Scholar]