Abstract
Previous studies have demonstrated that phosphorylation of human p53 on serine 15 contributes to protein stabilization after DNA damage and that this is mediated by the ATM family of kinases. However, cellular exposure to hypoxia does not induce any detectable level of DNA lesions compared to ionizing radiation, and the oxygen dependency of p53 protein accumulation differs from that of HIF-1, the hypoxia-inducible transcription factor. Here we show that, under severe hypoxic conditions, p53 protein accumulates only in S phase and this accumulation correlates with replication arrest. Inhibition of ATR kinase activity substantially reduces hypoxia-induced phosphorylation of p53 protein on serine 15 as well as p53 protein accumulation. Thus, hypoxia-induced cell growth arrest is tightly linked to an ATR-signaling pathway that is required for p53 modification and accumulation. These studies indicate that the ATR kinase plays an important role during tumor development in responding to hypoxia-induced replication arrest, and hypoxic conditions could select for the loss of key components of ATR-dependent checkpoint controls.
The tumor microenvironment affects both the malignant progression of transformed cells and their response to chemotherapy and radiotherapy. Tumor hypoxia develops in most solid tumors as a result of inefficient vascular development or abnormal vascular architecture (6). Previous studies have demonstrated that hypoxia is an independent prognostic factor of survival independent of other factors, including tumor grade or treatment modality (surgery or radiotherapy) (27). One insight into how oxygen deficiency can affect the aggressiveness of tumors is through the modulation of the p53 tumor suppressor gene (22). During tumor growth, hypoxia can act as a selective pressure for the elimination of cells with wild-type p53 and the clonal expansion of cells with mutant or otherwise inactive p53 protein (21). This observation provides a possible explanation for the more aggressive nature of hypoxic tumors compared to well-oxygenated ones and for the frequent occurrence of p53 mutations in advanced stages of tumor development. Thus, both hypoxia and genotoxic stresses like UV and ionizing radiation induce p53-dependent apoptosis.
Activation of p53 following genotoxic damage is achieved by induction of p53 levels and by modifications of the p53 protein (reviewed in references 19 and 41). Accumulation of p53 protein following genotoxic stress involves posttranscriptional mechanisms such as enhanced translation of p53 mRNA and decreased proteolytic degradation of the protein (32, 35, 38). The product of the mdm-2 oncogene, itself a transcriptional target of p53, was shown to bind to the N terminus of p53 and inhibit p53 transactivation properties as well as promote its proteolytic degradation (26, 31, 37). In cells that are exposed to genotoxic stress, interactions between p53 and mdm-2 are disrupted in large part due to posttranslational modifications of p53 and mdm-2. In contrast to genotoxic stress, a proposed mechanism for the accumulation of p53 by hypoxia is through the binding of the hypoxia-inducible factor 1 (HIF-1) to p53 (2). However, this hypothesis is problematic in that hypoxia-induced p53 accumulation can occur in HIF-1β−/− and HIF-1α−/− cells (53), suggesting that alternative mechanisms for the stabilization of p53 protein are also induced in hypoxic cells, such as through the regulation of mdm-2 (1).
In response to DNA damage, both the amino- and carboxy-terminal domains of p53 become phosphorylated at multiple sites. The prevailing thought is that phosphorylation of p53 on these different sites is important for regulating p53 protein stability and function. One of the first phosphorylation sites on p53 to be identified was serine 15 (4, 9, 44, 46). It has been suggested that serine 15 modification results in decreased binding affinity between mdm2 and p53, thereby disrupting this negative feedback loop and increasing the levels of p53 following DNA damage (44). p53 is also extensively phosphorylated at other sites in vitro and in vivo in response to genotoxic damage (19, 36, 41). Although some of these posttranslational modifications increase the sequence-specific DNA binding activity of p53 and its transactivation properties in vitro, the physiological significance of these modifications in vivo remains to be determined.
The phosphorylation of p53 on serine 15 is mediated by the ATM family of kinases (4, 10, 29). Cells deficient in ATM fail to exhibit rapid phosphorylation of serine 15 after gamma irradiation (γIR) but exhibit rapid phosphorylation of this site after UV irradiation by the ATR kinase, indicating that different stresses can signal individual ATM family members to phosphorylate serine 15. In some cell types, reduced phosphorylation of serine 15 correlates with decreased p53 stabilization. In fact, introduction of an alanine in place of the corresponding serine 15 residue in mouse p53 (serine 18) results in a substantial reduction of p53 stabilization but not complete elimination (11). It has also been demonstrated that ATR can partially complement the defective intra-S-phase checkpoint response of ATM cells, suggesting functional overlap between the two kinases. Despite this overlap in function, ATM primarily responds to DNA damage throughout the cell-cycle, whereas ATR has a more prominent role in the response to stresses that induce a replication arrest. However, while deficiency of ATM is compatible with both cellular and animal viability, loss of ATR is invariably lethal, which indicates that ATR plays an essential role in cellular homeostasis that cannot be complemented by ATM (5). The catalytic activity of ATM has been shown to increase rapidly, in a matter of minutes, in response to IR or radiomimetic drugs (4, 9). In contrast, there has been no increase in ATR activity in response to DNA damage reported when using the reagents available to date. This may be due to the fact that ATR relocalizes within the cell in response to stress, a phenomenon that is not observed for ATM (52). Thus, if ATM is important to the p53 damage-inducible stress response that occurs when cells are exposed to genotoxic damage either from the environment or during cancer therapy, we hypothesized that ATR plays an important role in modulating p53 in response to growth-inhibitory stresses such as hypoxia that occur during tumor evolution.
In this report we investigated how hypoxia-induced growth arrest could be a signal for modification of p53 at serine 15, as well as the involvement of the ATM or ATR kinases in this modification. We also compared hypoxia and other inhibitors of DNA replication in their modulation of p53 to determine whether growth arrest alone or the combination of growth arrest and DNA damage were prerequisite events in the modification of p53 under hypoxic conditions. These studies indicate that ATR plays an important role during tumor progression in modulating the activity of the p53 tumor suppressor gene in response to hypoxia.
MATERIALS AND METHODS
Cell lines and transfections.
The RKO, 293T, RCC4, and HCT116 cell lines were maintained in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum. GM1526 and GM536 were maintained in RPMI medium supplemented with 15% fetal bovine serum. Both GM1526 (ATM+/+, p53+/+) and GM536 (ATM−/− from an ataxia telangiectasia patient) are Epstein-Barr virus immortalized lymphoblastoid cell lines. For transfections 293T cells were plated at 105 cells/5-cm-diameter dish the day before use. Cells were transfected using the Lipofectamine reagent and protocol. Cells gamma irradiated in the presence of LY294002 were pretreated with the LY294002 for 8 h prior to treatment. No pretreatment was necessary for the hypoxia-treated cells.
Hypoxia treatment.
Cells were plated in glass dishes, and treatment was carried out in a hypoxia chamber (<0.2% O2) (Sheldon Corp., Cornelius, Oreg.).
Precipitation of [3H]thymidine-labeled DNA with TCA.
Cells were plated at 104/well. One hour prior to harvesting, cells were labeled with 1 μCi ml−1 of [3H]thymidine. Cells were lysed in 25 μM Tris-HCl (pH 8.0)-25 mM EDTA-0.5% sodium dodecyl sulfate. Trichloroacetic acid (TCA) (50%) was then added to a final concentration of 12%, and samples were incubated on ice for 20 min. Precipitated nucleic acids were collected on Whatman GF/C glass fiber filters (2.4-cm diameter). The filters were washed three times with ice-cold 5% TCA and 20 mM sodium pyrophosphate and once with 70% ethanol. The amount of radioactivity was determined after the addition of Ecolume scintillant. Each experiment was carried out in triplicate.
Microarray analysis.
Total RNA was extracted using Trizol (Invitrogen) after indicated periods of hypoxia. cRNA was then synthesized, labeled, hybridized to an oligo-based array, washed, and scanned according to standard Affymetrix protocols.
Immunoblotting.
For immunoblotting, cells were lysed in 9 M urea-75 mM Tris-HCl (pH 7.5)-0.15 M β-mercaptoethanol and sonicated briefly. Fifty micrograms of protein were electrophoresed on 7.5% polyacrylamide gels. Primary antibodies used were p53 DO-1, phospho-p53 Ser 15 (16G8 monoclonal; catalogue no. 9286), phospho-p53 Ser 37 (catalogue no. 9289), and phospho-chk1 Ser 345 (catalogue no. 2341) from Cell Signaling Technology, Gapdh (TRK5G4-6C5) from Research Diagnostics, and HIF-1α (H72320) from Transduction Laboratories.
Immunofluorescence.
Cells were grown and treated on eight-well chamber slides. After treatment, cells were fixed in methanol at −20°C for 20 min. Cells were then rehydrated in phosphate-buffered saline (PBS) and blocked in 20% heat-inactivated normal goat serum-0.1% bovine serum albumin-0.1% sodium azide in PBS for 30 min. Cells were incubated for 1.5 h at 37°C in a humidified box with protein G-purified α-ATR (49) at a final concentration of 1.0 μg ml−1 in blocking solution. Samples were washed with PBS-0.2% Tween 20 and then incubated with a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Sigma) for 1 h. After being washed, samples were counterstained with 0.1 μg of propidium iodide per ml and mounted with coverslips and an aqueous antifade mounting reagent (Vectashield; Vector Laboratories).
Comet assay.
Comet assays were carried out as previously described (14, 45). In brief 1 × 104 to 3 × 104 RKO cells were prepared as a single cell suspension in magnesium- and calcium-free PBS. Three volumes of a 1% low-melt agarose-2% dimethyl sulfoxide solution was added to the cells followed by mixing. The mixture was placed onto a microscope slide and allowed to set on a cold surface. When completely set, the slide was immersed in lysis buffer for 1 h (0.03 M NaOH, 1 M NaCl, 0.1% N-lauroylsarcosine) at room temperature. Hypoxia-treated cells were both harvested and lysed inside the hypoxia chamber to avoid reoxygenation. The propidium iodide-stained cells (comets) were visualized using a Nikon Optiphot microscope attached to an Ikegami 4612 CCD camera and fluorescence image analysis system. By using specially designed software (40), the tail moment of each cells was calculated as the product of the percentage of DNA in the tail multiplied by the length of the comet tail. Two hundred comets were scored for each treatment.
RESULTS
p53 accumulates in response to hypoxia independently of HIF-1α in S-phase cells.
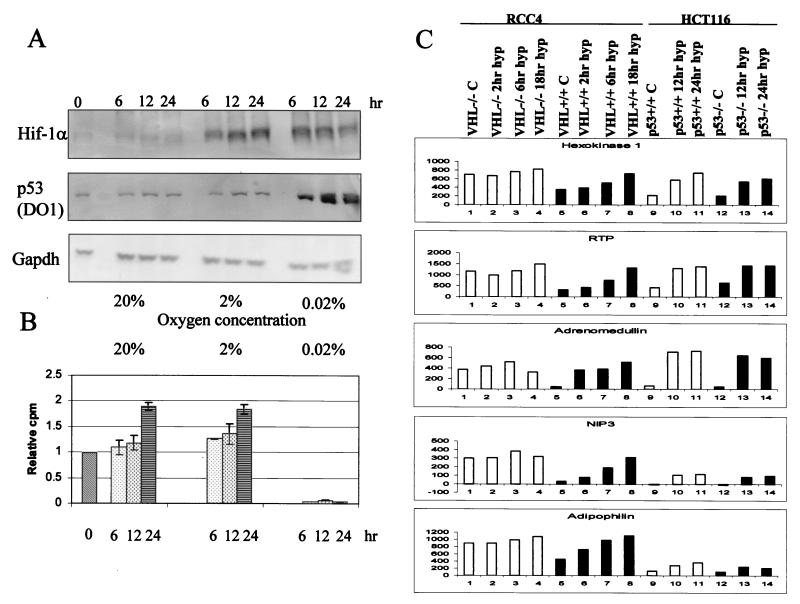
Previous reports have proposed that, in response to hypoxia, p53 is stabilized by a direct interaction between HIF-1α and p53 (2). We have tested this finding further in human tumor-derived cells, which have wild-type p53, after incubation in different oxygen concentrations (0.02, 2, and 20%). RKO cells were exposed to hypoxia for different periods of time, and then cell extracts were prepared to assess the levels of HIF-1α and p53 proteins (Fig. 1A). We used Gapdh as a loading control in these experiments. It should be noted that some reports have suggested that Gapdh gene expression is hypoxia inducible (56). However, in the cell lines used in this study no changes in Gapdh protein levels were observed. HIF-1α protein accumulated with similar kinetics in response to 0.02 and 2% oxygen, whereas p53 protein accumulated in response to 0.02% oxygen only. If HIF-1α protein levels were solely responsible for stabilizing p53, one would expect p53 to accumulate in response to 2% oxygen, as there are quantitatively similar amounts of HIF-1α induced in 2% oxygen and in 0.02% oxygen-treated cells. Thus, if HIF-1α has any role in the stabilization of p53 in response to hypoxia, it also requires another factor present at near-anoxic conditions that is absent in cells grown in 2% oxygen. We hypothesized that this additional factor could be induced by the inhibition of DNA synthesis that also occurs at near-anoxia. Figure 1B depicts the relative amounts of DNA synthesis in the RKO cell line as determined by [3H]thymidine incorporation over a period of 24 h. Cells grown in 0.02% oxygen show a decrease in DNA synthesis. After 6 h at 0.02% oxygen there was essentially no [3H]thymidine incorporation, indicative of an S-phase arrest as has been previously reported in studies using BrdU incorporation and fluorescence-activated cell sorter analysis (23). This arrest was not seen in response to growth in 2% oxygen and was not influenced by HIF-1α stabilization. Using the matched cell lines HCT 116 p53+/+ and HCT 116 p53−/−, we have demonstrated that the arrest seen in response to culture at 0.02% oxygen is independent of p53 status (data not shown).
FIG. 1.
In RKO cells HIF-1α is induced at oxygen concentrations of 2 and 0.02%, while p53 is stabilized only at 0.02%. (A) RKO cells were grown in glass dishes at the oxygen concentrations indicated (20, 2, or 0.02%) and harvested after 6, 12, and 24 h. The total levels of HIF-1α, p53 and Gapdh are shown. (B) RKO cells were grown as indicated for panel A. Growth at 0.02% oxygen induced a decrease in DNA synthesis as measured by [3H]thymidine incorporation. In contrast, cells grown in normal (20% O2) and 2% O2 concentrations continued to cycle and have similar profiles. (C) The cell lines shown were grown in glass dishes and treated with hypoxia for the indicated time periods. Shown are the expression levels of hexokinase I, RTP, adrenomedullin, NIP3, and adipophilin which were determined by hybridization to an oligo-based Affymetrix microarray.
It has been suggested that cells lacking p53 have enhanced levels of HIF-1α (42). The VHL (von Hippel-Lindau) tumor suppressor gene targets HIF-1α subunits for degradation under normoxic conditions. VHL-null cells have stabilized HIF-1α subunits, which leads to HIF-1 activation and a subsequent increase in HIF-responsive genes independent of oxygen status. We have carried out microarray screening experiments with the following cell lines: RCC4 VHL+/+, RCC4 VHL−/−, HCT116 p53+/+, and HCT116 p53−/−. The expression of five HIF-inducible genes, Hexokinase 1, RTP, Adrenomedullin, NIP 3, and Adipophilin are shown in Fig. 1C (8, 17; N. Denko, personal communication). These genes were selected based on their expression level and known hypoxia inducibility. We show that these representative HIF-1-induced genes are constitutively expressed in the VHL−/− cell line irrespective of hypoxia. This phenomenon was not seen with p53−/− cells, indicating that in these cell lines lack of p53 does not amplify the normal HIF-1-dependent response.
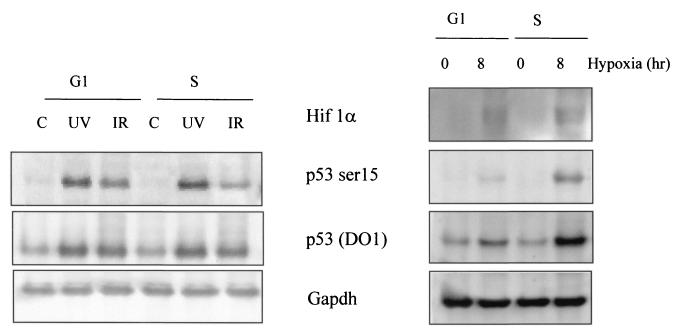
Although hypoxia induced an intra-S-phase arrest that was independent of p53 (20), the possibility exists that this arrest acts as the signal for p53 accumulation and modification. If replication arrest induced by hypoxia is the signal for p53 accumulation and modification, then the prediction would be that p53 protein accumulation should occur selectively during S phase in hypoxic cells. To test this hypothesis, we used the mitotic shake-off technique to generate synchronized populations of cells. [3H]thymidine incorporation assays indicate that RKO cells enter S phase approximately 8 h after mitotic shake-off, signifying a G1 phase of 8 h (data not shown). Experiments were therefore carried out with cells 30 min after mitotic shake-off (G1 phase) or with cells incubated for 8 to 10 h after mitotic shake-off (S phase). G1- or S-phase cells were treated with hypoxia (8 h), UV (5 h after treatment with 50 J/m2), or γIR (5 h after treatment with 8 Gy), and immunoblotting experiments were carried out to assess p53 and HIF-1α protein levels and modification of p53 protein. Figure 2 shows that p53 was stabilized and phosphorylated at serine 15 in response to UV and γIR in both G1- and S-phase cells. As predicted, there was a significant difference in the p53 responses to hypoxia between G1- and S-phase cells. In contrast to what was seen with γIR or UV treatment, S-phase cells induced significantly more p53 than the G1 cells. These same S-phase populations also display increased serine 15 phosphorylation after hypoxia compared to G1 cells. The slight accumulation and phosphorylation of p53 seen in the G1-phase cells under hypoxic conditions could be accounted for by the small 10% contamination of S-phase cells in the G1 population. These findings support the hypothesis that S-phase arrest triggered by severe hypoxia is the signal that ultimately leads to p53 accumulation and phosphorylation.
FIG. 2.
Hypoxia induces p53 stabilization and phosphorylation in S-phase cells rather than cells in G1. Mitotic RKO cells were harvested by shaking flasks of exponentially growing populations in order to loosen adhering cells. After shake-off, cells were plated in glass dishes and placed into a hypoxia chamber for 8 h. Greater than 90% of these cells are in the G1 phase of the cell cycle, which is approximately 8 h for RKO cells. Cells were plated and allowed to grow under normoxic conditions for 8 to 10 h after shake-off to produce a synchronized S-phase population. These cells were then placed in the hypoxia chamber for 8 h. Synchronized populations of G1- or S-phase cells were also treated with γIR (5 h after treatment with 8 Gy) and UV (5 h after treatment with 50 J m−2). The levels of p53 (total), p53 serine 15, and Gapdh were determined by Western blotting. The levels of HIF-1α protein are also shown for the hypoxia-treated samples.
Hypoxia-induced p53 is phosphorylated at the amino terminus in an ATR-dependent manner.
We have investigated the phosphorylation status of p53 in hypoxic extracts, initially focusing on serines 15 and 37, in wild-type p53 RKO cells, and wild-type p53 293T cells that also contain simian virus 40 large T antigen. Figure 3 shows that HIF-1α was induced in both cell lines in response to hypoxia and HIF-1-stabilizing drugs desferoxamine (DFO) and CoCl2. However, p53 was stabilized in the RKOs only after treatment with hypoxia, DFO, and hydroxyurea (Hu) but not after CoCl2. These results further reinforce the disconnection between the stabilization of p53 after hypoxia treatment and HIF-1α accumulation. It is unclear how DFO treatment induces p53 protein accumulation, although it has been suggested to be via its iron-chelating effects (3, 16). Previous studies have suggested that although the level of p53 remained unchanged in 293T cells due to the presence of large T antigen, modifications of p53 protein can still be detected and in some cases more easily so due to the elevated levels of p53. Phosphorylation of p53 at serines 15 and 37 was detected after hypoxia, DFO, and Hu treatments. CoCl2 induced HIF-1α protein accumulation but did not induce stabilization or modification of p53 protein.
FIG. 3.
p53 is phosphorylated at residues serine 15 and 37 in response to hypoxia. Total cell extracts were prepared from RKO (A) and 293T (B) cells, which had been grown in glass dishes. Cells were treated with hypoxia, DFO (100 μM; 24 h), CoCl2 (150 μM; 24 h) or Hu (1.5 mM; 24 h). Immunoblots were carried out to show the levels of HIF-1α, total p53, p53 serine 15, p53 serine 37, and Gapdh.
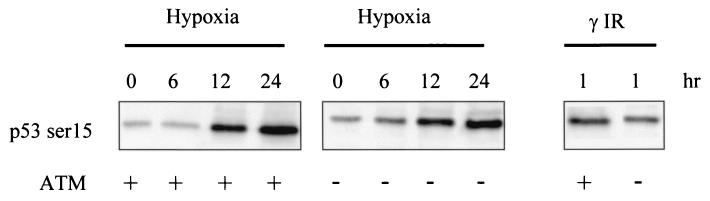
It is well established that serines 15 and 37 of p53 can be phosphorylated by members of the PI3 kinase-related kinase member in response to stress (29). Using isogenic ATM-deficient and reconstituted cell lines (GM1526 and GM536), we investigated whether ATM was the kinase responsible for hypoxia-induced phosphorylation of p53 at serine 15. In response to hypoxia, p53 was phosphorylated with identical kinetics and levels in both ATM-deficient and reintroduced cell lines (Fig. 4). In the absence of ATM, there was a lower level of serine 15 phosphorylation after γIR. As ATM is known to phosphorylate p53 with rapid kinetics after some stresses, we also investigated whether p53 in response to hypoxia was being phosphorylated at earlier time points than those shown. There was no observable phosphorylation at serine 15 of p53 until 4 h of hypoxia treatment in both the ATM+/+ and ATM−/− cell lines (data not shown). Taken together, these data demonstrate that ATM is not the kinase responsible for phosphorylation of p53 on serine 15 in hypoxic cells.
FIG. 4.
The phosphorylation of p53 at Serine 15 in response to hypoxia is independent of ATM. GM1526 ATM−/− and GM536 ATM+/+ were exposed to either hypoxia or 6 Gy of γIR. The lack of ATM had little or no effect on the levels of p53 serine 15 in response to hypoxia. In contrast, there was threefold less p53 serine 15 in the ATM-null cells after γIR than in the ATM wild-type cells. The relative increases in signal were determined by using a phosphoimager.
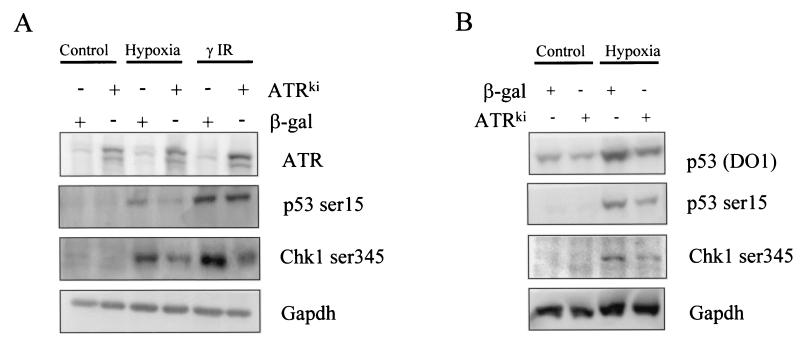
ATR homozygous knockouts result in both embryonic and cell lethality (5). ATR-null cells exhibit chromosomal abnormalities, display mitotic catastrophe, and cannot be passaged in culture (12). As a consequence of these findings, we have used a ATR kinase-dead (ATRKI) mutant, which can act as a dominant negative when overexpressed in mammalian cells, to investigate the role of ATR in serine 15 phosphorylation. The kinase-dead mutant was used in a transient-transfection experiment, the results of which are shown in Fig. 5A. A construct expressing β-galactosidase (β-Gal) was used as a negative control as well as to determine transfection efficiency, which was on average 80%. Hypoxia-induced serine 15 phosphorylation was significantly reduced in 293T cells when transfected with the ATR kinase-dead mutant and to a much lesser extent after γIR. Chk 1 has been shown to be a substrate of the ATR kinase and is phosphorylated at serines 317 and 345 in an ATR-dependent manner (34, 55). To demonstrate that the kinase-dead ATR dominant negative allele was acting to inhibit ATR kinase signaling, we examined Chk1 phosphorylation at serine 345 in the same cell lysates. Chk1 phosphorylation at residue 345 was induced in response to hypoxia and γIR, and this induction was reduced by ATRKI overexpression. Hypoxia-induced serine 15 phosphorylation of p53 and serine 345 phosphorylation on Chk1 was also significantly reduced in RKO cells when transfected with the ATR kinase-dead mutant (Fig. 5B). Significantly, the total level of hypoxia-induced p53 protein was reduced in the presence of the ATR kinase-dead mutant. These data provide evidence that ATR activity leads to not only p53 modification but also protein accumulation in response to hypoxia.
FIG. 5.
ATR is responsible for phosphorylating p53 at serine 15 during hypoxia exposure. (A) 293T cells were transiently transfected with either a β-Gal or an ATR kinase-dead construct. Cells were treated with either hypoxia or γIR (4 h after treatment with 6 Gy), and the Western blotting experiments shown were carried out. (B) RKO cells were transiently transfected with either a β-Gal or an ATR kinase-dead construct. After transfection, cells were treated with hypoxia for 24 h and the Western blotting experiments shown were carried out.
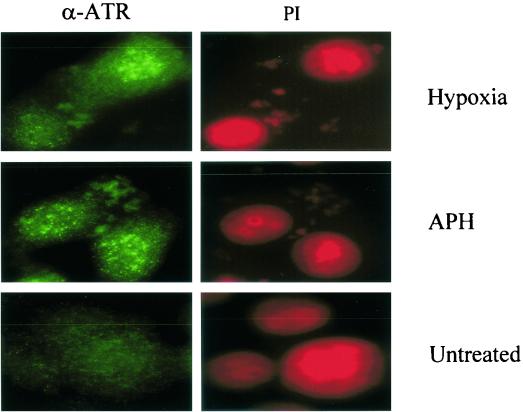
The protein kinase activity of ATR is not increased by cellular exposure to genotoxic stress; however, the same stimuli induce a dramatic relocalization of ATR into DNA damage-induced nuclear foci (49). We have examined whether ATR forms characteristic nuclear foci in response to hypoxia. RKO cells were treated with either hypoxia for 18 h or aphidicolin (APH), a known DNA replication inhibitor (5 μg/ml for 24 h), and stained for ATR localization. Cells grown in normoxic conditions showed a diffuse nuclear-staining pattern (Fig. 6). However, cells treated with either hypoxia or APH show distinct nuclear foci.
FIG. 6.
ATR forms distinct nuclear foci in hypoxic cells. RKO cells were grown on chamber slides and incubated in hypoxic conditions for 18 h. As a positive control RKOs were also treated with APH for 24 h (5 μg ml−1). After treatment, cells were fixed and permeabilized before staining with protein G-purified α-ATR (49). Slides were counterstained with propidium iodide to demonstrate that the foci seen were nuclear.
Hypoxia is a unique stress that induces a replication arrest in the absence of DNA damage.
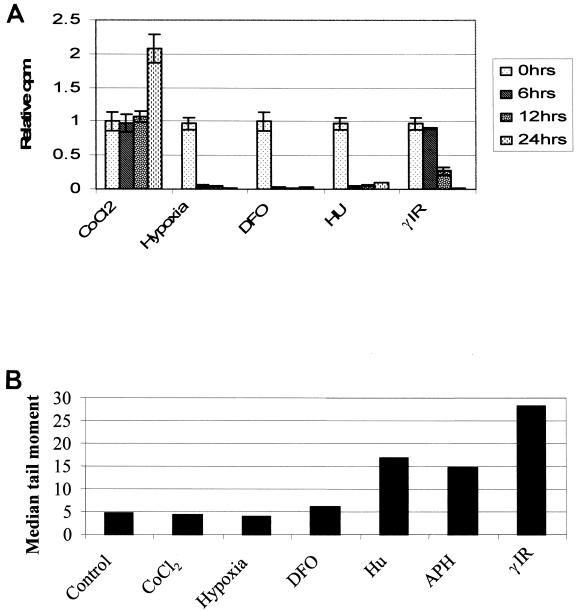
From our current understanding of the ATM and ATR kinases, we postulated that agents that induce replication arrest would cause activation of the ATR pathway and those that induce genotoxic stress would activate the ATM pathway. However, there is considerable overlap in this model, as many of the agents that induce growth arrest induce DNA damage. To determine whether hypoxia induced replication arrest and DNA damage, we compared the kinetics of DNA synthesis inhibition by hypoxia-mimetic agents CoCl2, DFO, Hu, and γIR with the kinetics of hypoxia. Within 6 h of exposure to hypoxia, RKO cells reduced their incorporation of thymidine into their DNA to less than 5% of that observed with untreated controls (Fig. 7A). Similar kinetics of [3H]thymidine inhibition were found for cells treated with Hu or DFO, both of which result in a depletion of ribonucleotide pools (39, 43). In contrast, the intra-S-phase and G2 checkpoints induced by γIR were gone by 6 h, and the decreased levels of DNA synthesis observed after 12 and 24 h were due to G1 checkpoint function. Most significantly, the inhibition of DNA synthesis in response to the hypoxia mimetic agents DFO and CoCl2 was markedly different. DFO induced an S-phase arrest similar to that seen after hypoxia, while CoCl2 had little effect on DNA synthesis as the cells continued to incorporate [3H]thymidine. These data demonstrate that the S-phase arrest seen in response to hypoxia is independent of HIF-1α accumulation as DFO, CoCl2, and hypoxia all induce HIF-1α but only hypoxia and DFO inhibit DNA synthesis. It is also clear that the effects seen here in response to DFO treatment, p53 protein accumulation and S-phase arrest, are unrelated to its function as a hypoxia-mimetic agent.
FIG. 7.
Hypoxia induces a rapid intra-S-phase arrest in the absence of any detectable DNA damage. RKO cells were treated with a variety of stresses, and a [3H]thymidine incorporation assay was carried out. The treatments were as follows: hypoxia (0.02% oxygen), 1.5 mM Hu, 8 Gy γIR, 100 μm DFO, and 150 μM CoCl2 for the times indicated. Comet assays were carried out to determine relative amounts of damage caused after each treatment. The median tail moment for each stress is shown. There was no increase in DNA damage over control levels after 24 h of hypoxia or CoCl2 treatment and only a slight increase with DFO. All the other stresses used have DNA damage associated with them to various degrees.
To determine the relative amounts of DNA damage induced by these same stresses, we examined treated cells with the comet assay (15, 40). This assay measures DNA breakage (alkaline sensitive sites, single- and double-strand breaks) through an increase in the electrophoretic mobility of denatured genomic DNA in an agarose gel. The relative amount of damage can be quantified by measuring the distance DNA moves in the gel or the length of the comet tail (40). Shown in Fig. 7B are the median tail moments for cells treated with hypoxia, DFO, CoCl2, Hu, APH, and γIR. Cells exposed to hypoxia were harvested and lysed inside a specially designed hypoxia chamber using equilibrated solutions. After 24 h of hypoxia, there was no detectable change in the electrophoretic mobility of DNA. Interestingly, while Hu and APH are commonly used to induce replication arrest, both of these agents induced significant DNA strand breakage to levels found with cells treated with γIR. It is unlikely that either Hu or APH directly damages DNA; rather, the breaks are probably due to incomplete and interrupted DNA synthesis. Thus, hypoxia represents a unique physiologic stress that induces ATR-dependent p53 protein accumulation and modification in a DNA damage-independent manner through replication arrest.
DISCUSSION
Serine 15 phosphorylation of p53 is associated with increased transcription efficiency, decreased affinity for mdm2, and increased nuclear localization (44, 54). While mdm2 protein levels decrease within cells exposed to hypoxia (1), it is conceivable that serine 15 phosphorylation of p53 represents a fail-safe mechanism to ensure sustained accumulation of p53 under hypoxia in the presence of residual mdm-2. Recently, Zhang and Xiong (54) identified a nuclear export signal in the amino terminus of p53 (residues 11 to 27). Furthermore, they demonstrated that this export signal is inhibited by phosphorylation of p53 at serine 15, allowing p53 to accumulate in the nucleus. We have demonstrated that overexpression of the ATR kinase-dead mutant significantly reduced the level of stabilized p53 protein induced by hypoxia. Phosphorylation of the amino terminus of p53 in response to hypoxic exposure could lead to inhibition of nuclear export of p53 and hence increase p53 nuclear protein levels. We have also made use of the PI3 kinase inhibitor LY294002, which inhibits both ATM and ATR kinase activities. Treatment of RKO cells with LY294002 during hypoxic exposure inhibited p53 serine 15 phosphorylation and p53 protein accumulation (data not shown). In contrast to hypoxia, LY294002 had a modest effect on p53 protein accumulation after γIR but had a more significant effect on the phosphorylation of p53 on serine 15. Thus, the hypoxia-induced ATR pathway is involved not only in modifying serine 15 but also in promoting p53 stabilization.
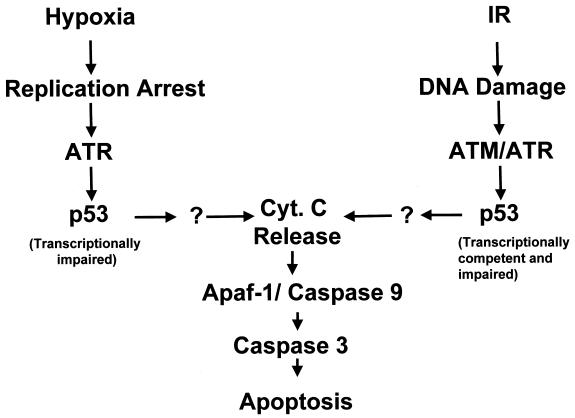
Hypoxia-induced p53 is transcriptionally impaired (30), and therefore it is unlikely that the role of serine 15 phosphorylation in this situation is to increase transactivation. Recent findings have demonstrated that p53 induced when DNA synthesis is blocked is also functionally impaired (20). This leads us to conclude that the induction of transactivation-impaired p53 is not necessarily a hypoxia-specific event but belongs to a group of stresses that induce p53 through inhibition of DNA replication. While DNA damage and strand breaks are sufficient to stimulate ATM-dependent p53 transactivation, agents that induce replication arrest and by definition affect S-phase cells result in transcriptionally impaired p53. These findings provide clear evidence that p53 can be stabilized and modified as a result of two types of stress, replication arrest and DNA damage (Fig. 8). Thus, one of the principal differences between ATM and ATR is the stress that they respond to, ATM sensing DNA damage and ATR sensing replication arrest (24, 34, 48). We have shown that many of the agents commonly used to induce p53 phosphorylation via a replication arrest also induce DNA damage to various degrees. Hypoxia is unique in that it is a stress that induces replication arrest in the absence of any detectable DNA damage. Based on the findings shown here and those of others (20, 30), it would seem that even in the presence of DNA damage induced by a replication block, p53 does not promote the transactivation of common target genes. Previously it has been shown that stalled replication forks can lead to DNA damage (7, 33). This is the probable cause of damage induced by both Hu and APH, which both stall replication forks but by different means, Hu by the depletion of ribonucleotide pools and APH by blocking DNA-polymerase α (28, 50). In contrast, hypoxia inhibits replicon initiation (18), and we speculate that this difference in the inhibition of replication can explain why there is no associated DNA damage under hypoxic conditions.
FIG. 8.
Model for the accumulation and activation of p53 by hypoxia and irradiation. Many genotoxic stresses lead to the accumulation of p53. These stresses can be divided into three groups: those that induce a replication arrest (hypoxia), those that induce DNA damage (IR and chemotherapeutic drugs), and those that do both (Hu and APH). See text for further details.
These findings raise important questions; for example, how does hypoxia induce rapid replication arrest and how does this arrest subsequently induce ATR activity? The lack of increased ATR kinase activity in response to replication stress points to its cellular partners and localization being critical for stress-induced activity. Further analysis of the ATR-containing foci in the nuclei of hypoxic cells may well lead to the identification of some of these critical partners. ATR and BRCA1 have been shown to form overlapping foci in response to APH. However, it is unclear whether all ATR-containing foci are the same. We have shown that APH induces significant DNA damage under conditions of dose level and time period that are commonly used by many investigators. The foci formed in response to replication arrest in the presence of DNA damage may well differ in composition from those formed in hypoxic cells and hence in the absence of damage. Thus, hypoxia will be a powerful tool in these studies, as it will allow investigators to study replication arrest in the absence of DNA damage.
Recent reports suggest that ATR plays a role in normal replication and that it is because of this role that ATR gene disruption leads to early embryonic lethality (5). In the absence of ATR, cells continue to cycle in the presence of incompletely replicated DNA, leading to mitotic catastrophe, implying that ATR plays a continuous role in genome surveillance and/or maintenance during DNA synthesis (5). It is noteworthy that ATR−/− and p53−/− mice do not have similar phenotypes; hence, it is unlikely that ATR functions exclusively through p53 regulation during normal embryonic development (13). However, BRCA1−/−, BRCA2−/−, RAD 51−/−, and Chk1−/− mice share with ATR−/− mice similar phenotypes of early embryonic lethality, which is consistent with the notion that this set of proteins is functionally linked during normal DNA replication (25, 34, 47, 51). While the increase in ATR function triggered by hypoxia-induced replication block is important for the regulation of p53 and apoptosis, this same ATR-dependent pathway may also regulate cell cycle checkpoints and recombinational DNA repair through interactions with the downstream target proteins, Chk1 and Rad51, respectively. These studies are currently under way. Clearly, the finding that hypoxia can modulate ATR kinase activity towards p53 and Chk1 substrates in a DNA damage-independent manner adds new insight into the important role of ATR during malignant progression. This study leads us to propose that hypoxia may select not only for transformed cells that have lost p53 functionality but also for cells that have lost key components of ATR-dependent checkpoint controls.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants CA67166 and CA88480 to A.J.G.
We thank Michael Kastan for the GM1526 and GM536 cell lines as well as the ATR dominant negative constructs and Richard Glynne (Eos Biotechnologies, South San Francisco) for assistance with the generation of microarray data.
REFERENCES
- 1.Alarcon, R., C. Koumenis, R. K. Geyer, C. G. Maki, and A. J. Giaccia. 1999. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 59:6046-6051. [PubMed] [Google Scholar]
- 2.An, W. G., M. Kanekal, M. C. Simon, E. Maltepe, M. V. Blagosklonny, and L. M. Neckers. 1998. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392:405-408. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft, M., Y. Taya, and K. H. Vousden. 2000. Stress signals utilize multiple pathways to stabilize p53. Mol. Cell. Biol. 20:3224-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 5.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. M., and A. J. Giaccia. 1998. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58:1408-1416. [PubMed] [Google Scholar]
- 7.Brox, L., D. Hunting, and A. Belch. 1984. Aphidicolin and deoxycoformycin cause DNA breaks and cell death in unstimulated human lymphocytes. Biochem. Biophys. Res. Commun. 120:959-963. [DOI] [PubMed] [Google Scholar]
- 8.Bruick, R. K. 2000. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc. Natl. Acad. Sci. USA 97:9082-9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canman, C. E., and D. S. Lim. 1998. The role of ATM in DNA damage responses and cancer. Oncogene 17:3301-3308. [DOI] [PubMed] [Google Scholar]
- 10.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 11.Chao, C., S. Saito, C. W. Anderson, E. Appella, and Y. Xu. 2000. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc. Natl. Acad. Sci. USA 97:11936-11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Klein, A., M. Muijtjens, R. van Os, Y. Verhoeven, B. Smit, A. M. Carr, A. R. Lehmann, and J. H. Hoeijmakers. 2000. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10:479-482. [DOI] [PubMed] [Google Scholar]
- 13.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 14.Dorie, M. J., M. S. Kovacs, E. C. Gabalski, M. Adam, Q. T. Le, D. A. Bloch, H. A. Pinto, D. J. Terris, and J. M. Brown. 1999. DNA damage measured by the comet assay in head and neck cancer patients treated with tirapazamine. Neoplasia 1:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairbairn, D. W., P. L. Olive, and K. L. O'Neill. 1995. The comet assay: a comprehensive review. Mutat. Res. 339:37-59. [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi, K., S. Tomoyasu, H. Watanabe, S. Kaetsu, N. Tsuruoka, and K. Gomi. 1995. Iron deprivation results in an increase in p53 expression. Biol. Chem. Hoppe-Seyler 376:627-630. [DOI] [PubMed] [Google Scholar]
- 17.Garayoa, M., A. Martinez, S. Lee, R. Pio, W. G. An, L. Neckers, J. Trepel, L. M. Montuenga, H. Ryan, R. Johnson, M. Gassmann, and F. Cuttitta. 2000. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol. Endocrinol. 14:848-862. [DOI] [PubMed] [Google Scholar]
- 18.Gekeler, V., J. Epple, G. Kleymann, and H. Probst. 1993. Selective and synchronous activation of early-S-phase replicons of Ehrlich ascites cells. Mol. Cell. Biol. 13:5020-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 20.Gottifredi, V., S. Shieh, Y. Taya, and C. Prives. 2001. p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc. Natl. Acad. Sci. USA 98:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88-91. [DOI] [PubMed] [Google Scholar]
- 22.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. Fornace, Jr., and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, S. L., R. A. Freiberg, and A. J. Giaccia. 2001. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol. Cell. Biol. 21:1196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakem, R., J. L. de la Pompa, C. Sirard, R. Mo, M. Woo, A. Hakem, A. Wakeham, J. Potter, A. Reitmair, F. Billia, E. Firpo, C. C. Hui, J. Roberts, J. Rossant, and T. W. Mak. 1996. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell 85:1009-1023. [DOI] [PubMed] [Google Scholar]
- 26.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 27.Hockel, M., C. Knoop, K. Schlenger, B. Vorndran, E. Baussmann, M. Mitze, P. G. Knapstein, and P. Vaupel. 1993. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 26:45-50. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami, S., T. Taguchi, M. Ohashi, M. Oguro, H. Nagano, and Y. Mano. 1978. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature 275:458-460. [DOI] [PubMed] [Google Scholar]
- 29.Kastan, M. B., and D. S. Lim. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 30.Koumenis, C., R. Alarcon, E. Hammond, P. Sutphin, W. Hoffman, M. Murphy, J. Derr, Y. Taya, S. W. Lowe, M. Kastan, and A. Giaccia. 2001. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21:1297-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 32.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 33.Li, J. C., and E. Kaminskas. 1987. Progressive formation of DNA lesions in cultured Ehrlich ascites tumor cells treated with hydroxyurea. Cancer Res. 47:2755-2758. [PubMed] [Google Scholar]
- 34.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 35.Maki, C. G., and P. M. Howley. 1997. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol. Cell. Biol. 17:355-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meek, D. W. 1998. Multisite phosphorylation and the integration of stress signals at p53. Cell. Signal. 10:159-166. [DOI] [PubMed] [Google Scholar]
- 37.Momand, J., G. P. Zambetti, D. C. Olson, D. George, and A. J. Levine. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69:1237-1245. [DOI] [PubMed] [Google Scholar]
- 38.Mosner, J., T. Mummenbrauer, C. Bauer, G. Sczakiel, F. Grosse, and W. Deppert. 1995. Negative feedback regulation of wild-type p53 biosynthesis. EMBO J. 14:4442-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyholm, S., G. J. Mann, A. G. Johansson, R. J. Bergeron, A. Graslund, and L. Thelander. 1993. Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J. Biol. Chem. 268:26200-26205. [PubMed] [Google Scholar]
- 40.Olive, P. L., J. P. Banath, and R. E. Durand. 1990. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 122:86-94. [PubMed] [Google Scholar]
- 41.Prives, C. 1998. Signaling to p53: breaking the MDM2-p53 circuit. Cell 95:5-8. [DOI] [PubMed] [Google Scholar]
- 42.Ravi, R., B. Mookerjee, Z. M. Bhujwalla, C. H. Sutter, D. Artemov, Q. Zeng, L. E. Dillehay, A. Madan, G. L. Semenza, and A. Bedi. 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 14:34-44. [PMC free article] [PubMed] [Google Scholar]
- 43.Reichard, P., and A. Ehrenberg. 1983. Ribonucleotide reductase-a radical enzyme. Science 221:514-519. [DOI] [PubMed] [Google Scholar]
- 44.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325-334. [DOI] [PubMed] [Google Scholar]
- 45.Siim, B. G., G. J. Atwell, R. F. Anderson, P. Wardman, S. M. Pullen, W. R. Wilson, and W. A. Denny. 1997. Hypoxia-selective antitumor agents. 15. Modification of rate of nitroreduction and extent of lysosomal uptake by polysubstitution of 4-(alkylamino)-5-nitroquinoline bioreductive drugs. J. Med. Chem. 40:1381-1390. [DOI] [PubMed] [Google Scholar]
- 46.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki, A., J. L. de la Pompa, R. Hakem, A. Elia, R. Yoshida, R. Mo, H. Nishina, T. Chuang, A. Wakeham, A. Itie, W. Koo, P. Billia, A. Ho, M. Fukumoto, C. C. Hui, and T. W. Mak. 1997. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 11:1242-1252. [DOI] [PubMed] [Google Scholar]
- 48.Takimoto, R., and W. S. El-Deiry. 2001. DNA replication blockade impairs p53-transactivation. Proc. Natl. Acad. Sci. USA 98:781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timson, J. 1975. Hydroxyurea. Mutat. Res. 32:115-132. [DOI] [PubMed] [Google Scholar]
- 51.Tsuzuki, T., Y. Fujii, K. Sakumi, Y. Tominaga, K. Nakao, M. Sekiguchi, A. Matsushiro, Y. Yoshimura, and MoritaT. 1996. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 93:6236-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watters, D., K. K. Khanna, H. Beamish, G. Birrell, K. Spring, P. Kedar, M. Gatei, D. Stenzel, K. Hobson, S. Kozlov, N. Zhang, A. Farrell, J. Ramsay, R. Gatti, and M. Lavin. 1997. Cellular localisation of the ataxia-telangiectasia (ATM) gene product and discrimination between mutated and normal forms. Oncogene 14:1911-1921. [DOI] [PubMed] [Google Scholar]
- 53.Wenger, R. H., G. Camenisch, I. Desbaillets, D. Chilov, and M. Gassmann. 1998. Up-regulation of hypoxia-inducible factor-1alpha is not sufficient for hypoxic/anoxic p53 induction. Cancer Res. 58:5678-5680. [PubMed] [Google Scholar]
- 54.Zhang, Y., and Y. Xiong. 2001. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292:1910-1915. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, H., and H. Piwnica-Worms. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong, H., and J. W. Simons. 1999. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem. Biophys. Res. Commun. 259:523-526. [DOI] [PubMed] [Google Scholar]