Abstract
Aluminum (Al) inhibits inward K+ channels (Kin) in both root hair and guard cells, which accounts for at least part of the Al toxicity in plants. To understand the mechanism of Al-induced Kin inhibition, we performed patch clamp analyses on Kin in guard cells and on KAT1 channels expressed in Xenopus oocytes. Our results show that Al inhibits plant Kin by blocking the channels at the cytoplasmic side of the plasma membrane. In guard cells, single-channel recording revealed that Al inhibition of Kin occurred only upon internal exposure. Using both “giant patch” recording and single-channel analyses, we found that Al reduced KAT1 open probability and changed its activation kinetics through an internal membrane-delimited mechanism. We also provide evidence that a Ca2+ channel–like pathway that is sensitive to antagonists verapamil and La3+ mediates Al entry across the plasma membrane. We conclude that Al enters plant cells through a Ca2+ channel–like pathway and inhibits K+ uptake by internally blocking Kin.
INTRODUCTION
Aluminum (Al) is one of the major components in the soil, and its trivalent ionic form released in acid soil is highly toxic to plant growth, which is responsible for significant reductions of crop production. Al has been found to inhibit root elongation within minutes after exposure, whereas over longer periods both cell division and elongation are inhibited (Jones and Kochian, 1995; Kochian, 1995). Despite extensive studies, the molecular mechanisms of Al toxicity remain poorly understood (Rengel, 1992; Delhaize and Ryan, 1995; Kochian, 1995).
One of the early effects of Al toxicity is a dramatic reduction in uptake of K+, Ca2+, NH4+, and other cations (Foy et al., 1978; Kinraide and Parker, 1987; Brady et al., 1993). The reduction of cation uptake can be correlated with the inhibition of root elongation because cation (especially K+) accumulation contributes to the expansion of cell volume, initiating turgor-driven cell elongation (Boyer, 1985; Frensch, 1997). Inhibition of ion channels and transporters in the plasma membrane often underlies the reduction of cation uptake. Indeed, Al has been shown to block inward K+ channels (Kin) in root hair cells (Gassmann and Schroeder, 1994), which is consistent with the observation of Al inhibition of K+ uptake and root elongation. In epidermal guard cells, Kin is one of the major components of the control of stomatal movements (Assmann, 1993; Maathuis et al., 1997). Al inhibition of Kin in guard cells also has been documented and can be correlated with the inhibition of light-induced stomatal opening (Schroeder, 1988; Schroeder et al., 1994).
Al inhibition of K+ uptake through Kin may be an important component of Al toxicity in plants. The mechanism underlying Al-induced Kin inhibition clearly deserves serious attention. Because Al inhibition of Kin is partially reversible and voltage independent upon external perfusion of root hair protoplasts, it has been proposed that Al may inhibit Kin by a direct external block (Gassmann and Schroeder, 1994; Schroeder et al., 1994). To test this hypothesis and to understand the molecular basis for Al action in plant cells, it is necessary to identify a target channel protein responsible for the Al inhibition of Kin in root hair or guard cells.
Since the first Kin genes in plants, AKT1 and KAT1, were isolated from Arabidopsis by functional complementation of a K+ uptake–deficient yeast mutant (Anderson et al., 1992; Sentenac et al., 1992), a number of other Kin genes have been cloned and functionally characterized in several heterologous systems. Among them, KAT1 is expressed mainly in Arabidopsis guard cells and AKT1 in root cells (reviewed in Czempinski et al., 1999). Some other homologous genes include KST1, a potato KAT1-like gene expressed mainly in guard cells, SKT1, a potato AKT1-like gene expressed in roots and guard cells, and LKT1, a tomato AKT1-like gene highly expressed in root hair cells (Czempinski et al., 1999). All of these genes can be expressed functionally in Xenopus oocyte or insect cell expression systems (Schachtman et al., 1992; Müller-Röber et al., 1995; Gaymard et al., 1996; Zimmermann et al., 1998; Hartje et al., 2000). Although extensive studies have examined the biophysical and regulatory properties of these expressed channels, none of the studies has successfully addressed Al inhibition of these channels (Hoshi, 1995; Hartje et al., 2000).
In the present article, we demonstrate that Al inhibits plant Kin by direct internal block. Both Kin in guard cells and KAT1 expressed in Xenopus oocytes are sensitive to Al on the cytoplasmic side of the plasma membrane. Further analysis of Al inhibition of the KAT1 channel unambiguously demonstrates that Al inhibits the channel by reducing open probability and modifying activation kinetics. Our study also indicates that Al may enter guard cells by a Ca2+ channel–like pathway.
RESULTS
Internal Al Block of Kin in Fava Bean Guard Cells
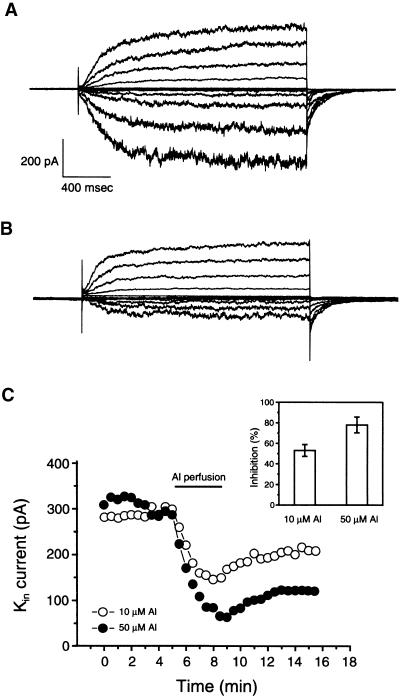
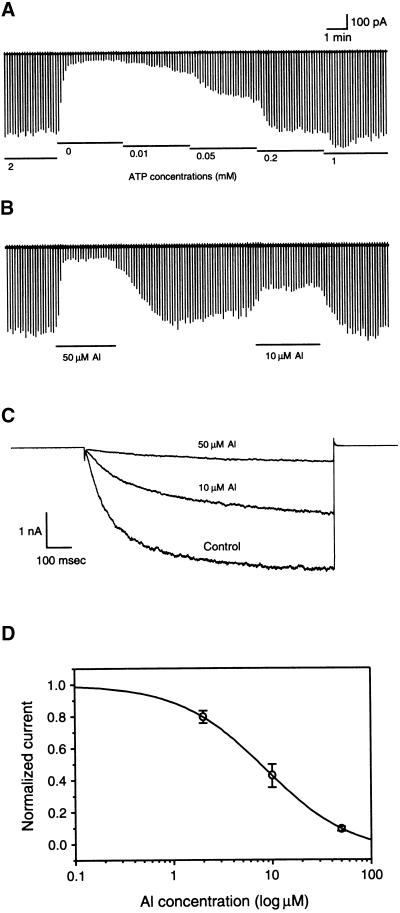
In whole-cell configuration, a series of voltage protocols from −160 to +80 mV elicited both Kin current and an outward K+ current across the plasma membrane of a fava bean guard cell protoplast (Figure 1A). Bath perfusion of 50 μM Al strongly inhibited Kin current but had little effect on the outward current (Figure 1B). The Kin current was inhibited at −160 mV by 78% from 385 pA (Figure 1A) to 86 pA (Figure 1B). To determine the time course of Al inhibition, Kin current was recorded at a constant interval of 30 sec by using a pulse protocol of 3 sec at −150 mV; the steady state currents from two representative cells were plotted against time (Figure 1C). The maximal inhibition occurred in the first 2 to 3 min after Al perfusion. With either 10 or 50 μM Al, the current was only partially reversed after Al was removed from the bath solution. During a period of 5 to 6 min, ∼20 to 40% of the inhibited current was recovered (Figure 1C). Typically, Al inhibition is concentration dependent (Figure 1C, inset). At 10 and 50 μM, Al inhibited Kin by 52.1 ± 8.8% (n = 10) and 78.3 ± 9.4% (n = 10), respectively. These results are consistent with previous observations (Schroeder, 1988; Gassmann and Schroeder, 1994).
Figure 1.
Al Inhibition of Kin in Guard Cells.
(A) Whole-cell Kin currents recorded in a guard cell protoplast under control conditions. The currents were elicited at membrane potentials from −160 to 80 mV with increments of 20 mV. The holding potential was −50 mV. Both pipette and bath solutions contained 100 mM K+.
(B) Whole-cell Kin currents from the same protoplast as in (A) perfused with 50 μM Al in the bath solution.
(C) Time courses of Al effects on Kin currents from two protoplasts perfused with 10 μM Al (open circles) and 50 μM Al (closed circles), respectively. Each data point represents the amplitude of whole-cell Kin current at −150 mV at steady state. The Al perfusion period is shown as a horizontal bar. The inset shows the inhibition of steady state Kin current by 10 μM Al (n = 10) and 50 μM Al (n = 10). Error bars indicate ±se.
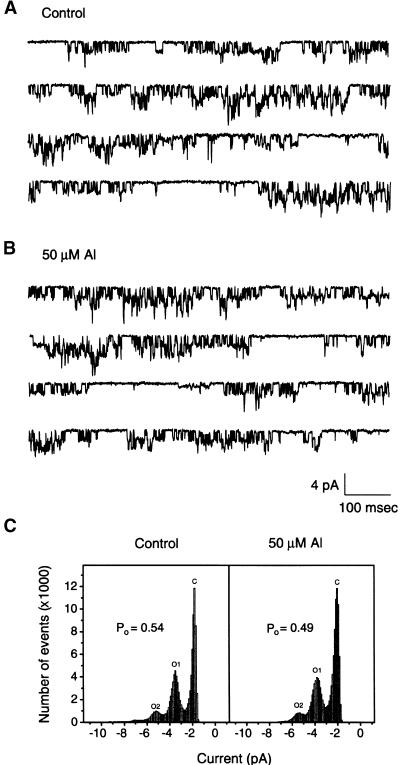
Using single-channel recording techniques, we determined whether Al inhibits Kin by external block, as suggested previously (Schroeder, 1988; Schroeder et al., 1994). As shown in Figure 2, we recorded the typical single-channel Kin currents in fava bean guard cells as characterized in previous studies (Liu and Luan, 1998). If Al externally blocks Kin, single-channel current should be inhibited in an outside-out configuration when the patches are bath perfused with Al-containing solution. Surprisingly, the single-channel activity did not respond to Al application during 10 min of bath perfusion (Figures 2A and 2B). Indeed, Figure 2C shows that Al did not have any effect on either open probability or single-channel current amplitude (n = 7). This finding suggests that Al inhibition of Kin is not caused by an external block. Instead, it suggests that intracellular components (either on the plasma membrane or in the cytoplasm) are required for Al action.
Figure 2.
Al Effect on Single-Channel Current of Guard Cell Kin in the Outside-Out Configuration.
(A) Single-channel current recorded at a pipette potential of −120 mV in an outside-out patch. The solutions in both sides of membrane patches contained 100 mM K+.
(B) Single-channel current from the same patch as in (A) in the presence of 50 μM Al in the bath solution.
(C) Open probability (Po) analyses of single-channel currents in (A) and (B). The open probability values were calculated from the ratio of total open time to total recording time. O, open state; C, closed state. The numbers after the Os denote different state levels.
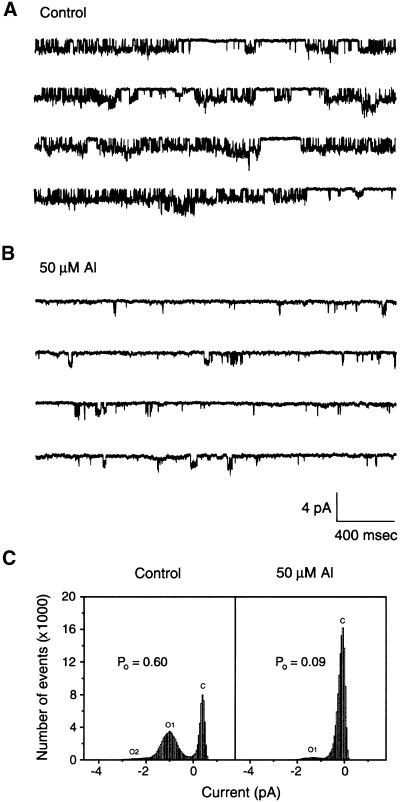
To determine whether Al inhibits Kin through an intracellular binding site on the plasma membrane, we performed single-channel recording in the inside-out configuration. When membrane patches were perfused with 50 μM Al on the cytoplasmic side, the single-channel activities of Kin were inhibited strongly (Figures 3A and 3B). As shown in Figure 3C, Al inhibited single-channel activities by suppressing the open probability, in this case, from 0.6 to 0.09, whereas the single-channel current amplitude remained unchanged (Figures 3A to 3C). Six individual membrane patches were analyzed, and all of them displayed significant Al inhibition. Results from one of the six patches are presented here. These results indicate that Al inhibits Kin by interacting with plasma membrane components on the cytoplasmic side. Because the cytoplasmic side solution contains 2 mM EGTA, the free Al3+ concentration from the 50 μM total Al was calculated to be only 0.168 pM at pH 7.2 (Schoenmakers et al., 1992).
Figure 3.
Al Effect on Single-Channel Current of Guard Cell Kin in the Inside-Out Configuration.
(A) Single-channel current recorded at a pipette potential of 120 mV in an inside-out patch configuration. The solutions in both sides of the patch contained 100 mM K+.
(B) Single-channel current from the same patch as in (A) in the presence of 50 μM Al in the bath solution. The same conditions were used as in (A). The time/current scale is the same in both (A) and (B).
(C) Open probability (Po) analyses of single-channel currents in (A) and (B). The open probability values were calculated from the ratio of total open time to total recording time. O, open state; C, closed state. The numbers after the Os denote different state levels.
Al Blocks KAT1 in Xenopus Oocytes
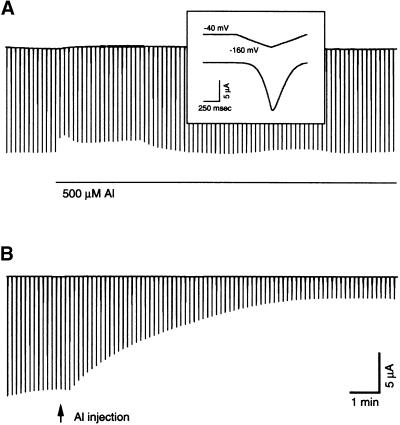
To further determine the molecular basis for the Al inhibition of Kin, we expressed one of the cloned K+ channel genes, KAT1, in Xenopus oocytes. KAT1 is expressed predominantly in guard cells (Nakamura et al., 1995) and is considered to be a major component of Kin in guard cells. When KAT1 copy RNA (cRNA) was introduced into oocytes by microinjection, a predominant Kin current that was not present in uninjected or water-injected oocytes was detected by two-electrode voltage clamp recording (data not shown). KAT1 current is activated by hyperpolarization, and the activation is both time and voltage dependent (Hoshi, 1995; Véry et al., 1995; Zei and Aldrich, 1998), like the Kin current in guard cells. To determine whether Al inhibits KAT1 current, we perfused KAT1-expressed oocytes with 500 μM Al while recording the currents activated by the voltage protocols. Under such conditions, we detected no significant effect of Al perfusion on KAT1 currents (Figure 4A; n = 9), consistent with an earlier report (Hoshi, 1995). In contrast to bath perfusion, injection of 23 nL of 50 mM Al into cytoplasm inhibited KAT1 current significantly (Figure 4B). Ten minutes after injection, KAT1 current was reduced by 86.4 ± 11.3% (n = 7). Compared with Kin inhibition in guard cells, Al inhibition of KAT1 currents progressed more slowly, possibly due to the large volume of the oocyte. These data suggest that Al inhibits the KAT1 channel by an intracellular pathway instead of by external block.
Figure 4.
Al Effect on Whole-Oocyte KAT1 Currents.
(A) Voltage clamp recording in a KAT1-expressing oocyte. The current traces in the main frame were produced by a number of voltage ramps from a holding potential of −40 mV to the testing potential of −160 mV with 10-sec intervals. Each voltage ramp and corresponding current trace are shown in the inset. The horizontal line represents bath perfusion with 500 μM Al in the bath solution.
(B) KAT1 current recorded from the same oocyte under the same protocol as in (A). The arrow indicates delivery of 23 nL of 50 mM AlCl3 into the oocyte cytosol. Time/current scales are shown for both (A) and (B).
To determine whether Al inhibition of KAT1 is a membrane-delimited process or requires cytoplasmic components, we conducted experiments using an excised inside-out patch recording procedure called giant patch recording. This powerful technique is used in studying ion channel regulation by intracellular factors in oocytes and other large cells (see Methods). It takes advantage of macroscopic currents derived from large excised membrane patches, with the bath solution perfusing the cytoplasmic side of the membrane.
We performed giant patch recording with KAT1-expressing oocytes and observed KAT1 currents. Consistent with an earlier study (Hoshi, 1995), KAT1 current displayed a significant rundown process after the patch was excised. The rundown current was restored partially or fully by cytosol-side application of ATP (Figure 5A). ATP activated KAT1 current in a concentration-dependent manner. At 10 μM, ATP began to be effective, and the effect was saturated in the presence of ≥200 μM ATP (Figure 5A). Because ATP is required to maintain KAT1 channel activity in excised patches, we performed the subsequent patch recordings in the presence of 2 mM ATP unless indicated otherwise. As shown in Figure 5B, bath perfusion of 50 μM Al strongly inhibited KAT1 current by up to 90%. The inhibition occurred rapidly and reached the maximum in <30 sec. In nine of 11 patches studied, Al inhibition was reversed fully upon removal of Al from the bath solution. This Al effect also was concentration dependent, because 10 μM Al was less effective than 50 μM. Figure 5C shows the steady state currents as affected by Al. The relationship of Al concentration and KAT1 current is shown in Figure 5D. The concentration resulting in 50% inhibition was ∼6.8 μM, close to that of Al inhibition on Kin in root hair cells (Gassmann and Schroeder, 1994).
Figure 5.
Al Effect on KAT1 Currents in Giant Patch Recording.
(A) ATP regulation of KAT1 activity. Macroscopic KAT1 currents were recorded in the presence of various concentrations of ATP in a giant oocyte membrane inside-out patch. The voltage protocol is similar to that in Figure 4, except that a positive voltage ramp to 20 mV was added after each testing ramp to monitor the leak current. Time/current scales are noted for both (A) and (B).
(B) Al inhibition of KAT1 activity. KAT1 current was recorded in the presence of 10 and 50 μM Al and 2 mM ATP in the solution perfusing the cytoplasmic side of the membrane.
(C) KAT1 currents elicited by a stepping voltage pulse from 0 to −160 mV in the presence of 0 (control), 10, and 50 μM Al.
(D) Concentration-dependent Al inhibition of KAT1 activity in giant patch recording. Currents are normalized as the percentage of the current before Al application. Al concentrations are presented in logphase. The smooth trace is the best fit of the data with the power logistic function I/I0 = 1/{1 + (C/C50)p}, where I denotes the KAT1 current amplitudes at different concentrations of Al, I0 is the I in the absence of Al, C denotes the concentrationof Al applied, C50 is the C resulting in 50% of inhibition, and p reflects the slope of the I–C curve. In this fit, C50 is 6.8 μM and p is 1.14. Error bars indicate ±se.
Al Modifies Activation Kinetics of the KAT1 Channel
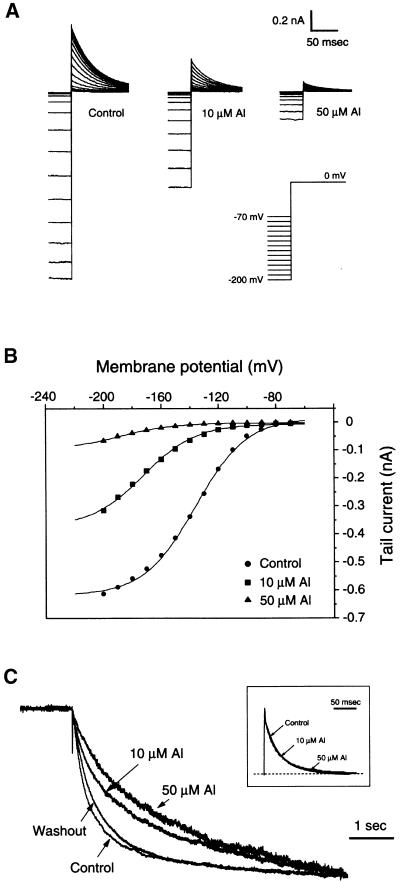
The open probability (Po) of KAT1 channels is deduced by tail currents elicited by a double voltage step protocol. The membrane potential was clamped at the first voltage step (V) to activate KAT1 channels in the range of −70 to −200 mV and then switched to the second step (0 mV) to close the channels and relax the tail currents. The amplitude of instantaneous tail current (It) at time 0 is proportional to the open probability of the channel at the end of the first activation voltage. According to the two-state model (closed and open), the It − V relationship can be described by the Boltzmann function:
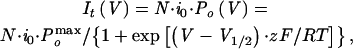 |
where N denotes the number of channels available to open, i0 denotes the single-channel current at the second voltage step (0 mV), Pomax denotes the maximal open channel probability, V1/2 denotes the half-maximal activation potential, and z denotes the gating charge. As shown in Figure 6A, Al significantly reduced the KAT1 tail current in a concentration-dependent manner. Fitting the It − V curves with the equation above indicates that the half-maximal activation potential shifted to less negative potentials with increasing Al concentrations, suggesting that open probability was affected (Figure 6B). The gating charge remained unmodified. Unlike the voltage-dependent block by Cs+ (Véry et al., 1995), Al inhibition of KAT1 was voltage independent between −70 and −200 mV (Figure 6B). Furthermore, the inhibition was accompanied by the slowing of the activation time course (Figure 6C), indicating that the gating kinetics were modified. However, Al did not affect the deactivation time course of KAT1 tail currents (Figure 6C, inset).
Figure 6.
Al Modification of KAT1 Activation Kinetics.
(A) KAT1 tail currents (It) from a giant inside-out patch of oocyte plasma membrane in the presence of 0 (control), 10, and 50 μM Al in the bath solution (cytoplasmic side). The tail currents were elicited by a two-step voltage protocol described at bottom right. The first 6-sec prepulse to voltages between −70 and −200 mV (shown partially) was applied and jumped to the second pulse at 0 mV. To induce clear tail currents in this condition, external (pipette) K+ concentration was reduced to 5 mM (see Methods).
(B) It − V curves of KAT1 tail currents from the data in (A). Data are fitted with a Boltzmann function of the form It(V) = I0 /{1 + exp[(V − V1/2) · zF/RT]}, where I0 represents the current amplitude at the maximal open probability. The other abbreviations are described in Results. The fitting parameters are as follows: control: I0, −0.62 nA; V1/2, −136.18 mV; z, 1.43 e; 10 μM Al: I0, −0.37 nA; V1/2, −172.22 mV; z, 1.41 e; 50 μM Al: I0, −0.095 nA; V1/2, −187.08 mV; z, 1.40 e. e stands for electronic charge.
(C) Activation time course of KAT1 in the presence of 0 (control), 10, and 50 μM Al and after removal of Al (washout). KAT1 currents were induced by the voltage pulse at −160 mV for 6 sec, and the traces were scaled to the same amplitude at the end of the test pulse. The inset shows a comparison of the deactivation time course from tail currents at the −180-mV prepulse shown in (A). The currents are normalized to the same amplitude at the beginning of the tail currents. The dotted line indicates the current basal level.
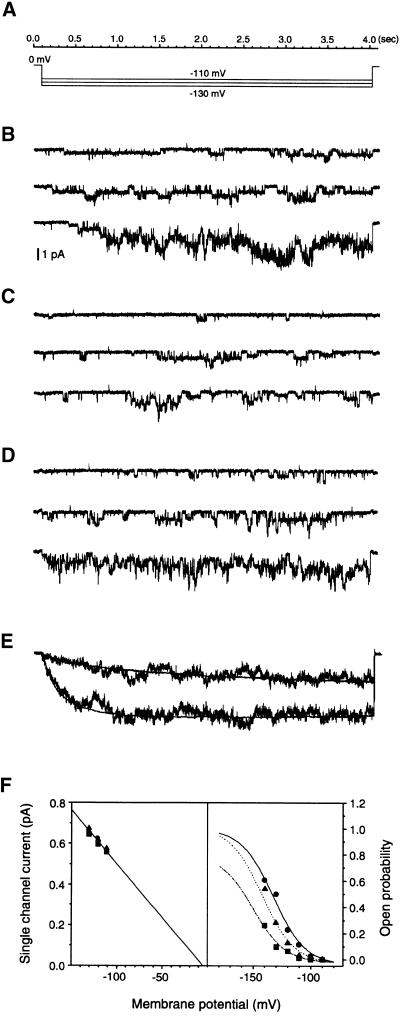
To further elucidate the mechanisms for Al action on the KAT1 channel, we conducted single-channel recording on membrane patches excised from KAT1-expressing oocytes. In the inside-out configuration, hyperpolarized membrane potentials elicited a voltage-dependent inward single-channel current (Figure 7B). In the presence of 2 mM ATP, the channel started to be activated by membrane potentials hyperpolarized to less than −100 mV. The activation process was both time dependent and voltage dependent. The ensemble average of single-channel currents closely matched KAT1 giant patch currents (Figure 7E). The reversal potential of this current was close to that of K+ (−3.9 mV), and the voltage dependence of steady state open probability can be fitted with a Boltzmann function using approximate values of the parameters for KAT1 macroscopic current from the giant patch recording (Figure 7F). This channel had a single-channel conductance of 5.5 picosiemens in the given condition, consistent with results described previously (Hoshi, 1995; Zei and Aldrich, 1998).
Figure 7.
Al Effect on Single-Channel Current of KAT1 in Oocyte Membranes.
(A) The time scale and voltage protocol used for single-channel recording. Three different voltage pulses (−110, −120, and −130 mV) were used as test potentials, and the holding potential was held at 0 mV.
(B) Single-channel current traces recorded from KAT1-expressing oocytes under the control conditions. The scale bar is applicable for (B) to (D).
(C) Single-channel current traces recorded from the same patch as in (B) in the presence of 10 μM Al in the bath.
(D) Single-channel current traces recorded from the same patch as in (B) and (C) after removal of Al from the bath solution.
(E) Matching single-channel currents with giant patch currents. The noisy traces represent ensemble averages of single-channel currents from 30 individual traces at −140 mV before (bottom) and after (top) application of 10 μM Al. The smooth traces were derived from giant patch recordings at −140 mV with (bottom) or without (top) 10 μM Al. The two traces from different recording configurations are scaled to the same amplitude at the end of test pulses to compare their time courses.
(F) Single-channel current amplitude and open probability plotted against membrane potentials. The data are collected from (B) (control; closed circle), (C) (10 μM Al; closed square), and (D) (washout; closed triangle). The open probabilities are fitted with the Boltzmann function PO = POmax/{1 + exp[(V − V1/2) · zF/RT]}. The symbols are described in Results. The Pomax for control is valued at 1. The fitting parameters are as follows: control: Pomax, 1; V1/2, −132 mV; z, 1.8 e; 10 μM Al: Pomax, 0.8; V1/2, −152 mV; z, 1.8 e; washout: Pomax, 0.98; V1/2, −140 mV; z, 1.9 e. e stands for electronic charge.
In the inside-out configuration, we applied Al in bath solution that perfused the cytoplasmic side of the membrane. As shown in Figure 7C, 10 μM Al significantly reduced the open probability of KAT1 single-channel currents. Upon removal of Al, the KAT1 currents recovered almost fully (Figure 7D). Five patches were studied, and they all showed significant Al inhibition and reversion. The ensemble average of single-channel currents in the presence of Al matched giant patch data well (Figure 7E). Figure 7F summarizes the effect of Al on single-channel conductance and open probability. Al had little effect on single-channel conductance but shifted the voltage dependence of open probability to less negative potentials. These data suggest that Al inhibits KAT1 current by modification of open probability in a membrane-delimited manner. In addition, the effect could be reversed fully and was voltage independent, implicating a direct internal block.
Al Block of KAT1 Is Caused by Specific Interaction
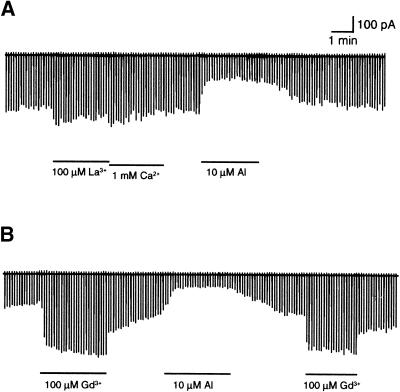
Al is capable of binding to a number of macromolecules, including membrane proteins and lipids (Kochian, 1995). Such binding often is caused by nonspecific metal chelation. For this reason, some trivalent metal ions can mimic Al effects on certain biological processes (Jones and Kochian, 1995). Thus, such nonspecific binding also may cause Al inhibition of KAT1. We tested the specificity of the Al effect on KAT1 by replacing Al with other metal ions, including La3+, Ca2+, and Gd3+. As shown in Figure 8A, the addition of 100 μM La3+ and 1 mM Ca2+ (0.32 μM free Ca2+ with 2 mM EGTA) had little effect on KAT1 current, whereas 10 μM Al inhibited KAT1 current strongly. As a trivalent analog to Al, Gd3+ had a very different effect on KAT1. Unlike Al, Gd3+ increased KAT1 activity in the membrane patch that responded to Al normally (Figure 8B). These findings suggest that Al inhibits the KAT1 channel upon specific interaction with KAT1 protein or a regulatory protein associated with the plasma membrane.
Figure 8.
Effects of Al Analogs and Ca2+ on KAT1 Currents in Oocyte Giant Patch Recording.
(A) Current spikes from giant patch recording in the presence of La3+, Ca2+, or Al in the bath (cytosolic side). The scales are noted for both (A) and (B). The total concentration of each ion is indicated.
(B) Giant patch current spikes in the presence of Gd3+ or Al.
Cytoplasmic Translocation and Redistribution of Al Involve Ca2+ Channel–like and Energy-Dependent Pathways
In both guard cells and oocytes, Al must reach the cytoplasmic side of the membrane to exert its inhibitory effects on Kin. In the whole-cell configuration, external perfusion of Al has no effect on KAT1 in oocytes but inhibits Kin in guard cells, suggesting that Al can enter guard cells but not oocytes. If Al influx across the plasma membrane of guard cells is inhibited, Kin inhibition by Al will not occur. Therefore, we can now use Al inhibition of Kin as an indirect measure to monitor Al entry into guard cells.
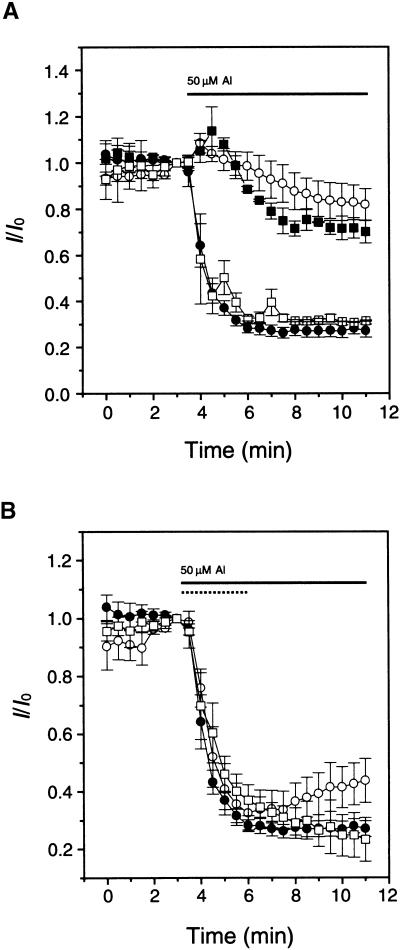
It has been speculated that Al uptake across the plasma membrane is not energy dependent (Zhang and Taylor, 1990; Taylor et al., 2000), implicating ion channels in the “passive” transport of Al into cells. In addition, our studies in this article clearly show that Al inhibition of Kin in guard cells can be detected immediately after external perfusion, indicating that Al entry must happen very quickly. The rapid occurrence also suggests that ion channel activities may mediate the Al influx. Earlier studies showed that Al blocks Ca2+-permeable channels by reducing the single-channel conductance, suggesting that Al may enter the channel pore to block the Ca2+ channel (Piñeros and Tester, 1995). This evidence led us to the hypothesis that Al may pass through a Ca2+ channel to enter the guard cells. To test this hypothesis, we measured the effects of several Ca2+ channel antagonists on Al inhibition of Kin in the whole-cell configuration. As shown in Figure 9A, preincubation with 50 μM verapamil, a typical Ca2+ channel blocker, for 30 min before recording reduced Al inhibition of Kin by 70%. Another Ca2+ channel blocker, La3+, also reduced Al effects by 50% when perfused at 100 μM. However, 50 μM nifedipine had no effect on Al inhibition of Kin current. These findings suggest a verapamil-sensitive pathway mediating Al uptake. This pathway may be related to Ca2+ channels.
Figure 9.
Effects of Calcium Channel Antagonists and DNP on Al Inhibition of Kin in Guard Cells.
(A) Verapamil and La3+ prevented Al inhibition of Kin in the whole-cell configuration. Verapamil, La3+, or nifedipine was added to bath solution 30 min before recording. Closed circles, control (n = 12); open circles, 50 μM verapamil (n = 14); closed squares, 100 μM La3+ (n = 6); and open squares, 50 μM nifedipine (n = 4). The horizontal line indicates perfusion with 50 μM Al in the bath solution. The current amplitude before the application of Al was treated as I0 for calculation of I/I0.
(B) DNP inhibited recovery of K in after Al inhibition. Open circles indicate the effect of short-term Al exposure (dotted horizontal line); closed circles indicate the effect after long-term application (solid horizontal line); and open squares denote the effect of 50 μM DNP on the current recovery after short-term exposure (dotted horizontal line).
Error bars indicate ±se.
Regarding Al toxicity, studies have shown that a metabolism-dependent mechanism contributes to Al resistance in Al-resistant cultivars, suggesting that detoxification may involve Al transport by an active pathway (Archambault et al., 1997). More recently, Taylor et al. (2000) showed that a dinitrophenol (DNP)-sensitive pathway is responsible for clearance of Al from the cytoplasm. Our studies suggest that Al inhibition of Kin in the excised membrane patches is fully reversible (Figure 5B). However, Kin current in the whole-cell configuration was only partially reversible after removal of Al from the external solution (Figure 1C). The sustained inhibition in the whole-cell configuration most likely was due to Al retention in the cytoplasm. Partial reversal may indicate that part of the Al pool is transported out of the cell or into the vacuole, thereby reducing the concentration of Al in the cytosol. The Al transport processes may be related to the energy-dependent detoxification process proposed earlier (Taylor et al., 2000). To test this idea, we removed ATP from the pipette solution and applied the same ATP synthesis inhibitor, DNP, in the bath solution. The absence of ATP in the pipette solution had no effect on Al inhibition (data not shown). However, preincubation with 50 μM DNP in the absence of ATP for 30 min prevented the recovery of Kin current from Al inhibition after Al removal (Figure 9B). These results suggest that the recovery from Al inhibition in the whole-cell configuration requires an energy-dependent mechanism that may be involved in Al clearance from the cytoplasm.
Verapamil Reduces Al Effects on Stomatal Opening
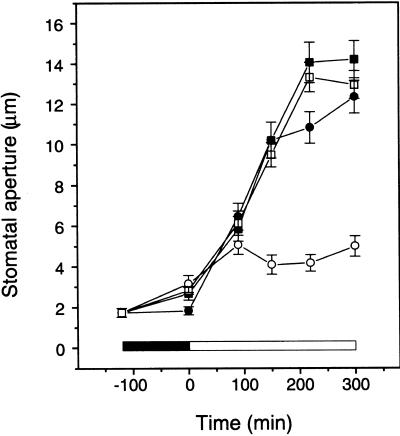
Al inhibits stomatal opening (Schnabl and Ziegler, 1975; Schroeder, 1988), although the effective concentration is higher compared with that required for the inhibition of Kin in this study. We tested whether Al inhibition of Kin can be correlated with its effect on stomatal movement. In particular, we wanted to determine whether verapamil, which prevents Al inhibition of Kin, also can compromise the Al effect on stomatal opening. Using a stomatal peel assay, we examined the Al effect on stomatal movements in the presence or absence of verapamil. As shown in Figure 10, 2 mM Al inhibited stomatal opening during a 4-hr incubation. Verapamil at 50 μM significantly reduced Al inhibition of stomatal opening, suggesting that a verapamil-sensitive pathway is required for Al action, as shown earlier in Kin inhibition assays. These results provide further evidence that Al inhibition of stomatal opening may be a result of Kin block in guard cells.
Figure 10.
Effect of Verapamil on Al-Inhibited Stomatal Opening.
Stomatal aperture was measured on epidermal peels incubated in the peel solution (closed circles), peel solution plus 2 mM Al (open circles), peel solution plus 50 μM verapamil (closed squares), and peel solution plus 2 mM Al plus 50 μM verapamil (open squares). The incubation started 2 hr before illumination. The solid bar and the open bar indicate dark and light conditions, respectively. Error bars indicate ±se.
DISCUSSION
Al toxicity is an important factor limiting crop production. Studies have identified several cellular processes that may contribute to Al toxicity. These include inhibition of cation uptake, interference of calcium homeostasis, and inhibition of cell division and elongation (Kinraide and Parker, 1987; Huang et al., 1992, 1996; Jones and Kochian, 1995; Kochian, 1995). To improve Al tolerance in plants by traditional breeding or genetic engineering, we must understand the mechanisms underlying Al action in these cellular processes. However, despite extensive studies of Al toxicity, little information on the molecular mechanism is available. This report presents a mechanistic analysis of Al inhibition of K+ uptake through Kin. We demonstrate that internal block of Kin reduces the open probability and alters the activation kinetics of Kin. This finding provides evidence for an intracellular target site for Al action. This study also directly links Al inhibition of a native channel in plant cells and Al block of a cloned channel protein expressed in oocytes, providing a vital model system for further molecular analysis of Al regulation of plant Kin. Some results in this study also provide clues to the mechanism of Al–Ca2+ interaction in plant cells.
Al inhibits root elongation and changes cellular K+, Ca2+, and H+ concentrations within minutes of exposure (Jones and Kochian, 1995; Lindberg and Strid, 1997), suggesting a link between Al toxicity and inhibition of cation uptake. Potassium deficiency caused by Al can be correlated with the inhibitory effect on Kin in roots (Gassmann and Schroeder, 1994). Kin has been shown to contribute up to 63% of total potassium permeability in Arabidopsis root cells. Plants lacking Kin may lose 50% of fresh weight (Hirsch et al., 1998). These studies further link Kin activity to K+ nutrition in plants. If Kin is inhibited by Al, K+ uptake is compromised, which in turn reduces turgor-driven cell elongation. Therefore, Al block of Kin in roots may be a critical component of Al toxicity in plants (Frensch, 1997).
How does Al block Kin in plant cells? The answer to this question may provide information on the mechanism of Al toxicity in plants. Earlier studies have shown that Al inhibits Kin in both guard cell and root hair protoplasts when applied to the external solution (Schroeder, 1988; Gassmann and Schroeder, 1994). The inhibition occurs rapidly and can be reversed partially after removal of Al from the bath solution, supporting the hypothesis of direct external block (Schroeder et al., 1994). However, this hypothesis has not been confirmed by further studies using single-channel recording and/or molecular biological approaches.
To date, all known Kin proteins belong to the animal Shaker family (Czempinski et al., 1999). The channels in this family have similar molecular structures. For example, the KAT1 channel expressed in guard cells and the AKT1 channel in root hairs share the same six–transmembrane domain topology and >40% amino acid sequence identity. If KAT1 and AKT1 are major components of Kin currents in guard cells and root hairs, respectively, the structural similarity between the two channels is consistent with the similarity in Al-induced inhibition of Kin in these two cell types. With cloned channels, it should be possible to determine whether an inward channel protein is the target of Al inhibition. To date, however, none of the studies have detected Al inhibition of any cloned Kin. In particular, Al has been shown not to block KAT1 (Hoshi, 1995), LKT1, and SKT1 (Hartje et al., 2000) in Xenopus oocytes and insect cells. These studies used the external perfusion of Al as described in studies of endogenous guard cell and root hair Kin (Schroeder, 1988; Gassmann and Schroeder, 1994). These negative results could be interpreted in at least three ways. First, KAT1, LKT1, and SKT1 may not be the major channel types in guard cells and root hair cells that contain Al-sensitive Kin currents. Second, these channels expressed in heterologous systems may have altered properties toward Al inhibition. Third, the Al inhibition of Kin may not be caused by external block, and the plant channels expressed in these heterologous systems may not be accessible to external Al applications.
Using single-channel recording and molecular biological approaches, our study has clearly demonstrated that Al inhibits Kin by internal block. Several findings support the notion that the internal Al block is an intrinsic property of Kin proteins. The Al effect is membrane delimited, suggesting that the cytoplasmic component is not involved. Both Kin in guard cells and KAT1 in oocytes are blocked by Al through a similar internal pathway, making it more likely that Al interacts directly with KAT1 proteins. Al inhibition of Kin in guard cells and KAT1 in oocytes is reversible, indicating a simple direct interaction between Al and the channels. In addition, Al effects are rather specific and cannot be mimicked by other metal ions, excluding the possible effect of nonspecific binding to membrane lipids or other components.
It has been shown that KAT1 is expressed preferentially in guard cells (Nakamura et al., 1995). Although the KAT1 gene is cloned from Arabidopsis, its homology with Kin in fava bean has been shown to be quite high (Hoth et al., 1997). Al inhibits Kin in guard cells and KAT1 expressed in oocytes by similar mechanisms, further supporting the idea that KAT1-type channels may be a major component of Kin in guard cells. Because Al blocks Kin in several different cell types, internal Al block may be a common property of Kin in plants. Our finding will provide a useful tool for studying KAT1 and other Kin at the molecular level. Indeed, few of the K+ channel blockers have been shown to block the KAT1 channel internally. Some classic K+ channel antagonists, such as tetraethylammonium and Cs+, which potently block animal K+ channels on both sides, have little effect on KAT1 if applied internally (Hoshi, 1995; Ichida and Schroeder, 1996). Therefore, Al can be used as an internal blocker for KAT1 and other plant Kin to further determine the structure–function relationship of these channels. Regarding Al toxicity in roots, Al block of KAT1 could be physiologically relevant considering that KAT1 is also expressed in the vascular tissues of roots as previously described (Nakamura et al., 1995). Clearly, further study on Al block of root-specific channels will provide more insight into the mechanism of Al toxicity in plants.
If Al inhibits Kin by internal block, it needs to be transported across the membrane before its action. In this regard, guard cells behave differently compared with oocytes. Al readily enters guard cell protoplasts but not oocytes. Although there is little information about Al transport into oocytes, it has been shown that Al can accumulate in plant cells rapidly (Lazof et al., 1994; Taylor et al., 2000). However, the mechanism for Al transport across the plasma membrane has not been identified in any organism. Internal Al block of Kin in guard cells offers a unique model to explore the Al entry process. Because verapamil and La3+, two Ca2+ channel blockers, prevent Al-induced Kin inhibition, we propose that a Ca2+ channel–like molecule mediates Al entry into guard cells. Because the Ca2+ channels in guard cell plasma membrane were identified recently electrophysiologically (Hamilton et al., 2000; Pei et al., 2000), it is possible that Al enters the cell through these channels but also transiently blocks them during permeation. Further studies are required to test this hypothesis. The results presented here provide initial information on the mechanism of Al uptake in plants.
Internal block of Kin in guard cells may explain Al inhibition of stomatal opening. However, it takes an almost 100-fold higher concentration to inhibit stomatal movement than to block Kin, and this inhibition takes hours to occur, as opposed to the 2 min required for Kin inhibition (Figures 1 and 10). This difference may be attributed to the different kinetics of Al transport into protoplasts and epidermal peels. Indeed, a recent study (Taylor et al., 2000) of Al uptake shows that the cell wall strongly hinders both short-term and long-term uptake of Al across the plasma membrane. Most of the applied Al (>90%) was trapped in the cell wall. This finding may explain the difference in Al concentrations required to inhibit Kin activity and to block stomatal opening. Results from experiments with verapamil also support the idea that the Al effects on both stomatal opening and Kin activity require Al transport into guard cells. If some of the Al toxicity is due to Al entry into the cytoplasm, detoxification of Al may involve clearance from the cytoplasm. Our studies show that partial reversal of Al inhibition of whole-cell Kin current may be a result of Al clearance from the cytoplasm, which is an energy-dependent process.
METHODS
Preparation of Guard Cell Protoplasts
Vicia faba seeds were germinated in potting mix, and plants were grown in a growth chamber for 4 weeks under a 10-hr-light/14-hr-dark cycle (180 μmol m−2 sec−1). Guard cell protoplasts were isolated as described by Kruse et al. (1989). The protoplasts were kept in 0.45 M sorbitol, 1 mM CaCl2, and 5 mM Mes medium on ice in the dark for at least 1 hr before patch clamp recording.
Whole-Cell and Single-Channel Recordings in Guard Cells
Whole-cell patch clamp recording and single-channel recording were performed as described previously (Liu and Luan, 1998). For whole-cell recording, the pipette solution contained 100 mM K-glutamate, 2 mM EGTA, 2 mM MgCl2, 10 mM Hepes, pH 7.2, and 2 mM Mg-ATP. The osmolarity was adjusted to 500 mosmol with d-sorbitol. Protoplasts were bathed in a solution consisting of 100 mM K-glutamate, 1 mM CaCl2, 4 mM MgCl2, and 10 mM Mes, pH 5.7. The osmolarity was adjusted to 450 mosmol with d-sorbitol. For experiments with Ca2+ channel antagonists and dinitrophenol (DNP), the bath solution contained freshly prepared chemicals at the concentrations indicated in Results. AlCl3 was used as the aluminum (Al) source and prepared freshly just before use. The voltage clamp protocols were generated using pClamp 6.0 software (Axon Instruments, Foster City, CA). Whole-cell currents were low-pass filtered at 1 kHz during measurements. For single-channel recording, the same solutions as those described for whole-cell recording were used in the outside-out configuration, and the solutions for pipette and bath were switched for the inside-out configuration. pClamp 6.0 software was used for data analyses. The open probabilities were calculated from the ratio of total open time to total recording time. For patches in which more than one channel was recorded, open probability was defined as the average of the open probability values calculated from each of the open channels. Single-channel current amplitude was obtained from Gaussian fitting of open-state amplitude histograms derived from data recorded for 10 to 30 sec.
Measurement of Stomatal Aperture
Stomatal aperture assay was performed as described previously (Liu and Luan, 1998). In brief, the epidermis was peeled and floated in the peel solution containing 50 mM KCl and 10 mM Mes, pH 5.7. After 1 hr of incubation in the dark, the peels were transferred to different Petri dishes with the peel solutions containing 2 mM Al, 100 μM verapamil, 2 mM Al plus 100 μM verapamil, and no Al or verapamil as a control and incubated in the dark for 120 min. Then, white light at 300 μmol m−2 sec−1 was applied (defined as time 0). The images of stomatal aperture in the peel strips were captured at −120, 0, 90, 150, 220, and 300 min with a TEA/CCD-1317K/2 camera (Princeton Instruments, Trenton, NJ) and a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) controlled by IPLab software (Signal Analytics, Vienna, VA). The maximal diameter of stomata was measured on a Power Mac G3 computer (Apple, Cupertino, CA) using NIH Image software (National Institutes of Health, Bethesda, MD). At least four images for each treatment with 15 to 20 stomata in each image were processed. The experiments were repeated three times. The data are presented as means ±se, and Student's t test was used to determine significant differences between each pair of groups.
Expression of KAT1 in Xenopus Oocytes and Electrophysiological Analysis
KAT1 cDNA was isolated from an Arabidopsis thaliana cDNA library as described previously (Bei and Luan, 1998). For construction of the oocyte expression plasmid, the KAT1 cDNA was inserted into the BamHI site of the expression vector pGEMHE (Liman et al., 1991). The copy RNA (cRNA) was transcribed with T7 RNA polymerase using the mMessage mMachine transcription protocol (Ambion, Austin, TX). The cRNA quality was checked by loading a small sample on agarose gel. The concentration was determined by OD260/280 and adjusted to a final concentration of 0.5 μg/μL.
Freshly isolated Xenopus laevis oocytes were kind gifts from Dr. E.Y. Isacoff (University of California, Berkeley). Using the Nanoject II oocyte injector (Drummond Scientific, Broomall, PA), we injected each oocyte with 23 nL of KAT1 cRNA. Electrophysiological measurements were made 3 to 10 days after injection. For whole-oocyte recording, membrane currents were recorded with a two-electrode voltage clamp amplifier (model TEV-200A; Dagan Corp., Minneapolis, MN) connected to a Digidata 1200 interface (Axon Instruments). The 0.5- to 1-MΩ pipettes filled with 3 M KCl were used, and the oocytes were incubated in the bath solution containing 50 mM KCl, 90 mM NaCl, 2 mM MgCl2, and 10 mM Hepes, pH 7.2. For injection of Al during recording, oocytes were impaled by the injecting pipette containing 50 mM AlCl3 before establishment of the recording configuration. A 23-nL aliquot of Al solution was injected into the oocytes as needed. The filter frequency for recording was set at 0.5 kHz. The voltage protocols used are described in the figure legends.
For giant patch recording, the currents were measured with the setup described above in the section on whole-cell recording in guard cells. The pipettes were forged in a microforge (model MF90; Narishige, Tokyo, Japan) with a tip opening of 20 to 30 μm and coated with a mix of mineral oil and Parafilm before use. The giant inside-out membrane patches were obtained in the bath solution containing 100 mM K-glutamate, 20 mM KCl, 2 mM MgCl2, 2 mM EGTA, and 10 mM Hepes, pH 7.2. Mg-ATP was included at 2 mM unless indicated otherwise. The pipette solution contained 140 mM K-glutamate, 2 mM MgCl2, and 10 mM Hepes, pH 7.2. For tail current analysis, the pipette solution contained 5 mM K-glutamate, 135 mM N-methyl-d-glucamine, 2 mM MgCl2, and 10 mM Hepes, pH 7.2 (with HCl). For clarity, we sometimes use the terms internal solution or external solution to refer to the solution at the cytoplasmic side or the extracellular side of the membrane, respectively.
The single-channel recording in oocytes was performed similar to that in guard cells. Inside-out patches were obtained using the pipettes with a tip opening of 1 to 2 μm in diameter. The solutions for the pipette and bath were the same as those described for giant patch recording. The single-channel data were analyzed using pClamp6.0 software and calculated as described above.
Acknowledgments
We thank Dr. E.Y. Isacoff for providing oocytes used in this study. We thank Dr. Legong Li for helping with the construction of KAT1 plasmid. This work was supported in part by a grant from the United States Department of Agriculture National Research Initiative program to S.L.
References
- Anderson, J.A., Huprikar, S.S., Kochian, L.V., Lucas, W.J., and Gaber, R.F. (1992). Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89, 3736–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault, D.J., Zhang, G., and Taylor, G.J. (1997). Spatial variation in the kinetics of aluminium (Al) uptake in roots of wheat (Triticum aestivum L.) exhibiting differential resistance to Al: Evidence for metabolism-dependent exclusion of Al. J. Plant Physiol. 151, 668–674. [Google Scholar]
- Assmann, S.M. (1993). Signal transduction in guard cells. Annu. Rev. Cell Biol. 9, 345–375. [DOI] [PubMed] [Google Scholar]
- Bei, Q., and Luan, S. (1998). Functional expression and characterization of a plant K+ channel gene in a plant cell model. Plant J. 13, 857–865. [DOI] [PubMed] [Google Scholar]
- Boyer, J.S. (1985). Water transport. Annu. Rev. Plant Physiol. 36, 473–516. [Google Scholar]
- Brady, D.J., Edwards, D.G., Asher, C.J., and Blamey, F.P.C. (1993). Calcium amelioration of aluminium toxicity effects on root hair development in soybean Glycine max (L.). Merr. New Phytol. 123, 531–538. [DOI] [PubMed] [Google Scholar]
- Czempinski, K., Gaedeke, N., Zimmermann, S., and Müller-Röber, B. (1999). Molecular mechanisms and regulation of plant ion channels. J. Exp. Bot. 50, 955–966. [Google Scholar]
- Delhaize, E., and Ryan, P.R. (1995). Aluminum toxicity and tolerance in plants. Plant Physiol. 107, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy, C.D., Chaney, R.L., and White, M.C. (1978). The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol. 29, 511–566. [Google Scholar]
- Frensch, J. (1997). Primary responses of root and leaf elongation to water deficits in the atmosphere and soil solution. J. Exp. Bot. 48, 985–999. [Google Scholar]
- Gassmann, W., and Schroeder, J.I. (1994). Inward-rectifying K+ channels in root hairs of wheat: A mechanism for aluminum-sensitive low-affinity K+ uptake and membrane potential control. Plant Physiol. 105, 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard, F., Cerutti, M., Horeau, C., Lemaillet, G., Urbach, S., Ravallec, M., Devauchelle, G., Sentenac, H., and Thibaud, J.B. (1996). The baculovirus/insect cell system as an alternative to Xenopus oocytes: First characterization of the AKT1 K+ channel from Arabidopsis thaliana. J. Biol. Chem. 271, 22863–22870. [DOI] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Kohler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartje, S., Zimmermann, S., Klonus, D., and Müller-Röber, B. (2000). Functional characterization of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta 210, 723–731. [DOI] [PubMed] [Google Scholar]
- Hirsch, R.E., Lewis, B.D., Spalding, E.P., and Sussman, M.R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. [DOI] [PubMed] [Google Scholar]
- Hoshi, T. (1995). Regulation of voltage dependence of the KAT1 channel by intracellular factors. J. Gen. Physiol. 105, 309–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, S., Dreyer, I., Dietrich, P., Becker, D., Müller-Röber, B., and Hedrich, R. (1997). Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. USA 94, 4806–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J.W., Shaff, J.E., Grunes, D.L., and Kochian, L.V. (1992). Aluminum effects on calcium fluxes at the root apex of aluminum-tolerant and aluminum-sensitive wheat cultivars. Plant Physiol. 98, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J.W., Pellet, D.M., Papernik, L.A., and Kochian, L.V. (1996). Aluminum interactions with voltage-dependent calcium transport in plasma membrane vesicles isolated from roots of aluminum-sensitive and -resistant wheat cultivars. Plant Physiol. 110, 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida, A.M., and Schroeder, J.I. (1996). Increased resistance to extracellular cation block by mutation of the pore domain of the Arabidopsis inward-rectifying K+ channel KAT1. J. Membr. Biol. 151, 53–62. [DOI] [PubMed] [Google Scholar]
- Jones, D.L., and Kochian, L.V. (1995). Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: A role in aluminum toxicity? Plant Cell 7, 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide, T.B., and Parker, D.R. (1987). Cation amelioration of aluminum toxicity in wheat. Plant Physiol. 83, 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian, L.V. (1995). Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 237–260. [Google Scholar]
- Kruse, T., Tallman, G., and Zeiger, E. (1989). Isolation of guard cell protoplasts from mechanically prepared epidermis of Vicia faba leaves. Plant Physiol. 90, 1382–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof, D.B., Goldsmith, J.G., Rufty, T.W., and Linton, R.W. (1994). Rapid uptake of aluminum into cells of intact soybean root tips: A microanalytical study using secondary ion mass spectrometry. Plant Physiol. 106, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman, E.R., Hess, P., Weaver, F., and Koren, G. (1991). Voltage-sensing residues in the S4 region of a mammalian potassium channel. Nature 353, 752–756. [DOI] [PubMed] [Google Scholar]
- Lindberg, S., and Strid, H. (1997). Aluminium induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum). Physiol. Plant. 99, 405–414. [Google Scholar]
- Liu, K., and Luan, S. (1998). Voltage dependent K+ channels as targets of osmosensing in guard cells. Plant Cell 10, 1957–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis, F.J.M., Ichida, A.M., Sanders, D., and Schroeder, J.I. (1997). Roles of higher plant K+ channels. Plant Physiol. 114, 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber, B., Ellenberg, J., Provart, N., Willmitzer, L., Busch, H., Becker, D., Dietrich, P., Hoth, S., and Hedrich, R. (1995). Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 14, 2409–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, R.L., McKendree, W.L., Jr., Hirsch, R.E., Sedbrook, J.C., Gaber, R.F., and Sussman, M.R. (1995). Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Piñeros, M., and Tester, M. (1995). Characterization of a voltage-dependent Ca2+-selective channel from wheat roots. Planta 195, 478–488. [Google Scholar]
- Rengel, Z. (1992). Role of calcium in aluminium toxicity. New Phytol. 121, 499–513. [DOI] [PubMed] [Google Scholar]
- Schachtman, D.P., Schroeder, J.I., Lucas, W.J., Anderson, J.A., and Gaber, R.F. (1992). Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258, 1654–1658. [DOI] [PubMed] [Google Scholar]
- Schnabl, H., and Ziegler, H. (1975). The influence of aluminum ions on the movement of stomata in Vicia faba epidermis strips. Pflanzenphysiologie 74, 394–403. [Google Scholar]
- Schoenmakers, T.J.M., Visser, G.J., Flik, G., and Theuvenet, A.P.R. (1992). CHELATOR: An improved method for computing metal ion concentrations in physiological solutions. Biotechnology 12, 870–879. [PubMed] [Google Scholar]
- Schroeder, J.I. (1988). Potassium transport properties of potassium channels in the plasma membrane of Vicia faba guard cells. J. Gen. Physiol. 92, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Ward, J.M., and Gassmann, W. (1994). Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: Biophysical implications for K+ uptake. Annu. Rev. Biophys. Biomol. Struct. 23, 441–471. [DOI] [PubMed] [Google Scholar]
- Sentenac, H., Bonneaud, N., Minet, M., Lacroute, F., Salmon, J.M., Gaymard, F., and Grignon, C. (1992). Cloning and expression in yeast of a plant potassium ion transport system. Science 256, 663–665. [DOI] [PubMed] [Google Scholar]
- Taylor, G.J., McDonald-Stephens, J.L., Hunter, D.B., Bertsch, P.M., Elmore, D., Rengel, Z., and Reid, R.J. (2000). Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol. 123, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry, A.A., Gaymard, F., Bosseux, C., Sentenac, H., and Thibaud, J.B. (1995). Expression of a cloned plant K+ channel in Xenopus oocytes: Analysis of macroscopic currents. Plant J. 7, 321–332. [DOI] [PubMed] [Google Scholar]
- Zei, P.C., and Aldrich, R.W. (1998). Voltage-dependent gating of single wild-type and S4 mutant KAT1 inward rectifier potassium channels. J. Gen. Physiol. 112, 679–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G., and Taylor, G.J. (1990). Kinetics of aluminum uptake in Triticum aestivum L.: Identity of the linear phase of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars. Plant Physiol. 94, 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, S., Talke, I., Ehrhardt, T., Nast, G., and Müller-Röber, B. (1998). Characterization of SKT1, an inwardly rectifying potassium channel from potato, by heterologous expression in insect cells. Plant Physiol. 116, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]