Abstract
We examined the biogenesis of the von Hippel-Lindau (VHL) tumor suppressor protein (pVHL) in vitro and in vivo. pVHL formed a complex with the cytosolic chaperonin containing TCP-1 (CCT or TRiC) en route to assembly with elongin B/C and the subsequent formation of the VCB-Cul2 ubiquitin ligase. Blocking the interaction of pVHL with elongin B/C resulted in accumulation of pVHL within the CCT complex. pVHL present in purified VHL-CCT complexes, when added to rabbit reticulocyte lysate, proceeded to form VCB and VCB-Cul2. Thus, CCT likely functions, at least in part, by retaining VHL chains pending the availability of elongin B/C for final folding and/or assembly. Tumor-associated mutations within exon II of the VHL syndrome had diverse effects upon the stability and/or function of pVHL-containing complexes. First, a pVHL mutant lacking the entire region encoded by exon II did not bind to CCT and yet could still assemble into complexes with elongin B/C and elongin B/C-Cul2. Second, a number of tumor-derived missense mutations in exon II did not decrease CCT binding, and most had no detectable effect upon VCB-Cul2 assembly. Many exon II mutants, however, were found to be defective in the binding to and subsequent ubiquitination of hypoxia-inducible factor 1α (HIF-1α), a substrate of the VCB-Cul2 ubiquitin ligase. We conclude that the selection pressure to mutate VHL exon II during tumorigenesis does not relate to loss of CCT binding but may reflect quantitative or qualitative defects in HIF binding and/or in pVHL-dependent ubiquitin ligase activity.
Inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene product, pVHL, results in inherited forms of cancer of the kidney and pancreas, vascular tumors, including benign hemangioblastomas of the cerebellum, spine, brain stem, and retina (23, 41), as well as sporadic clear-cell renal carcinoma (34, 37). pVHL functions, at least in part, by regulating the activity of the hypoxia-inducible transcription factor 1α (HIF-1α). Specifically, pVHL controls the stability of HIF-1α in response to available oxygen levels. Under conditions of normoxia HIF-1α is targeted for destruction by a ubiquitin-dependent proteasome degradation pathway (4, 29, 42, 43, 59). Under hypoxic conditions, however, the oxygen dependent degradation of HIF is blocked, resulting in HIF-1α stabilization and increased transcription of hypoxia-inducible genes (e.g., vascular endothelial growth factor, glucose transporter 1, and many others) (for reviews, see references 53, 54, and 63). Transcription of these genes is part of a protective mechanism against damage resulting from hypoxia. Many pVHL mutants are unable to mediate the oxygen-regulated proteolysis of HIF-1α. Consequently, transcription of hypoxia-inducible genes under conditions of normoxia is observed (4, 15, 21, 40, 42, 43, 56). This dysregulation of HIF-1α likely plays a central role in processes related to carcinogenesis.
pVHL participates in the regulation of HIF-1α stability through formation of a multimeric ubiquitin ligase complex that specifically conjugates ubiquitin to HIF-1α and HIF-2α (2, 4, 29, 42, 43, 59). The VHL-dependent ubiquitin ligase complex is comprised of pVHL, elongin B, elongin C, cullin-2 (Cul2), and a RING-H2 box protein, Rbx1 (25, 28, 38, 40, 46). We refer to this complex here collectively as VCB-Cul2. The interaction of pVHL with Cul2 is indirect, requiring that pVHL first interact with elongin C of the elongin B/C heterodimer before subsequent assembly with Cul2 (40, 47). Once fully assembled, the VCB-Cul2 complex catalyzes the processive conjugation of ubiquitin to the target substrate, in this case, HIF-1α. The ubiquitinated HIF-1α substrate now is targeted for degradation via the ubiquitin-dependent proteasome pathway.
pVHL is a 213-amino-acid protein that is encoded by three exons. Mutations in all three exons can result in a VHL-associated syndrome (27, 32, 41). Solution of the crystal structure of the VCB complex revealed that pVHL is comprised of two domains (58). The largely α-helical domain (α-domain) is comprised of amino acids 157 to 189 and is encoded by exon III. Amino acids 64 to 154, encoded by exons I and II, make up the β-domain. The α-domain, specifically, α-helical residues 157 to 172, is in close contact with elongin C. This structural observation is consistent with previous studies of the biochemical defects resulting from mutations within exon III. Specifically, residues 157 to 172 were shown to comprise an elongin B/C binding site, and mutations in this region decreased or abolished pVHL binding to elongin B/C (7, 30, 31, 44). This impaired assembly of exon III mutants in the VCB complex (and therefore the subsequent VCB-Cul2 complex) results in decreased ubiquitination of HIF-1α (24, 27, 36, 40). Mutations in exon I (β-domain) decrease the affinity of pVHL for HIF-1α and impair ubiquitination of HIF-1α, thereby leading to HIF-1α stabilization under conditions of normoxia (4, 43). Taken together, these studies of pVHL structure-function reveal that the α- and β-domains of pVHL participate in regulating the stability of target proteins through domain-specific interactions with ubiquitin ligase components (i.e., elongin B/C, Cul2, and Rbx1) and the substrate protein, respectively.
A search for other proteins that might interact with pVHL (by using a yeast two-hybrid interaction assay) identified a number of other putative pVHL-binding proteins (VBP) (61). One protein identified, VBP-1, is a homologue of subunit 3 of a molecular chaperone complex termed prefoldin (also referred to as GimC) (12, 62). Prefoldin cooperates with the cytosolic chaperonin containing TCP-1 (CCT or TRiC) (10, 11; for a recent review, see reference 36) in the folding of actins and tubulins (12, 17, 55, 62). CCT is a homologue of the bacterial chaperonin, GroEL, a molecular chaperone with a double toroidal structure that contains a central cavity in which protein folding is assisted. The reported interaction between pVHL and VBP-1 raised the interesting possibility that the prefoldin/CCT pathway may mediate pVHL folding and/or VCB-Cul2 assembly. Indeed, a subsequent study that examined the synthesis and maturation of pVHL concluded that CCT was required for the proper folding of pVHL (9). Moreover, amino acids 100 to 155, which contain the residues encoded by exon II, were shown to be necessary and sufficient for the interaction of pVHL with CCT. A deletion mutant of pVHL, lacking exon II, was no longer able to assemble into the VCB complex. Based upon these findings, Feldman et al. proposed that mutations in exon II lead to the VHL disease phenotype because the mutant protein products failed to interact with CCT, thereby resulting in misfolding severe enough to prevent the assembly of a stable VCB complex (9).
In the present study, we examined the biogenesis of pVHL in vitro and in vivo, paying particular attention to the effects of different disease-associated mutations within exon II and the potential role of both prefoldin and CCT in the folding or assembly of pVHL. First, we were unable to detect any interaction of newly synthesized pVHL with prefoldin. Second, consistent with the aforementioned earlier report (9), newly synthesized pVHL was observed to interact with CCT prior to its binding to elongin B/C and assembly of the VCB-Cul2 complex. Moreover, inhibiting the interaction of pVHL with elongin B/C caused pVHL to accumulate in a complex with CCT. The functional consequences of different mutations in exon II of the VHL gene were diverse. First, deletion of exon II decreased the interaction of pVHL with CCT, as expected based on the observations of Feldman et al. (9). Nevertheless, pVHL assembly into complexes with elongin B/C and elongin B/C-Cul2 was relatively unaffected. Second, six exon II pVHL missense mutants (all disease related) were found to interact with CCT and assemble into the VCB and VCB-Cul2 complexes. Finally, most of the exon II mutants displayed a lower affinity for HIF-1α. Based upon the results presented here, we suggest that loss of CCT binding does not underlie the development of VHL disease in association with exon II VHL mutations. Rather, the development of VHL disease in this setting may reflect quantitative or qualitative defects in HIF binding and/or pVHL-dependent ubiquitin ligase activity.
MATERIALS AND METHODS
Antibodies.
Rat anti-glucose regulated protein 94 (GRP94) antibody 9G10 and anti-CCT-1α antibody 23C (StressGen Biotechnologies, Vancouver, British Columbia, Canada) were purified on protein G-Sepharose (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's instructions. The other antibodies were obtained as follows: rabbit anti-Cul2 (Zymed, South San Francisco, Calif.), goat anti-elongin B (A-19) and mouse anti-Gal4 (DBD RK5C1) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), mouse anti-elongin B (J. Conaway, Oklahoma Medical Research Foundation, Oklahoma City, Okla.), rabbit anti-prefoldin (N. Cowan, New York University Medical School, New York), rabbit anti-GLUT1 antibody (GT-11A; Alpha Diagnostic International, San Antonio, Tex.), mouse monoclonal anti-hemagglutinin (anti-HA; Roche Diagnostics, Indianapolis, Ind.), and rabbit anti-HIF-2α (Novus Biologicals, Littleton, Colo.). The anti-pVHL antibodies, monoclonal Ig32 antibody and polyclonal R98 antibody, have been described previously (30).
Plasmids.
A vector containing a wild-type VHL gene under the transcriptional regulation of the SP6 promoter was obtained from Robert Stearman and Richard Klausner (National Cancer Institute, Bethesda, Md.). pRc-CMV-HA-VHL (wild-type [WT], W117R, and C162F) and pcDNA3-GAL4-HA-HIF-1α[ODD] (in which ODD represents the HIF-1α oxygen-dependent degradation domain) were described previously (20, 40, 43-45). pRc-CMV-HA-truncated-VHL mutants were described previously (40). pRc-CMV-HA-VHL (G114S, F119S, D121G, L128F, and A149T) plasmids were made by using a Clontech (Palo Alto, Calif.) Site-Directed Mutagenesis Kit according to the manufacturer's instructions. Plasmids were verified by direct DNA sequence analysis. Wild-type and mutant VHL genes were subcloned as BamHI-EcoRI fragments into a pBABE-Puro retroviral vector modified so as to encode an N-terminal HA epitope tag (J. Klco, unpublished data).
Cell culture, stable cell lines, and retroviral transfections.
Renal carcinoma cell (RCC) line 786-O obtained from the American Type Culture Collection (Manassas, Va.) was grown in Dulbecco modified Eagle medium containing 10% heat-inactivated defined and/or supplemented bovine calf serum (HyClone) at 37°C in a humidified, 10% CO2 atmosphere. The preparation of cell extracts (30) and RCC 786-O subclones stably expressing pVHL, W117R, and C162F (40, 45) was as described previously. Retroviral vectors were transfected into the Phoenix packaging cell line (Gary Nolan, Department of Molecular Pharmacology, Stanford University). Tissue culture supernatant was harvested 48 h later, passed through a 0.45-μm-pore-size filter, and used to infect 786-O cells in the presence of 4 μg of Polybrene/ml. Infected cells were selected by growth in the presence of puromycin (1 μg/ml).
In vitro transcription and translation, native-polyacrylamide gel electrophoresis (PAGE), antibody-induced gel shifts, and peptidic perturbation of pVHL complex stability.
GpppG-capped mRNA templates, used for time course experiments (33), were generated by in vitro transcription with SP6 RNA polymerase as described previously (16) and were used without purification. Maturation of pVHL in the rabbit reticulocyte lysate (RRL) was examined after synthesis at 24°C. Translation reactions were synchronized by the incubation with 4 mM 7-methylguanosine monophosphate and 10 μM edeine. Nascent polypeptide chains were released from the ribosome by incubation with 0.5 mM puromycin.
Native-PAGE and antibody-induced gel shifts were performed at 4°C as described previously (17), except that the discontinuous gels used were optimized to fractionated species with native masses of ∼20 to ∼1,000 kDa (i.e., a 5.5, 9, 17.5% discontinuous acrylamide gradient was used). For immunodepletion experiments antibodies were immobilized on a mixture of protein A- and protein G-Sepharose (Amersham Pharmacia Biotech) that had been previously incubated in 2 mg of bovine serum albumin/ml. The beads were washed five times with phosphate-buffered saline and then incubated with the pVHL-containing complexes.
In order to block the assembly of pVHL with elongin B/C, synthetic pVHL (amino acids 157 to 172) wild-type and C162-F mutant peptides (30) (at the concentrations indicated in each figure) were incubated in the RRL during the translation of VHL mRNA. In experiments involving VCB disassembly, recombinant VCB was produced in Escherichia coli and purified as described previously (58). The recombinant VCB was added to the RRL and then incubated in the absence or presence of either the wild-type pVHL peptide or the mutant pVHL peptide. The incubations were performed in the presence of an ATP-regenerating system for 60 min, ATP was depleted by the incubation with apyrase (Sigma, St. Louis, Mo.), and the reaction products were analyzed by native-PAGE.
Experiments comparing the assembly of wild-type and mutant pVHL into complexes with CCT, elongin B/C, and elongin B/C-Cul2 were carried out in the TNT T7-coupled transcription-translation system (Promega Corp., Madison, Wis.) according to the manufacturer's instructions. A composite fluorogram of the native gel is shown after multiple exposures to film in order to correct for the differences in the total counts per minute of radiolabel incorporated into the various pVHL mutant polypeptides.
Generation of VCB from purified CCT-pVHL complexes.
mRNA encoding pVHL was translated in RRL at 24°C in the presence of the elongin C-binding pVHL peptide. CCT-pVHL complexes were isolated by gel filtration through Sephacryl-S200 HR (Amersham Pharmacia Biotech). The isolated CCT-pVHL complexes were incubated with RRL and an ATP regeneration system in the absence or presence of the elongin C-binding VHL peptide. After 90 min of incubation at 24°C, the reaction products were analyzed by native-PAGE.
In vitro assays for HIF-1α binding and ubiquitination.
Assays for binding of HIF-1α to pVHL and for pVHL-dependent ubiquitination of HIF-1α were performed as described previously (43). For the HIF-1α binding assay, TNT reticulocyte lysate translation products (5 μl) synthesized in the presence (for pVHL) or absence (for Gal4-HIF-1α[ODD]) of [35S]methionine were incubated with anti-Gal4 antibody and protein A-Sepharose in 700 μl of EBC buffer (50 mM Tris [pH 8], 120 mM NaCl, 0.5% NP-40). After five washes with NETN buffer (20 mM Tris [pH 8], 100 mM NaCl, 0.5% NP-40, 1 mM EDTA), the bound proteins were resolved by sodium dodecyl sulfate (SDS)-PAGE and detected by fluorography. Binding of pVHL to the hydroxylated HIF-1α ODD peptide (amino acids 556 to 575) was performed as described previously (24). A biotinylated, hydroxylated HIF-derived peptide (0.1 μg) was bound to 30 μl of monomeric avidin agarose (Pierce Chemical Company, Rockford, Ill.) and incubated with 5 or 10 μl of [35S]methionine-labeled pVHL in vitro translation products in a final volume of 500 μl of EBC for 1 h at 4°C. After four washes with NETN, bound proteins were eluted by boiling in SDS-containing sample buffer, resolved by SDS-PAGE, and detected by fluorography.
For the ubiquitination assay, [35S]methionine-labeled TNT reticulocyte lysate Gal4-HIF-1α[ODD] translation products (4 μl) were incubated in RCC 786-O S100 extracts (100 to 200 μg of protein) (prepared as previously described [43]) supplemented with 8 μg of ubiquitin (Sigma)/μl, 100 ng of ubiquitin aldehyde (BostonBiochem, Cambridge, Mass.)/μl, an ATP-regenerating system (20 mM Tris [pH 7.4], 2 mM ATP, 5 mM MgCl2, 40 mM creatine phosphate, 0.5 μg of creatine kinase/μl), and the indicated unlabeled pVHL reticulocyte lysate translation products (2 μl) in a reaction volume of 20 to 30 μl for 1 to 2 h at 30°C. Gal4-HIF-1α[ODD] was immunoprecipitated with anti-Gal4 antibody, resolved by SDS-PAGE, and detected by fluorography.
RESULTS
Role of the cytosolic chaperonin in the maturation of pVHL and the assembly of VCB and VCB-Cul2 ubiquitin ligase complexes. (i) Order of events in the biogenesis of pVHL, VCB, and the VCB-Cul2 ubiquitin ligase in vitro.
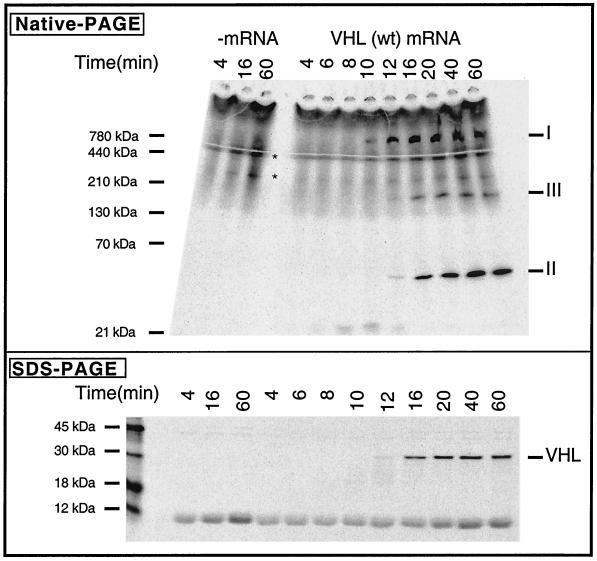
The pathway of pVHL maturation was investigated by using translation in vitro in RRL. Wild-type VHL mRNA was translated in the presence of [35S]methionine, and the reaction products were analyzed by both SDS-PAGE and native-PAGE. Full-length VHL mRNA was translated for 4 min, inhibitors of translational initiation (see Materials and Methods) were added, and the translation reactions continued to allow for polypeptide chain completion. At various times, aliquots were removed and then incubated with apyrase (to deplete ATP and stabilize putative chaperone-nascent chain complexes) and puromycin (to release incomplete polypeptides from the ribosome). Analysis of the reaction products by SDS-PAGE revealed a small amount of full-length pVHL after 12 min of translation (Fig. 1, bottom panel). Maximal amounts of full-length pVHL had accumulated between 20 and 40 min. Native-PAGE analysis (Fig. 1, top panel) showed that, over time, the reaction products consisted of at least three major pVHL-containing complexes with apparent masses of between ∼50 and 800 kDa (indicated as I, II, and III). pVHL-containing species I appeared first, followed by species II and III.
FIG. 1.
Order of events in the assembly pathway of the VCB and VCB-Cul2 complexes. (Top) VHL mRNA was translated in the RRL in the presence of [35S]methionine for 4 min, and then inhibitors of initiation of translation were added, followed by continued incubation. Equal aliquots were removed at the times indicated after the beginning of the reaction and analyzed by native-PAGE. A fluorogram of the gel is shown. The migration positions of different pVHL-containing complexes are indicated on the right as I, II, and III (based on their apparent order of appearance). Native molecular mass standards are shown on the left. A control translation reaction in which no mRNA was added (−mRNA) was analyzed on the left. The position of migration of species not dependent upon VHL mRNA (i.e., which was present in the no-added mRNA column) is indicated by an asterisk. (Bottom) Aliquots of the same samples examined in the top panel were analyzed by SDS-PAGE. A fluorogram of the gel is shown. The migration positions of molecular mass standards are shown on the left.
(ii) Identification of the complexes formed containing pVHL translated in vitro.
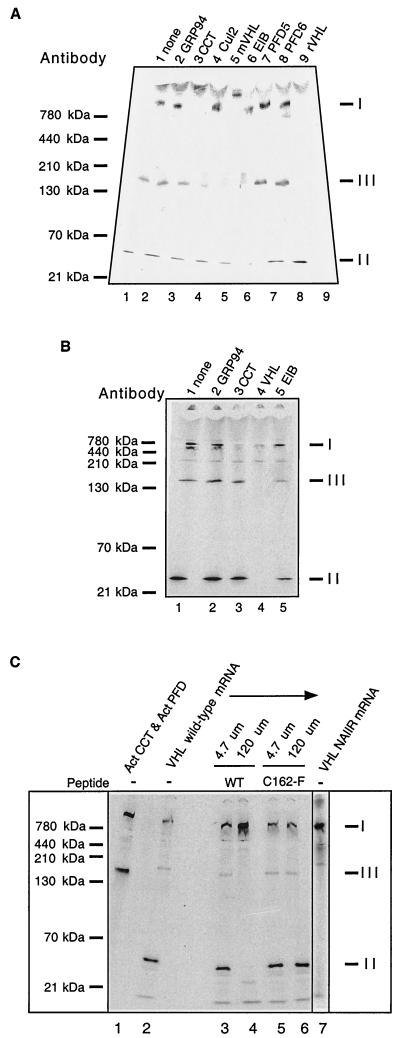
As mentioned earlier, a complex of pVHL with the cytosolic chaperonin (CCT) (Mr ∼800 kDa) has been detected previously by an independent assay (9). Based upon this previous report and the apparent mass of complex I (∼800 kDa), we suspected that species I likely represents pVHL bound to CCT. The nature of complex II, based upon its apparent mass (ca. 40 to 50 kDa), is likely to be the well-characterized VCB complex comprised of pVHL (24 kDa) with elongin B and C (18 and 14 kDa, respectively) (8, 30). Complex III, with an apparent mass ca. 140 to 150 kDa, may represent VCB-Cul2, the complex of VCB with the ∼87-kDa cullin-2 protein (40, 46) or, alternatively, a complex containing pVHL and prefoldin (ca. 120 to 130 kDa). In order to test these proposed compositions of the pVHL-containing complexes shown in Fig. 1, we used an immunochemical approach. If the migration of a pVHL complex during native-PAGE is altered by the presence of a specific antibody (i.e., a gel shift is observed), then the specific antigen is likely to be a component of the complex. A mixture of the pVHL-containing complexes (i.e., complexes I, II, and III) was incubated with antibodies that recognize proteins known to interact directly or indirectly with pVHL (see Materials and Methods). The results of these gel shift analyses are shown in Fig. 2 and can be summarized as follows: compared to the starting materials, anti-GRP94 antibody (a control) had no effect upon the mobility of any of the pVHL complexes (Fig. 2A, lane 1, no added antibody, and lane 2, added GRP94 antibody 2); complex I was shifted by an antibody against CCT or by two antibodies specific for pVHL (lanes 3, 5, and 9, respectively); complex II was shifted by antibodies to elongin B and a rabbit polyclonal antibody to pVHL (lanes 6 and 9); and finally, antibodies to pVHL (lanes 5 and 9), Cul2 (lane 4), and elongin B (lane 6) all altered the mobility of complex III. The reason why the mouse anti-pVHL monoclonal antibody caused a gel shift of complexes I and III but not of complex II is not known. Among several possibilities, perhaps the epitope recognized by this antibody is masked in complex II under these assay conditions. Antibodies recognizing prefoldin, the aforementioned molecular chaperone that has been shown to participate in the maturation of other CCT substrates (e.g., actin and tubulin) (12, 17, 55, 62) and that was suggested to interact with pVHL in yeast (via the two-hybrid assay) (61), had no effect upon the mobility of any of the pVHL-containing complexes (lanes 7 and 8). Under the same experimental conditions, these prefoldin antibodies do in fact alter the mobilities of complexes containing prefoldin bound to tubulin or actin (17). Similarly, we and others have failed to detect prefoldin subunits in anti-pVHL immunoprecipitates as assayed by an anti-prefoldin immunoblot analysis (M. Ohh and W. G. Kaelin, unpublished data; W. Krek, unpublished data). Taken together with results obtained with antibodies to elongin B and Cul2 (see also below), it is unlikely that complex II is pVHL-prefoldin. Contrary to what is observed for the actin and tubulin folding pathways, pVHL folding or assembly appears to be assisted by CCT but apparently without an intermediate step mediated by prefoldin.
FIG. 2.
Identification of pVHL-containing complexes formed during in vitro translation of VHL mRNA. (A) Antibody-induced gel shifts of pVHL-containing complexes. [35S]methionine-labeled pVHL-containing complexes were generated by in vitro translation of VHL mRNA in the RRL. Cycloheximide then was added to a final concentration of 0.5 mM, and equal aliquots of the reaction products were incubated at 4°C for 30 min with the following antibodies: lane 1, noantibody; lane 2, rat monoclonal anti-GRP94 (negative control); lane 3, rat monoclonal anti-CCT-1α; lane 4, rabbit polyclonal anti-Cul2; lane 5, mouse monoclonal anti-pVHL; lane 6, mouse monoclonal and goat anti-elongin B; lane 7, rabbit anti-prefoldin subunit 5; lane 8, rabbit anti-prefoldin subunit 6; and lane 9, rabbit anti-pVHL. The reaction products were then analyzed by native-PAGE. A fluorogram of the gel is shown. The migration positions of the pVHL complexes are indicated at the right (indicated by I, II, and III), and those of native molecular mass standards are shown on the left. (B) Binding of pVHL-containing complexes to immobilized antibodies. pVHL-containing complexes similar to those in panel A were generated by in vitro translation of VHL mRNA in the RRL. Equal aliquots of the reaction products were incubated at 4°C for 30 min with the following antibodies immobilized on a mixture of protein A- and protein G-Sepharose: lane 1, no antibody; lane 2, rat monoclonal anti-GRP94 (negative control); lane 3, rat monoclonal anti-CCT-1α; lane 4, mouse monoclonal anti-VHL and rabbit VHL antibodies; and lane 5, mouse monoclonal and goat anti-elongin B. Proteins remaining in the unbound fraction were then analyzed by native-PAGE. A fluorogram of the gel is shown. The migration positions of the pVHL complexes are indicated on the right (by I, II, and III), and those of the native molecular mass standards are shown on the left. (C) A peptide derived from pVHL, known to interact with elongin C, blocks the formation of complex II and III and results in the accumulation of VHL with CCT (complex I). VHL mRNA was translated in the presence of [35S]methionine in the RRL for 60 min in the absence or presence of two different synthetic pVHL peptides (at the concentrations indicated at the top). The first peptide was derived from wild-type pVHL (amino acids 157 to 172) and is known to bind to elongin C (WT), and the second was derived from a mutant form of pVHL that fails to interact with elongin C (C162F). The lane on the far right contains the products of a translation reaction programmed with VHL mRNA encoding a mutant that contains an insertion of the sequence NAIIRS in the elongin C binding region of pVHL. A fluorogram of the native gel is shown. The migration positions of native molecular mass standards are indicated at the far left. For reference purposes, a reaction wherein newly synthesized actin bound to both CCT and prefoldin is included in the first lane. The position of migration of the pVHL complexes is indicated on the right (I, II, and III).
We also used an alternative immunochemical approach to identify the pVHL-containing complexes. Different antibodies were immobilized on protein A-Sepharose and then used to deplete or remove the different pVHL complexes. After incubation of the radiolabeled pVHL mixture with the various immobilized antibodies, the unbound fraction (i.e., the supernatant fraction) was analyzed by native-PAGE. When compared to the starting materials, the anti-GRP94 antibody (a control) had no effect upon the amount of any of the pVHL complexes (Fig. 2B, lanes 1 and 2); complex I was depleted by antibodies to CCT and pVHL (lanes 3 and 4); complex II was depleted by antibodies to pVHL (lane 4) and diminished by antibodies to elongin B (lane 5); and finally, antibodies to pVHL (lane 4) and elongin B (lane 5) diminished complex III. Although an anti-Cul2 peptide antibody was observed to gel shift complex III (Fig. 2A), once immobilized this antibody to the Cul2 peptide did not deplete complex III (W. J. Hansen and W. J. Welch, unpublished data). Among several possibilities, perhaps the immobilized antibody is unable to interact with Cul2 present within complex III for steric reasons.
Two additional means were used to verify the identity of the pVHL-containing complexes. The molecular contacts between pVHL and the elongin B/C complex are known because the crystal structure of VCB complex has been determined (58). Therefore, we used a synthetic peptide derived from pVHL (VHL157-172) that binds to elongin B/C and prevents the full-length pVHL from assembling with elongin B/C (30, 38, 44) to help in the identification of the different pVHL complexes. VHL mRNA was translated in vitro, this time in either the presence or the absence of this synthetic peptide. Again, three major pVHL complexes were formed in the absence of the peptide (Fig. 2C, lane 2, VHL wild-type mRNA). For reference purposes, newly synthesized actin bound to both CCT and prefoldin is shown in Fig. 2C, lane 1. When the elongin B/C binding peptide was included in the reaction, however, a dose-dependent decrease in the amount of both complexes II and III was observed (Fig. 2C, wild type, lanes 3 and 4). In addition, the pVHL molecules now unable to assemble into complex II and III were found to accumulate in complex I (VHL-CCT). Note that a point mutant peptide VHL157-172-(C162F) that cannot bind to elongin B/C had no effect upon the amount of any of the complexes detected (Fig. 2C, C162F, lanes 5 and 6). Finally, we examined the translation products of an mRNA encoding a pVHL mutant containing a six-amino-acid insertion within the elongin B/C binding region of pVHL. This insertion mutant does not form a complex with either elongin B/C or elongin B/C-Cul2 (40). After translation of this VHL mRNA, complexes II and III no longer were detected. Rather, all of the newly synthesized pVHL again accumulated in the VHL-CCT complex (Fig. 2C, VHL NAIIRS mRNA, lane 7). It is important to note that pVHL was only observed to assemble into the Cul2-containing complex if it had previously bound to elongin B/C (40, 46, 47). Thus, based upon the results shown here and those of previous studies (see also Fig. 3B, below), we conclude (i) that complex I contains pVHL and CCT, (ii) that complex II represents pVHL bound to elongin B/C, and (iii) that complex III contains pVHL-elongin B/C bound to Cul2. It has not yet been determined whether pVHL binds first to elongin B/C or whether it interacts with preformed complexes containing both elongin B/C and Cul2. Whenever pVHL is prevented from binding to elongin B/C, either by mutation of the pVHL elongin B/C binding box or by the inhibitory elongin B/C binding peptide, pVHL remains firmly bound to CCT. It is therefore likely that CCT functions, at least in part, by retaining pVHL chains pending the availability of elongin B/C for final folding and/or assembly.
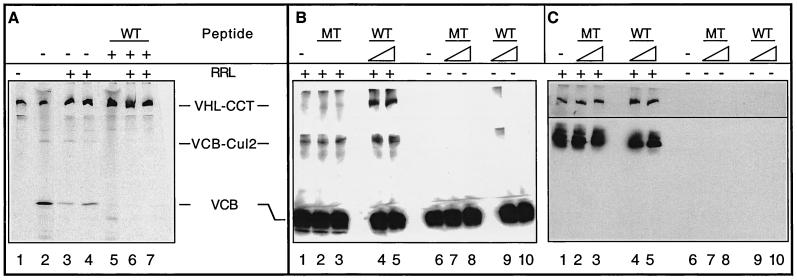
FIG. 3.
A role for CCT in the assembly and disassembly of VCB. (A) Generation of VCB from purified VHL-CCT complexes. VHL-CCT complexes assembled in RRL were isolated by gel filtration chromatography and analyzed by native-PAGE (lane 1). To mark the position of migration of the VCB and VCB-Cul2 complexes, a sample of the unfractionated RRL reaction also was analyzed (lane 2). Purified VHL-CCT was incubated in the presence of RRL and an ATP-regeneration system (lanes 3 and 4), which resulted in the appearance of both VCB and VCB-Cul2. The migration position of VHL-CCT is marked by pVHL synthesized in the presence of the elongin C binding peptide (lane 5). When purified VHL-CCT was incubated in RRL in the presence of the elongin C binding peptide, VCB and VCB-Cul2 formation now was blocked (lanes 6 and 7). (B and C) pVHL present in the VCB complex rebinds to CCT upon VCB disassembly. (B) Recombinant VCB (see Materials and Methods) was added to rabbit reticulocyte lysate (+RRL, lanes 1 to 5) and then incubated in the absence (lane 1) or presence of either the pVHL peptide (WT, lanes 4 and 5) or the mutant pVHL peptide (MT, lanes 2 and 3) described in Fig. 2C (either 120 or 600 μM as indicated by the triangles). As a control, the recombinant VCB was incubated in the absence (lane 6) or presence of either wild type (WT, lanes 9 and 10) or mutant (MT, lanes 7 and 8) peptide in the absence of RRL (−RRL, lanes 6 to 10). After 60 min of incubation, the reaction products were analyzed by native-PAGE. The proteins in the gel were denatured, transferred to nitrocellulose, and analyzed in panel B by Western blotting with a monoclonal antibody specific for pVHL. The positions of the pVHL-containing complexes are indicated at the left. (C) The membrane from the blot shown in panel B was stripped and reprobed with anti-CCT-1α (top) or anti-Cul2 antibodies (bottom). The lane designations are the same as for panel B.
(iii) Potential CCT-assisted assembly-disassembly cycle.
It remained possible that VHL bound to CCT could represent a dead-end off-pathway complex rather than a true intermediate in the assembly of pVHL with elongin B/C and Cul2. In order to determine whether VHL bound to CCT can serve as a productive intermediate in the VCB assembly pathway, VHL-CCT complexes were isolated by gel filtration chromatography and analyzed by native-PAGE (Fig. 3A, lane 1). To mark the position of migration of the VCB and VCB-Cul2 complexes, a sample of the unfractionated RRL reaction was also analyzed (Fig. 3A, lane 2). When the isolated VHL-CCT was incubated in the presence of RRL (to provide a source of elongin B/C and Cul2) and an ATP-regeneration system, we now observed the formation of both the VCB and VCB-Cul2 complexes (Fig. 3A, lanes 3 and 4). pVHL synthesized in the presence of the elongin C binding peptide (pVHL157-172) was run in lane 5. We used the elongin C binding peptide to verify that the complexes formed from the purified VHL-CCT complexes contained elongin C. When purified VHL-CCT was incubated in RRL in the presence of the elongin C binding peptide, VCB and VCB-Cul2 formation was blocked (Fig. 3A, lanes 6 and 7). Based upon these results, we conclude that pVHL can exchange productively from the VHL-CCT complex to form VCB and VCB-Cul2. Hence, VHL-CCT represents an intermediate in the assembly of VCB and VCB-Cul2.
The results of Fig. 2C and 3A demonstrate that newly synthesized pVHL remains associated with CCT if the binding of pVHL to elongin B/C is inhibited. Thus, we examined whether there might exist a disassembly-assembly cycle in which pVHL released from VCB may reform the VHL-CCT complex. Purified recombinant VCB was produced by coexpression in E. coli as described previously (58). The purified VCB was incubated with the pVHL peptide (VHL157-172), the mutant peptide (C162F), or buffer alone. After resolution of the reaction products by native-PAGE, the position of pVHL-containing complexes was determined by immunoblotting with an anti-VHL antibody. In each case, the purified recombinant VCB still migrated as a single band with a mobility similar to that of complex II (Fig. 3B, right side of panel, RRL−, lanes 6 to 10). The small amount of pVHL detected at the top of the 5.5 and 9% acrylamide layers used for the native-PAGE (Fig. 3B, lane 9) may represent pVHL-containing aggregates. Whenever the incubations were done in the presence of rabbit reticulocyte lysate, however, a new species with the same mobility as complex III (i.e., the VCB-Cul2 complex) was observed to form (Fig. 3B, left side of panel, RRL+, lanes 1 to 5). Note that when the purified VCB was added to the RRL in the presence of the elongin C binding peptide, complex I (i.e., VHL-CCT) could be detected (Fig. 3B, left panel, RRL+, lanes 4 and 5). Addition of the wild-type peptide, but not of the mutant peptide (Fig. 3B, left panel, RRL+, lanes 2 and 3), was sufficient to displace a portion of the full-length VHL protein from the recombinant VCB. Once displaced, the free VHL protein apparently rebinds to the cytosolic chaperonin CCT. The position of migration of the reticulocyte CCT and Cul2 proteins in the native-PAGE was confirmed by stripping the Western blot in Fig. 3B and then reprobing it with antibodies specific for CCT (Fig. 3C, top panel) or Cul2 (Fig. 3C, bottom panel). Based upon these observations, an assembly-disassembly pathway for VCB likely exists in which CCT serves as a reservoir of the pVHL monomer until its subsequent assembly with elongin B/C.
(iv) Interaction of pVHL with CCT in vivo and determination of a possible CCT binding site.
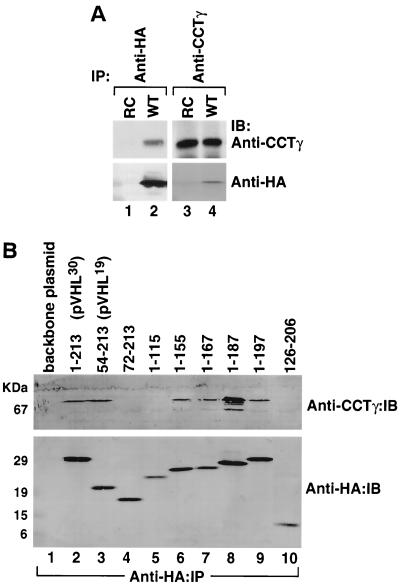
pVHL maturation and possible interactions with the cytosolic chaperonin were examined in vivo. RCC 786-O cells (lacking pVHL) were transfected with a pRc-CMV vector (control) or a vector encoding HA-tagged pVHL. Cell extracts were prepared from the transfected cells and then immunoprecipitated with antibodies specific for either the HA-tagged pVHL or CCT-1γ, a subunit of the chaperonin complex. Proteins precipitated by the anti-HA antibody (i.e., pVHL) (Fig. 4A, lane 2) also contained CCT-1γ as detected by Western blotting. Similarly, proteins precipitated by the anti-CCT-1γ antibody (i.e., cytosolic chaperonin) (Fig. 4A, lane 2) also contained HA-immunoreactive protein (i.e., pVHL). Thus, like the situation in vitro, at least some pVHL interacts with CCT in vivo. Some polypeptides that fold via a CCT-mediated pathway (e.g., tubulins and actins) contain particular subdomains that display an affinity for CCT (6, 19, 49, 50). Relevant to the present study, a 55-amino-acid region (residues 100 to 155) of pVHL has been shown to be necessary and sufficient for binding to CCT (9). To further examine this CCT binding site within pVHL, a series of deletion mutants within the VHL gene were generated and expressed in RCC 786-O cells. Potential interaction of these different pVHL polypeptides with CCT was determined via immunoprecipitation of the HA-tagged pVHL mutants and subsequent Western blotting of the proteins captured with the anti-CCT-1γ antibody (Fig. 4B). Full-length pVHL (i.e., amino acids 1 to 213) and pVHL lacking the first 53 amino acids (i.e., amino acids 54 to 213) (1, 22, 51) both bound to CCT (Fig. 4B, lanes 2 and 3). Likewise, carboxyl-terminal pVHL truncations from amino acids 1 to 155, 1 to 167, 1 to 187, and 1 to 197 were observed to bind to CCT in this assay (Fig. 4B, lanes 6 to 9). In contrast, pVHL amino acids 72 to 213, 1 to 115, and 126 to 206 did not bind to CCT (Fig. 4B, lanes 4, 5, and 10). Based upon these results, we conclude that amino acids 54 to 155, which include the β-domain of pVHL, are important determinants for the interaction of pVHL with CCT.
FIG. 4.
(A) Interactions of wild-type and mutant pVHL with the cytosolic chaperonin in vivo. RCC 786-O cells (lacking endogenous pVHL) transfected with and stably expressing HA-tagged wild-type pVHL (WT) or cells transfected with empty plasmid (RC) were lysed and used for immunoprecipitation reactions (IP) employing an anti-HA antibody (lanes 1 and 2) or an anti-CCTγ antibody (lanes 3 and 4). The resultant pVHL immunoprecipitates were resolved by SDS-PAGE, transferred onto a polyvinylidene difluoride membrane, and then immunoblotted (IB) with anti-CCTγ (top panels) or anti-HA (bottom panels) antibodies. (B) The region encompassing amino acids 54 to 155 of pVHL contributes to the binding of CCT. RCC 786-O cells expressing HA-tagged versions of either wild-type pVHL or a series of pVHL deletion mutants were lysed and used for immunoprecipitation reactions with the anti-HA antibody. The resultant pVHL immunoprecipitates were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and then analyzed for the presence of both the cytosolic chaperonin (Anti-CCTγ:IB) and HA-pVHL proteins (Anti-HA:IB) via Western blotting. The amino acids included in each pVHL polypeptide are listed at the top. Lane 1 represents cells transfected with empty plasmid. p30 represents the full-length pVHL that was initiated at the first methionine codon, while p19 represents a naturally occurring pVHL that was initiated at the second methionine codon.
Diverse effects of mutations in exon II of VHL upon CCT binding, assembly into VCB/VCB-Cul2 complexes and VCB-Cul2 ubiquitin ligase function.
VHL exon II encodes pVHL residues 114 to 154. An alternatively spliced VHL mRNA characterized by the exclusion of exon II is produced in several tissues and cell lines (13, 14, 48). Feldman et al. (9) showed that pVHL encoded by an exon II deletion mutant could not bind to CCT. Moreover, these authors found that this mutant was impaired for VCB complex assembly, suggesting that the two phenomena were linked. Finally, these authors suggested that several disease-causing point mutations in exon II likely interfered with pVHL function by decreasing its affinity for CCT. We therefore examined the consequences of different exon II mutations on pVHL maturation, assembly formation, and substrate interactions.
(i) Assembly of pVHL exon II mutants into pVHL-CCT, VCB, and VCB-Cul2 complexes.
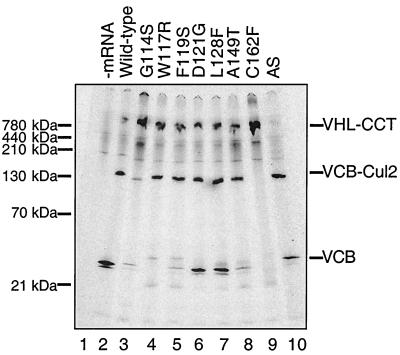
The assembly of wild-type pVHL and the various pVHL mutants into complexes with CCT, elongin B/C, and elongin B/C-Cul2 in vitro was examined by native-PAGE. The particular mutants chosen are all known to be associated with VHL cancer syndrome. These include the pVHL exon II deletion mutant (Δexon II), as well as six point mutants within exon II. Finally, an exon III mutant, C162F, that is unable to form VCB or VCB-Cul2 complexes also was included as a control (40, 44). Analysis of wild-type pVHL again showed the presence of all of the three complexes, VHl-CCT, VCB, and VCB-Cul2 (Fig. 5, lane 2). Consistent with previous observations (9) (and those shown here in Fig. 4B), the pVHL-Δexon II mutant, lacking amino acids 114 to 154 (indicated by “AS” for alternatively spliced), failed to interact with CCT (Fig. 5, lane 10). However, all of the exon II point mutants were observed to interact with CCT (Fig. 5, lanes 2 to 8). Note that the exon II mutants appeared to assemble into the VCB complex with differing efficiencies. In agreement with previous studies, the exon III mutant C162F, which served as a negative control, failed to assemble into a complex with elongin B/C (Fig. 5, lane 9). Failure of this C162F mutant to interact with elongin B/C was accompanied by its increased binding to CCT. Similar results were obtained when a naturally occurring mutant in which all of exon III is deleted was analyzed (Hansen and Welch, unpublished). Finally, note that some of the VCB complexes displayed an aberrant electrophoretic mobility in the native-PAGE analysis (e.g., Fig. 5, lane 10). We suspect that this is due to a somewhat altered conformation of the VCB complex containing the different exon II mutants. The nature of the Δexon II (AS) complex that migrated near VCB containing wild-type pVHL (Fig. 5, lane 10) (i.e., whether it is indeed an VCB complex) was confirmed by showing that the elongin C binding peptide (pVHL157-172) prevented formation of this complex (Hansen and Welch, unpublished).
FIG. 5.
Effects of mutations in VHL exon II on the binding of pVHL to CCT and the assembly of VCB/VCB-Cul2. mRNAs encoding either wild-type or various mutant forms of pVHL were translated in the RRL in the presence of [35S]methionine for 45 min, and aliquots of the reactions were removed and analyzed by native-PAGE. The migration positions of the pVHL-containing complexes are indicated on the right, and the positions of native molecular mass standards are shown on the left. A control translation reaction (−mRNA) was analyzed in lane 1. An exon III mutant, C162F, which does not form VCB and VCB-Cul2 was analyzed as a negative control in lane 9. AS is the naturally occurring alternatively spliced mutant in which all of exon II is deleted. The particular pVHL polypeptide synthesized in each reaction is indicated at the top of the panel.
All of the exon II mutants formed complexes with apparent native masses similar or identical to the VCB-Cul2 complex, whereas as expected the C162F mutant did not (Fig. 5). The pVHL157-172 peptide was used to confirm the identity of the VCB-Cul2 complexes indicated in Fig. 5. Specifically, addition of excess peptide prevented the formation of VCB-Cul2 complex (Hansen and Welch, unpublished). One mutant, G114S (Fig. 5, lane 3), appeared to assemble into the VCB-Cul2 complex to a lesser extent than that observed for the other exon II mutants. The majority of this mutant instead was found to accumulate with CCT. Interestingly, while the levels of the VCB complex varied among the different exon II mutants, the overall amount of the VCB-Cul2 complexes appeared similar (except for G114S). Perhaps this reflects a higher stability of the mutant VCB complex when bound to Cul2. Support for this idea, i.e., that one subunit of the ubiquitin ligase complex may be stabilized by the presence of other members of the complex, has been presented elsewhere (3, 52). Thus, these data indicated that all of exon II point mutants interacted with the cytosolic chaperonin and that all were capable of forming the VCB-Cul2 complex. In addition, the Δexon II (AS) mutant, although apparently unable to interact with CCT, still proceeded to form the VCB-Cul2 complex.
(ii) Binding and ubiquitination of HIF-1α by the VCB-Cul2 ubiquitin ligase complexes containing pVHL with mutations in exon II.
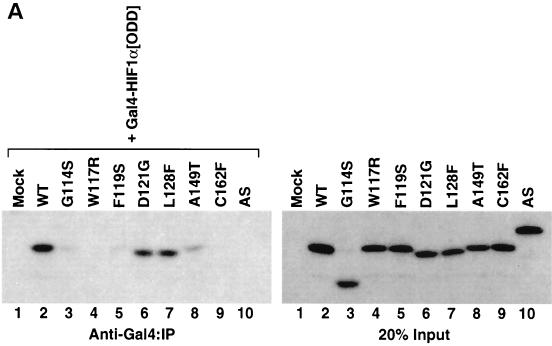
If point mutations within exon II of VHL do not impair the assembly of the VCB-Cul2 complex, why do these mutations cause VHL disease? As mentioned earlier, the VCB-Cul2 ubiquitin ligase complex binds to and mediates the ubiquitination of the HIF-1α, an important transcription factor activated during hypoxia (4, 29, 42, 43, 59). Therefore, mutations in exon II of VHL, while not influencing adversely the formation of VCB-Cul2, might potentially cause disease because of a decreased affinity for HIF-1α. This hypothesis was tested by assaying the binding of the different exon II mutants to HIF-1α. A vector encoding a Gal4 epitope fused in frame with the ODD (18, 43) of HIF-1α was transcribed and translated in RRL in the absence of radiolabel. This ODD is the site within HIF-1α that is recognized by the VCB-Cul2 complex (4, 18, 43, 59). The Gal4-HA-HIF-1α[ODD] was then incubated in the presence of in vitro-translated [35S]methionine-labeled wild-type or mutant pVHL (Fig. 6A, right panel). After incubation, the Gal4-HA-HIF-1α[ODD] was immunoprecipitated with an antibody to Gal4, the proteins captured were separated by SDS-PAGE, and the amount of [35S]methionine-labeled pVHL present was determined. Appreciable levels of wild-type pVHL were found to coprecipitate with the HIF-1α substrate (Fig. 6A, left panel). Similarly, the D121G and L128F pVHL mutants were bound by HIF-1α. In contrast, binding of the A149T pVHL mutant to HIF-1α was barely detectable, and binding of G114S, W117R, F119S, and Δexon II (AS) mutants was greatly diminished or undetectable (Fig. 6A, left panel).
FIG. 6.
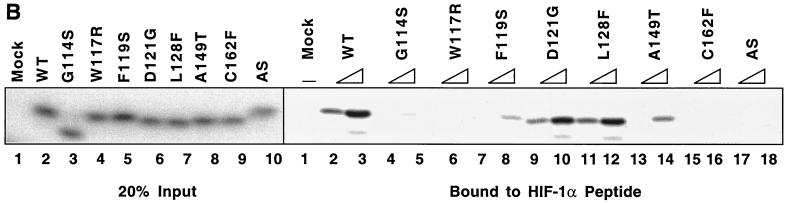
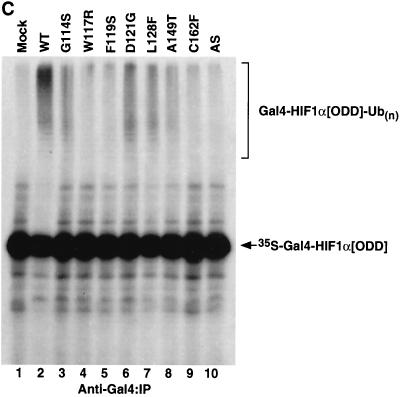
Diverse effects of mutations in exon II of VHL on ability of VCB-Cul2 to interact with the HIF-1α sequence implicated in oxygen-dependent proteolysis. (A) Wild-type and mutant VHL mRNAs were translated in the RRL in the presence of [35S]methionine. In a separate reaction, Gal4-HIF-1α[ODD] mRNA was translated (in the absence of [35S]methionine). Equal portions of the two reactions were mixed together in order to allow the 35S-labeled VCB-Cul2 to interact with the Gal4-HIF-1α[ODD] substrate. After a 30-min incubation, the Gal4-HIF-1α[ODD] was captured by using an anti-Gal4 antibody bound to protein A-Sepharose. The amount of 35S-labeled pVHL coprecipitating was determined by SDS-PAGE and fluorography. In the right panel, 20% of the input radiolabeled pVHL proteins used in the analysis are shown. In the left panel, the radiolabeled pVHL proteins immunoprecipitated by the anti-Gal4 antibodies are shown. The particular pVHL polypeptide synthesized in each reaction is indicated at the top of the panel. Note that the G114S mutant pVHL (lane 3) was observed to migrate faster than the wild-type (lane 2) and that the exon II deletion mutant (lane 10) migrated slightly slower than the wild-type protein under these conditions during SDS-PAGE in Tris-glycine buffer. The reason for this anomalous migration is not known. These anomalies were not observed when Tris-Tricine gels were used. (B) Binding of pVHL encoded by exon II mutants to a hydroxylated HIF-derived peptide. The indicated wild-type and mutant VHL mRNAs were translated in RRL in the presence of [35S]methionine. Then, 5 or 10 μl of radiolabeled translation products, as indicated by the triangles, was incubated with 0.1 μg of an immobilized biotin-conjugated hydroxylated HIF-derived peptide (a substrate for the VCB-Cul2 complex) (see Materials and Methods) to allow 35S-labeled VCB-Cul2 to interact with the hydroxylated peptide. After a 1-h incubation at 4°C, the amount of bound 35S-labeled pVHL was determined by SDS-PAGE and fluorography (right panel). In the left panel, 20% of the input radiolabeled pVHL proteins used in the analysis is shown. “Mock” indicates a reaction that was programmed with empty plasmid. (C) The ability of VHL exon II mutants to stimulate the ubiquitination of HIF-1α correlates well with the binding of the pVHL variants to HIF-1α. In an experiment similar to that shown in panels A and B, Gal4-HIF-1α[ODD] was synthesized in RRL, this time in the presence of [35S]methionine. In parallel, the wild type and the different pVHL mutants were translated separately in the absence of radiolabel. Portions of the translation reactions (4 μl of 35S-labeled Gal4-HIF-1α[ODD] and 2 μl of each unlabeled pVHL) were mixed together, along with an in vitro system capable of ubiquitin conjugation (see Materials and Methods). The Gal4-HIF-1α[ODD] was isolated by immunoprecipitation with anti-Gal4 antibodies, and the resultant immunoprecipitates were analyzed by SDS-PAGE and fluorography. The particular pVHL polypeptide synthesized in each reaction is indicated at the top of the panel. Indicated near the top of the gel is the position of ubiquitin-conjugated Gal4-HIF-1α[ODD] [UB(n)]. “Mock” indicates a reaction that was programmed with empty plasmid.
The interaction between pVHL and a specific domain of the HIF-1α subunit has recently been shown to be regulated through hydroxylation of a specific proline residue (HIF-1α P564) by an enzyme termed HIF-1α prolyl-hydroxylase (24, 26). Thus, pVHL can bind to a short HIF-derived synthetic peptide when this conserved proline residue is hydroxylated (24, 26). To further characterize mutant pVHL-HIF-1α interaction, we measured the binding of either the wild-type or mutant forms of pVHL to the hydroxylated HIF-derived peptide. A biotinylated, proline-hydroxylated, HIF-derived peptide was immobilized on avidin agarose and incubated with [35S]methionine-labeled in vitro-translated wild-type or mutant pVHL. After multiple washes, the bound pVHL was resolved by SDS-PAGE and detected by fluorography (Fig. 6B). Wild-type pVHL, D121G pVHL, and L128F pVHL all bound avidly to the HIF-derived peptide. The A149T mutant bound less well, while the G114S, W117R, F119S, and Δexon II (AS) mutants exhibited little or no binding. Thus, these two assays examining the interaction of the different pVHLs with HIF-1α yielded essentially the same results: several of the exon II pVHL mutants display a reduced capacity to bind to the HIF-1α substrate.
We next measured the ability of the different VCB-Cul2 complexes to ubiquitinate HIF-1α in an in vitro assay. [35S]methionine-labeled Gal4-HA-HIF-1α[ODD] was translated in the RRL. In parallel, the wild-type or mutant pVHL proteins were synthesized in the RRL in the absence of radiolabel. The reaction products were then mixed, along with an S100 fraction from RCC 786-O cells (lacking pVHL) (to provide ubiquitin activating and conjugating enzymes), ubiquitin, and an ATP-regenerating system (see Materials and Methods). Since ubiquitin isopeptidases can remove ubiquitin from target proteins, ubiquitin aldehyde was included to inhibit the activity of such isopeptidases and thereby facilitate the detection of the ubiquitinated products. The reaction products were analyzed by SDS-PAGE. Reaction products produced in the presence of wild-type pVHL were comprised of a high-molecular-weight smear of [35S]methionine-labeled Gal4-HA-HIF-1α[ODD], which is (Fig. 6C, lane 2) indicative of ubiquitination of this substrate (4, 29, 43, 59). In contrast, when the ubiquitination reactions were performed in the presence of the different pVHL exon II mutants, the ubiquitination of Gal4-HA-HIF-1α[ODD] was either undetectable [for W117R, F119S, and Δexon II (AS)] (Fig. 6C, lanes 4, 5, and 10) or markedly diminished (for G114S and A149T) (Fig. 6C, lanes 3 and 8). The D121G and L128F mutants appeared to support HIF-1α ubiquitination, albeit less than that observed for wild-type pVHL (Fig. 6C, lanes 6 and 7). Finally, the exon III mutant, C162F, which does not interact with elongin B/C-Cul2, served as a negative control. The C162F mutant was unable to support the ubiquitination of HIF-1α (Fig. 6C, lane 9). Taken together, the results of the HIF-1α binding and ubiquitination experiments support a model in which some exon II-encoded sequences impact either the binding to and/or ubiquitination of the substrate by the VCB-Cul2 ubiquitin ligase.
(iii) Stability and function of wild-type and representative exon II mutant pVHL polypeptide complexes with CCT and elongin B/C-Cul2 formed in vivo.
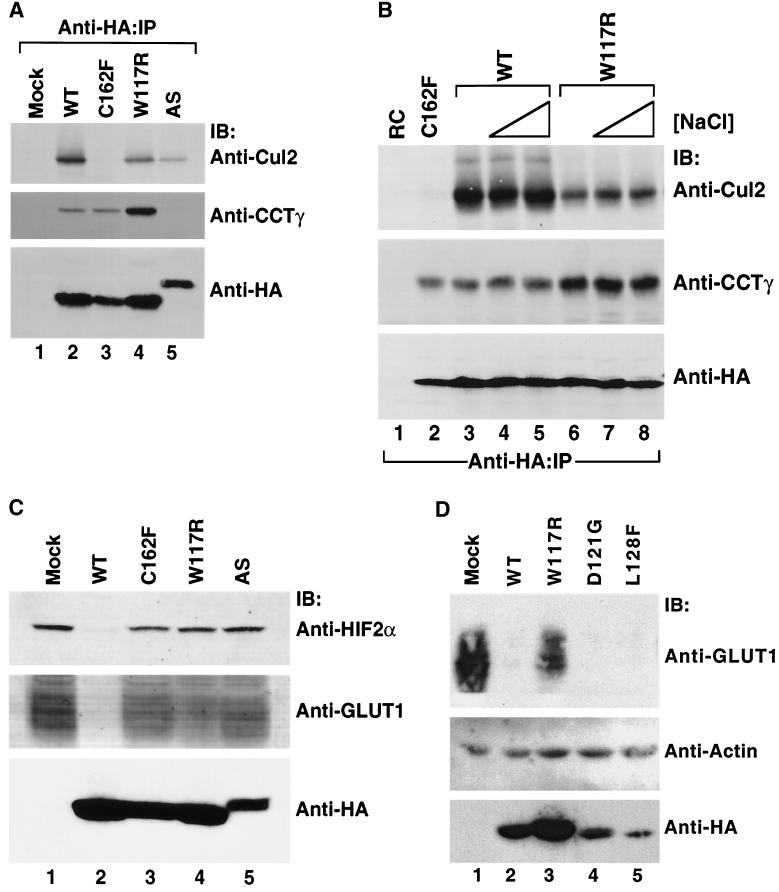
The in vitro biochemical experiments described above revealed that many of the VHL exon II point mutations affected pVHL's ability to bind to and target HIF for ubiquitination, while none affected CCT binding. To test the relevance of these findings in vivo, 786-O RCC cells (lacking endogenous pVHL) were stably transfected to produce HA-tagged forms of a representative exon II pVHL mutant (W117R), an exon III pVHL mutant (C162F), or the exon II deletion mutant (AS). In parallel, cells were transfected with HA-wild-type pVHL (WT) or with an empty expression plasmid (Mock). Cell extracts were prepared (48 h posttransfection), and the HA-tagged pVHL was captured by immunoprecipitation with an anti-HA antibody. Proteins present within the immunoprecipitates were analyzed by immunoblotting (Fig. 7A) with anti-Cul2, anti-CCTγ, or anti-HA antibodies as indicated in the figure. As would be predicted by our in vitro studies, the W117R mutant interacted with both Cul2 and CCTγ (Fig. 7A, lane 4). In contrast, pVHL containing the C162F mutation, known to disrupt the interaction of pVHL with elongin B/C, did not bind to Cul2 (Fig. 7A, lane 3), in keeping with earlier studies (40, 44), but did interact with CCT. Finally, the Δexon II mutant did not interact with CCT but did bind to Cul2 (Fig. 7A, lane 5).
FIG. 7.
Stability and function of wild-type and representative exon II mutant pVHL polypeptide complexes with CCT and elongin B/C-Cul2 formed in vivo. (A) Effects of mutations in VHL exon II upon pVHL-CCT and VCB assembly. RCC 786-O cells expressing empty plasmid (Mock) or HA-tagged versions of wild-type or mutant pVHL were lysed and then immunoprecipitated with the anti-HA antibody (Anti-HA-IP). Resultant immunoprecipitates were resolved by SDS-PAGE and then Western blotted with anti-Cul2 (top panel), anti-CCTγ (middle panel), and anti-HA (bottom panel) antibodies. The particular pVHL polypeptide synthesized is indicated at the top of the panel. (B) Effects on stability of pVHL-CCT and VCB-Cul2 complexes. RCC 786-O cells expressing HA-tagged wild-type pVHL (WT) (lanes 3 to 5) or an exon II pVHL point mutant (W117R) (lanes 6 to 8) were lysed and immunoprecipitated with the anti-HA antibody under increasing NaCl concentrations (125 mM [lanes 1, 2, 3, and 6], 500 mM [lanes 4 and 7], and 900 mM [lanes 5 and 8]). Lysates from cells transfected with plasmid alone (RC) (lane 1) and the HA-tagged exon III mutant (C162F) (lane 2) were immunoprecipitated with the anti-HA antibody under the lowest NaCl stringency condition. Proteins present within the immunoprecipitates were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed via Western blotting (IB) with the anti-Cul2 (top panel), anti-CCTγ (middle panel), and anti-HA (bottom panel) antibodies. (C) Exon II mutants that do not bind to HIF in the in vitro assays are unable to suppress expression of a HIF 2α regulated gene product, GLUT1. In parallel, the same cell lysates analyzed in B were examined for their expression of HIF-2α and GLUT1, a gene product whose expression is regulated by the HIF-2α transcription factor. Whole-cellextracts were prepared from the cells described above, and the proteins (200 μg) were resolved by SDS-PAGE and then Western blotted (IB) with anti-HIF-2α (top panel), anti-GLUT1 (middle panel), or anti-HA (bottom panel) antibodies. The GLUT1 protein likely appears as a rather diffuse band due to heterogeneity in its glycosylation and because of its many (>10) transmembrane segments (35, 57). (D) Exon II mutants that do bind to HIF in the in vitro assays are able to suppress expression of an HIF-2α-regulated gene product. RCC 786-O cells were infected with retroviral vectors encoding HA-tagged versions of the wild type or the indicated pVHL mutants. Whole-cell extracts were prepared, and proteins (200 μg) were resolved by SDS-PAGE and Western blotted (IB) with anti-GLUT1 (top panel), anti-actin (middle panel), or anti-HA (bottom panels) antibodies.
We next assayed the interaction of wild-type and W117R pVHL with CCT in the presence of increasing salt concentrations (Fig. 7B). Even in the presence of high salt (900 mM NaCl), the W117R pVHL mutant remained bound to CCT (Fig. 7B, lanes 6 to 8). The Cul2-W117R complexes also were stable in high-salt conditions, although the overall amount of Cul2 detected was diminished compared with that observed for the wild-type pVHL. Similar results were obtained when the stringency was increased by varying the concentration of SDS (Ohh and Kaelin, unpublished).
Due to their failure to express pVHL, 786-O cells contain relatively high levels of the HIF-2α transcription factor under normoxic conditions (4, 42). This allowed us to examine both the steady-state levels of HIF-2α and of GLUT1 (a gene product whose transcription is dependent upon HIF-2α) after transfection of the cells with either wild-type or mutant pVHL. As shown by immunoblot analysis, in cells expressing wild-type pVHL there was little or no expression of HIF-2α (Fig. 7C, top, lane 2) or GLUT1 (Fig. 7C, middle, lane 2). In contrast, cells expressing the C162F mutant (which fails to bind to elongin B/C and serves as a negative control) (Fig. 7C, top and middle, lane 3), the W117R mutant (which cannot support ubiquitination of HIF) (Fig. 7C, top and middle lane 4), and finally the Δexon II mutant (Fig. 7C, top and middle, lane 5) all showed relatively high levels of both HIF-2α and GLUT1. Hence, the W117R and Δexon mutants are unable to properly regulate HIF-2α and GLUT1 under conditions of normoxia.
Two other exon II mutants, D121G and L128F, were observed to bind to HIF in the in vitro assays used here as well as the wild-type pVHL. Consequently, we assessed whether expression of these two mutants, like the wild-type pVHL protein, would lead to HIF degradation and correspondingly low levels of a downstream target of HIF-2α, GLUT1. In cells expressing wild-type pVHL (Fig. 7D, lane 2) GLUT1 levels were suppressed compared to cells either mock transfected or expressing the W117R mutant (Fig. 7D, lanes 1 and 3). Cells expressing D121G and L128F mutants appeared to suppress the level of GLUT1 as well as did cells expressing wild-type pVHL under these experimental conditions. Thus, two mutants that measurably interact with HIF in vitro retained the ability to suppress expression of an HIF-regulated gene product.
DISCUSSION
A collection of in vitro and in vivo experiments have helped us define the maturation pathway of pVHL. Newly synthesized pVHL formed a complex with elongin B/C (VCB) that subsequently assembled with Cul2 (VCB-Cul2). After its synthesis and prior to these assembly events, pVHL was found to associate with the cytosolic chaperonin containing TCP-1 or CCT, a molecular chaperone that may assist in the maturation of the pVHL polypeptide chain. Our data are consistent with a role for CCT in mediating the assembly of pVHL into the oligomeric VCB and VCB-Cul2 protein complexes. Specifically, CCT appears to retain the pVHL monomer until a stable VCB complex has formed. Once VCB has assembled with Cul2, the resultant VCB-Cul2 ubiquitin ligase can bind to and facilitate the ubiquitination of its substrates (e.g., HIF-1/2α) under conditions of normoxia, thereby targeting them for destruction by the proteasome.
How and why might pVHL fold and/or assemble via a chaperonin-mediated pathway? pVHL shares one structural feature with actin, a polypeptide that also folds by a CCT-assisted pathway. Specifically, α-helical sequences that reside near the carboxyl terminus of both proteins interact in the final folded structure with β-sheet elements encoded >80 amino acids earlier in the amino acid sequence (i.e., they are discontiguous sequences). It is of interest that the β-strands S5, S6, and S6′ of pVHL (see the structure presented in reference 58) that interact with the carboxyl-terminal α-helix are encoded by exon II and comprise what has been determined here and by others previously (9) to be the main site of interaction with CCT. Why pVHL might interact with CCT may relate to the role CCT plays in pVHL folding and complex assembly. First, whenever VCB assembly was inhibited, either by sequestering elongin B/C with an elongin B/C binding pVHL peptide (Fig. 2C) or by mutations within the elongin B/C binding region of pVHL (Fig. 2C and 5), pVHL accumulated with the cytosolic chaperonin. In either circumstance, we were unable to detect any appreciable amount of the pVHL monomer. Second, when the preformed VCB complex was dissociated by the presence of the elongin B/C binding pVHL peptide, again pVHL was observed to rebind to CCT. Thus, we think it is likely that CCT stabilizes the pVHL monomer pending the availability of elongin B/C for final folding or assembly of the VCB complex. In the absence of elongin B/C, it is possible that the structure of pVHL may represent a compact “quasi-native” assembly intermediate similar to those described for the CCT-bound states of tubulin and actin (5, 6, 39, 60). Hence, the final and native folded state of pVHL might only be formed upon its assembly into the VCB complex. Consistent with this idea is the observation that three α-helices of pVHL (H1, H2, and H3) pack together with one α-helix of elongin C (H4) to form a four-helix cluster in the crystal structure of the VCB complex (58). Hence, it has been suggested that the three-helix cluster of pVHL would be unstable in the absence of the fourth helix contributed by elongin C (58).
Our data indicated the pVHL-Δexon II mutant failed to interact with CCT, a finding that is in agreement with the results of Feldman et al. (9). However, all of the tumor-derived pVHL exon II point mutants examined here were observed to bind to CCT. Our finding that single point mutations, within the region of pVHL that interacts with CCT, do not block interaction of the substrate with the chaperonin has precedent. Specifically, many single-amino-acid substitutions that were generated within a region of tubulin that interacts with CCT (e.g., between residues 263 and 384) had only minor effects upon the binding of tubulin to CCT (49). Hence, the previous conclusion that mutations in exon II are likely to result in VHL disease because of a failure to interact with CCT (9) appears unlikely.
All of the VHL exon II mutants examined here (both the Δexon II and the exon II point mutants) assembled into VCB and VCB-Cul2 complexes. These findings do not support the conclusion of the previous report that the exon II-encoded region of VHL is essential for assembly with elongin B/C (9). However, the present data are in agreement with a study that determined that the pVHL-Δexon II mutant expressed in yeast assembled with elongin B/C and Cul2 as efficiently as did wild-type pVHL (47). In addition, while this report was in preparation a study was published that examined the impact of exon II deletion on pVHL function (2). The authors of that study also concluded that deletion of exon II did not impair assembly of the VCB-Cul2 complex but that it did compromise efficient binding and ubiquitination of substrates of the VCB-Cul2 ubiquitin ligase (i.e., HIF-1/2α). The VCB-Cul2 complex containing pVHL lacking sequences encoded by exon II was competent to synthesize a homopolymer of ubiquitin in the absence of a recipient target protein (i.e., the complex retained the catalytic activity of the ubiquitin ligase) (2). Finally, their study showed that the pVHL exon II deletion mutant was incapable of stimulating the conjugation of NEDD8, a homologue of ubiquitin, to Cul2. It has been suggested that this modification of Cul2 accelerates the rate of ubiquitination of substrates by Cul2 (2). We have not yet determined whether the exon II point mutants examined here support the conjugation of NEDD8 to Cul2.
It remains possible that the CCT binding region of pVHL (i.e., that encoded by exon II) might interfere with the folding of the elongin B/C binding region (i.e., the domain encoded by exon III) of pVHL. If this is the case, then interaction with CCT might help circumvent potential negative impacts of this region upon folding of the elongin B/C binding site. In such a scenario, deletion of exon II might be expected to allow pVHL to assemble with elongin B/C and subsequently into a catalytically active ubiquitin ligase complex. Such a complex, however, would be unable to bind to specific substrates (e.g., HIF-1/2α).
We conclude that mutations within exon II of VHL are responsible for functional defects of the β-domain distinct from those caused by mutations in the exon III-encoded α-domain. Mutations in exon III lead to improper regulation of HIF-1α and/or HIF-2α due to impaired assembly of VCB and VCB-Cul2 (40, 44, 46). In contrast, mutations in exon II do not block VCB or VCB-Cul2 assembly. Rather, we have determined that many mutations in exon II (G114S, W117R, F119S, A149T, and Δexon II) likely impair degradation of HIF-1α because these pVHL mutants display defects in substrate binding. The biochemical defects appear similar to those displayed by the β-domain mutants in exon I of VHL (43, 59). The resultant stabilization of HIF-1α in vivo leads to expression of hypoxia-inducible genes under normoxia.
The finding that two disease-associated exon II mutants, pVHL D121G and L128F, retained the ability to bind to HIF in vitro and downregulated the expression of a gene controlled by HIF in cells was surprising given the current view that the development of the classical stigmata of VHL disease, consisting of blood vessel tumors of the eye and the central nervous system, is linked to deregulation of HIF and its downstream targets (32). One possibility is that these two mutants are associated with a quantitative defect in HIF regulation that is not revealed in all of our assays. That these two mutants displayed diminished HIF polyubiquitination activity compared to the wild type supports such a view. Alternatively, these two mutants might be defective for the polyubiquitination of some other, as-yet-unidentified substrate or perhaps lack a function of pVHL distinct from its role as a ubiquitin ligase.
Acknowledgments
This work was supported by the National Institutes of Health (GM33551 and GM27345-20 to W.J.W.). M.O. is a recipient of a National Cancer Institute of Canada Fellowship, J.M. is a recipient of a Sarnoff Scholarship, and W.G.K. is a Howard Hughes Assistant Investigator.
We thank Gary Nolan (Department of Molecular Pharmacology, Stanford University) for Phoenix cells and technical advice and Jeff Klco for unpublished plasmids.
W.J.H. and M.O. contributed equally to this work.
REFERENCES
- 1.Blankenship, C., J. G. Naglich, J. M. Whaley, B. Seizinger, and N. Kley. 1999. Alternate choice of initiation codon produces a biologically active product of the von Hippel Lindau gene with tumor suppressor activity. Oncogene 18:1529-1535. [DOI] [PubMed] [Google Scholar]
- 2.Bonicalzi, M. E., I. Groulx, N. de Paulsen, and S. Lee. 2001. Role of exon 2-encoded β-domain of the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 276:1407-1416. [DOI] [PubMed] [Google Scholar]
- 3.Botuyan, M. V., C. M. Koth, G. Mer, A. Chakrabartty, J. W. Conaway, R. C. Conaway, A. M. Edwards, C. H. Arrowsmith, and W. J. Chazin. 1999. Binding of elongin A or a von Hippel-Lindau peptide stabilizes the structure of yeast elongin C. Proc. Natl. Acad. Sci. USA 96:9033-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockman, M. E., N. Masson, D. R. Mole, P. Jaakkola, G. W. Chang, S. C. Clifford, E. R. Maher, C. W. Pugh, P. J. Ratcliffe, and P. H. Maxwell. 2000. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275:25733-25741. [DOI] [PubMed] [Google Scholar]
- 5.Cowan, N. J., and S. A. Lewis. 1999. A chaperone with a hydrophilic surface. Nat. Struct. Biol. 6:990-991. [DOI] [PubMed] [Google Scholar]
- 6.Dobrzynski, J. K., M. L. Sternlicht, G. W. Farr, and H. Sternlicht. 1996. Newly-synthesized beta-tubulin demonstrates domain-specific interactions with the cytosolic chaperonin. Biochemistry 35:15870-15882. [DOI] [PubMed] [Google Scholar]
- 7.Duan, D. R., J. S. Humphrey, D. Y. Chen, Y. Weng, J. Sukegawa, S. Lee, J. R. Gnarra, W. M. Linehan, and R. D. Klausner. 1995. Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations. Proc. Natl. Acad. Sci. USA 92:6459-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan, D. R., A. Pause, W. H. Burgess, T. Aso, D. Y. Chen, K. P. Garrett, R. C. Conaway, J. W. Conaway, W. M. Linehan, and R. D. Klausner. 1995. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science 269:1402-1406. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, D. E., V. Thulasiraman, R. G. Ferreyra, and J. Frydman. 1999. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell 4:1051-1061. [DOI] [PubMed] [Google Scholar]
- 10.Frydman, J., E. Nimmesgern, H. Erdjument-Bromage, J. S. Wall, P. Tempst, and F. U. Hartl. 1992. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 11:4767-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, Y., J. O. Thomas, R. L. Chow, G. H. Lee, and N. J. Cowan. 1992. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell 69:1043-1050. [DOI] [PubMed] [Google Scholar]
- 12.Geissler, S., K. Siegers, and E. Schiebel. 1998. A novel protein complex promoting formation of functional alpha- and gamma-tubulin. EMBO J. 17:952-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnarra, J. R., D. R. Duan, Y. Weng, J. S. Humphrey, D. Y. Chen, S. Lee, A. Pause, C. F. Dudley, F. Latif, I. Kuzmin, L. Schmidt, F. M. Duh, T. Stackhouse, F. Chen, T. Kishida, M. H. Wei, M. I. Lerman, B. Zbar, R. D. Klausner, and W. M. Linehan. 1996. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim. Biophys. Acta 1242:201-210. [DOI] [PubMed] [Google Scholar]
- 14.Gnarra, J. R., K. Tory, Y. Weng, L. Schmidt, M. H. Wei, H. Li, F. Latif, S. Liu, F. Chen, F. M. Duh, et al. 1994. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 7:85-90. [DOI] [PubMed] [Google Scholar]
- 15.Gnarra, J. R., S. Zhou, M. J. Merrill, J. R. Wagner, A. Krumm, E. Papavassiliou, E. H. Oldfield, R. D. Klausner, and W. M. Linehan. 1996. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc. Natl. Acad. Sci. USA 93:10589-10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, W., P. D. Garcia, and P. Walter. 1986. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell 45:397-406. [DOI] [PubMed] [Google Scholar]
- 17.Hansen, W. J., N. J. Cowan, and W. J. Welch. 1999. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J. Cell Biol. 145:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, L. E., J. Gu, M. Schau, and H. F. Bunn. 1998. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 95:7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes, G. M., and K. R. Willison. 2000. Individual subunits of the eukaryotic cytosolic chaperonin mediate interactions with binding sites located on subdomains of β-actin. J. Biol. Chem. 275:18985-18994. [DOI] [PubMed] [Google Scholar]
- 20.Iliopoulos, O., A. Kibel, S. Gray, and W. G. Kaelin. 1995. Tumour suppression by the human von Hippel-Lindau gene product. Nat. Med. 1:822-826. [DOI] [PubMed] [Google Scholar]
- 21.Iliopoulos, O., A. P. Levy, C. Jiang, W. G. Kaelin, and M. A. Goldberg. 1996. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc. Natl. Acad. Sci. USA 93:10595-10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliopoulos, O., M. Ohh, and W. G. Kaelin. 1998. pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc. Natl. Acad. Sci. USA 95:11661-11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivan, M., and W. G. Kaelin, Jr. 2001. The von Hippel-Lindau tumor suppressor protein. Curr. Opin. Genet. Dev. 11:27-34. [DOI] [PubMed] [Google Scholar]
- 24.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIF-α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 25.Iwai, K., K. Yamanaka, T. Kamura, N. Minato, R. C. Conaway, J. W. Conaway, R. D. Klausner, and A. Pause. 1999. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 96:12436-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 27.Kaelin, W. G., and E. R. Maher. 1998. The VHL tumour-suppressor gene paradigm. Trends Genet. 14:423-426. [DOI] [PubMed] [Google Scholar]
- 28.Kamura, T., D. M. Koepp, M. N. Conrad, D. Skowyra, R. J. Moreland, O. Iliopoulos, W. S. Lane, W. G. Kaelin, S. J. Elledge, R. C. Conaway, J. W. Harper, and J. W. Conaway. 1999. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284:657-661. [DOI] [PubMed] [Google Scholar]
- 29.Kamura, T., S. Sato, K. Iwai, M. Czyzyk-Krzeska, R. C. Conaway, and J. W. Conaway. 2000. Activation of HIF-1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc. Natl. Acad. Sci. USA 97:10430-10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kibel, A., O. Iliopoulos, J. A. DeCaprio, and W. G. Kaelin. 1995. Binding of the von Hippel-Lindau tumor suppressor protein to elongin B and C. Science 269:1444-1446. [DOI] [PubMed] [Google Scholar]
- 31.Kishida, T., T. M. Stackhouse, F. Chen, M. I. Lerman, and B. Zbar. 1995. Cellular proteins that bind the von Hippel-Lindau disease gene product: mapping of binding domains and the effect of missense mutations. Cancer Res. 55:4544-4548. [PubMed] [Google Scholar]
- 32.Kondo, K., and W. G. Kaelin, Jr. 2001. The von Hippel-Lindau tumor suppressor gene. Exp. Cell Res. 264:117-125. [DOI] [PubMed] [Google Scholar]
- 33.Krieg, P. A., and D. A. Melton. 1984. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 12:7057-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latif, F., K. Tory, J. Gnarra, M. Yao, F. M. Duh, M. L. Orcutt, T. Stackhouse, I. Kuzmin, W. Modi, L. Geil, et al. 1993. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260:1317-1320. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S., M. Neumann, R. Stearman, R. Stauber, A. Pause, G. N. Pavlakis, and R. D. Klausner. 1999. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol. Cell. Biol. 19:1486-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroux, M. R., and F. U. Hartl. 2000. Protein folding: versatility of the cytosolic chaperonin TRiC/CCT. Curr. Biol. 10:R260-R264. [DOI] [PubMed] [Google Scholar]
- 37.Linehan, W. M., M. I. Lerman, and B. Zbar. 1995. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA 273:564-570. [PubMed] [Google Scholar]
- 38.Lisztwan, J., G. Imbert, C. Wirbelauer, M. Gstaiger, and W. Krek. 1999. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 13:1822-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llorca, O., J. Martin-Benito, M. Ritco-Vonsovici, J. Grantham, G. M. Hynes, K. R. Willison, J. L. Carrascosa, and J. M. Valpuesta. 2000. Eukaryotic chaperonin CCT stabilizes actin and tubulin folding intermediates in open quasi-native conformations. EMBO J. 19:5971-5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonergan, K. M., O. Iliopoulos, M. Ohh, T. Kamura, R. C. Conaway, J. W. Conaway, and W. G. Kaelin. 1998. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell. Biol. 18:732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher, E. R., and W. G. Kaelin. 1997. von Hippel-Lindau disease. Medicine 76:381-391. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 43.Ohh, M., C. W. Park, M. Ivan, M. A. Hoffman, T. Y. Kim, L. E. Huang, N. Pavletich, V. Chau, and W. G. Kaelin. 2000. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2:423-427. [DOI] [PubMed] [Google Scholar]
- 44.Ohh, M., Y. Takagi, T. Aso, C. E. Stebbins, N. P. Pavletich, B. Zbar, R. C. Conaway, J. W. Conaway, and W. G. Kaelin. 1999. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein. J. Clin. Investig. 104:1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohh, M., R. L. Yauch, K. M. Lonergan, J. M. Whaley, A. O. Stemmer-Rachamimov, D. N. Louis, B. J. Gavin, N. Kley, W. G. Kaelin, and O. Iliopoulos. 1998. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell 1:959-968. [DOI] [PubMed] [Google Scholar]
- 46.Pause, A., S. Lee, R. A. Worrell, D. Y. Chen, W. H. Burgess, W. M. Linehan, and R. D. Klausner. 1997. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl. Acad. Sci. USA 94:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pause, A., B. Peterson, G. Schaffar, R. Stearman, and R. D. Klausner. 1999. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc. Natl. Acad. Sci. USA 96:9533-9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards, F. M., P. N. Schofield, S. Fleming, and E. R. Maher. 1996. Expression of the von Hippel-Lindau disease tumour suppressor gene during human embryogenesis. Hum. Mol. Genet. 5:639-644. [DOI] [PubMed] [Google Scholar]
- 49.Ritco-Vonsovici, M., and K. R. Willison. 2000. Defining the eukaryotic cytosolic chaperonin-binding sites in human tubulins. J. Mol. Biol. 304:81-98. [DOI] [PubMed] [Google Scholar]
- 50.Rommelaere, H., M. De Neve, R. Melki, J. Vandekerckhove, and C. Ampe. 1999. The cytosolic class II chaperonin CCT recognizes delineated hydrophobic sequences in its target proteins. Biochemistry 38:3246-3257. [DOI] [PubMed] [Google Scholar]
- 51.Schoenfeld, A., E. J. Davidowitz, and R. D. Burk. 1998. A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc. Natl. Acad. Sci. USA 95:8817-8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoenfeld, A. R., E. J. Davidowitz, and R. D. Burk. 2000. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc. Natl. Acad. Sci. USA 97:8507-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semenza, G. L. 2000. HIF-1 and human disease: one highly involved factor. Genes Dev. 14:1983-1991. [PubMed] [Google Scholar]
- 54.Semenza, G. L. 1999. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15:551-578. [DOI] [PubMed] [Google Scholar]
- 55.Siegers, K., T. Waldmann, M. R. Leroux, K. Grein, A. Shevchenko, E. Schiebel, and F. U. Hartl. 1999. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 18:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siemeister, G., K. Weindel, K. Mohrs, B. Barleon, G. Martiny-Baron, and D. Marme. 1996. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 56:2299-2301. [PubMed]
- 57.Simpson, I. A., S. J. Vannucci, M. R. DeJoseph, and R. A. Hawkins. 2001. Glucose transporter asymmetries in the bovine blood-brain barrier. J. Biol. Chem. 276:12725-12729. [DOI] [PubMed] [Google Scholar]
- 58.Stebbins, C. E., W. G. Kaelin, and N. P. Pavletich. 1999. Structure of the VHL-elongin C-elongin B complex: implications for VHL tumor suppressor function. Science 284:455-461. [DOI] [PubMed] [Google Scholar]
- 59.Tanimoto, K., Y. Makino, T. Pereira, and L. Poellinger. 2000. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19:4298-4309. [DOI] [PMC free article] [PubMed]
- 60.Tian, G., I. E. Vainberg, W. D. Tap, S. A. Lewis, and N. J. Cowan. 1995. Specificity in chaperonin-mediated protein folding. Nature 375:250-253. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchiya, H., T. Iseda, and O. Hino. 1996. Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product. Cancer Res. 56:2881-2885. [PubMed] [Google Scholar]
- 62.Vainberg, I. E., S. A. Lewis, H. Rommelaere, C. Ampe, J. Vandekerckhove, H. L. Klein, and N. J. Cowan. 1998. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 93:863-873. [DOI] [PubMed] [Google Scholar]
- 63.Zhu, H., and H. F. Bunn. 1999. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir. Physiol. 115:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]