Abstract
Objective:
To determine whether the elimination of bile reflux in the established esophagojejunostomy model of Barrett's esophagus (BE) will reduce or eliminate the risk of developing esophageal adenocarcinoma.
Summary Background Data:
Reflux of duodenal juice as well as gastric acid plays an important role in the pathogenesis of BE and adenocarcinoma. Duodenoesophageal reflux (DER) per se induces these diseases without carcinogen. However, it is unclear whether antireflux surgery induces regression of BE and prevents adenocarcinoma.
Methods:
Two hundred F344 male rats underwent one of following 3 operations: (1) total gastrectomy and esophagojejunostomy to induce DER, followed by killing after 20 (n = 13), 30 (n = 12), and 50 weeks (n = 30); (2) biliary diversion procedure, converted to Roux-en-Y method, to avoid bile regurgitation into the esophagus at 20 (n = 29) and 30 weeks (n = 32) after the operation to induce DER, followed by killing 50 weeks after initial operation; or (3) total gastrectomy and Roux-en-Y esophagojejunostomy followed by killing after 50 weeks served as controls (n = 28).
Results:
BE developed in more than half of the animals exposed to DER for 20 weeks, in more than 90% of rats with DER for 30 weeks, and in 100% of animals exposed to DER for 50 weeks. In the incidence and the length of BE, there is no difference between the animals that underwent biliary diversion at 20 (62%) and 30 weeks (94%) and those that had DER for 20 (54%) and 30 weeks (92%), respectively. Incidence of adenocarcinoma was significantly lower in the rats that underwent the biliary diversion procedure after 30 (19%) and 20 weeks (3%) than in the rats that had DER for 50 weeks (60%) (P < 0.005). None of the control animals that underwent Roux-en-Y esophagojejunostomy developed BE and carcinoma.
Conclusions:
It is likely that the converting procedure from the esophagojejunostomy to induce DER to biliary diversion does not lead to regression of BE but prevents the development of esophageal adenocarcinoma in the rats.
In an experimental study in rats, duodenoesophageal reflux caused Barrett's esophagus and eventually esophageal adenocarcinoma. Interruption of reflux by the biliary diversion procedure prevented carcinogenesis but esophageal metaplasia did not revert.
The incidence of esophageal adenocarcinoma has increased rapidly in the past few decades,1,2 and the major risk factor for esophageal adenocarcinoma is Barrett's esophagus (BE).1,3 BE, also known as columnar-lined esophagus, is a condition in which the normal squamous epithelial lining of the esophagus is damaged by chronic gastroesophageal reflux and replaced by metaplastic columnar epithelium.4,5 Several authors have shown that not only gastric, but also duodenal, reflux plays an important role in the pathogenesis of BE6–9 and adenocarcinoma.10–12 Duodenoesophageal reflux (DER) has been shown to promote esophageal carcinogenesis in the experimental model.13–16 More recent work has demonstrated that duodenal or duodenogastric reflux per se causes BE and esophageal carcinoma in rats, even without exposure to carcinogens.17–22 Conversely gastric reflux alone does not cause this metaplasia-carcinoma sequence.18 These experimental observations emphasize the importance of exposure to duodenal contents in carcinogenesis of the esophageal adenocarcinoma.
The effect of antireflux surgery on BE is controversial. Complete regression of BE23–26 and a decreased risk of dysplasia and cancer25–31 after antireflux surgery have been cited in several reports. However, many other reports found that antireflux surgery rarely leads to the complete regression of BE.24,28,30–32 A review of the English literature by DeMeester33 found that 74% of 340 patients who underwent antireflux surgery had no change in their BE, and 9% had progression of BE, whereas complete and partial regression occurred in only 4% and 12% of patients, respectively. However, some investigators have shown that progression from BE to esophageal carcinoma might be prevented by antireflux surgery.25–28 In contrast, a recent nationwide population-based retrospective cohort study revealed that the risk of developing esophageal adenocarcinomas remained higher than in the general population after antireflux surgery.34 Consequently, it remains to be determined whether antireflux surgery induces the regression of BE and prevention of carcinoma.
The aim of this study is to investigate experimentally whether the biliary diversion procedure as an antireflux intervention leads to regression of BE induced by DER and whether it prevents the subsequent development of esophageal adenocarcinoma in rats.
MATERIALS AND METHODS
Experimental Animals
The Committee on Animal Experimentation of Kanazawa University, Takara-machi Campus approved this animal study. Every effort was made to minimize animal suffering and reduce the number of animals used. F344 male rats weighing approximately 200 g were used. The animals were housed 3 to a cage and maintained under conditions of 22 ± 3°C and 55 ± 5% humidity with a 12-hour light-dark cycle. They were fed a standard solid chow CRF-1 (Charles River, Japan) and tap water free of carcinogens.
Surgical Procedure
After a 24-hour fast, an upper abdominal incision was made under diethyl ether inhalation anesthesia. Then, one of the surgical procedures illustrated in Figure 1 was performed. The rats were divided into 6 groups according to the operation performed (Fig. 2).
FIGURE 1. Schematic diagrams illustrating the surgical procedures performed in this study.

FIGURE 2. Experimental design. BD, biliary diversion procedure; K, killing.
All intestinal anastomoses were performed using 4 to 6 interrupted full-thickness sutures in a line using 7-0 silk-braided sutures on an atraumatic needle. All animals had free access to both water and food beginning 24 to 48 hours after surgery.
Duodenoesophageal reflux group (the DER group; n = 80). Total gastrectomy was performed, and the duodenal stump was sutured. The esophageal stump was anastomosed to the jejunum approximately 4 cm distal to the ligament of Treitz in the end-to-side fashion. This procedure permitted reflux of duodenal contents into the esophagus through the stoma. The DER20, DER30, and DER50 groups were defined as subgroups of animals that were killed at 20, 30, and 50 weeks after surgery, respectively.
Biliary diversion procedure group (the BD group; n = 80). The animals of this group were subjected to the same surgical procedure as those of the DER groups at the first operation, and the reconstruction of intestinal continuity was converted to Roux-en-Y type to avoid bile regurgitation into esophagus at the second time. This group consisted of 2 subgroups of BD20 and BD30. The timing of conversion of BD20 and BD30 groups were designed at 20 and 30 weeks after the first operation, respectively. The animals were killed 50 weeks after the initial operation.
Roux-en-Y procedure group (the RY group; n = 40). After total gastrectomy, the duodenal stump was sutured. The jejunum was divided approximately 4 cm distal to the ligament of Treitz, and the distal end was sutured. The esophageal stump was anastomosed to the distal jejunum near its sutured end in the end-to-side fashion. The oral jejunal cut end was anastomosed to the jejunum approximately 10 cm distal to the esophagojejunal anastomosis in the side-to-side fashion. This procedure prevented DER. The animals were killed 50 weeks after surgery.
Pathologic Evaluation
After the animals were killed by diethyl ether inhalation, the abdomen was opened. A ligation suture was placed around the afferent and the efferent jejunal loop near the esophagojejunal stoma, and the esophagus was ligated at the level of the thyroid cartilage through a thoracotomy. The esophagus and the anastomosed jejunum were removed in continuity.
Excised organs were washed clean with 10% formalin, spread, and pinned on a cork plate with the mucosal side up. After fixation in a 10% formalin solution for 24 hours, the esophagus was cut at 3-mm intervals along its length, embedded in paraffin, and 5-μm sections of each block were prepared for histologic evaluation using hematoxylin and eosin staining.
Definition of Pathologic Findings
Esophageal histology was classified into one of the following 4 categories:
Erosion. Erosion is defined as defects in the epithelium.
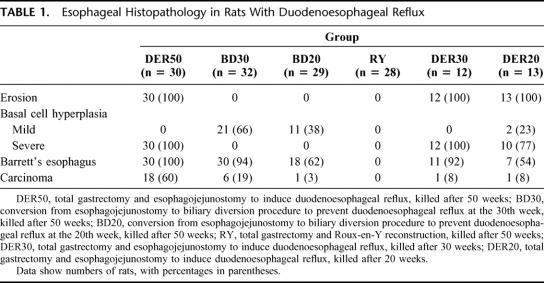
Basal cell hyperplasia. Here, the basal layer in the squamous epithelium is thickened and occupies more than 15% of the epithelial layer (Fig. 3A). These findings were graded into “mild” or “severe” according to the length of the lesions (mild: <3 mm, and severe: ≥3 mm).
Barrett’s esophagus. Esophageal squamous epithelium is replaced with columnar-lined epithelium comprising absorptive cells with brush borders and goblet cells. Mucus glands similar to the pyloric glands are present in the mucosa (Fig. 3B-D).
Adenocarcinoma. Carcinoma is defined as an epithelial growth with cellular and structural atypism. Adenocarcinoma is characterized by dysplastic glandular cell growth with both atypia and invasiveness. Lakes of extracellular mucin are observed (Fig. 3E and F).
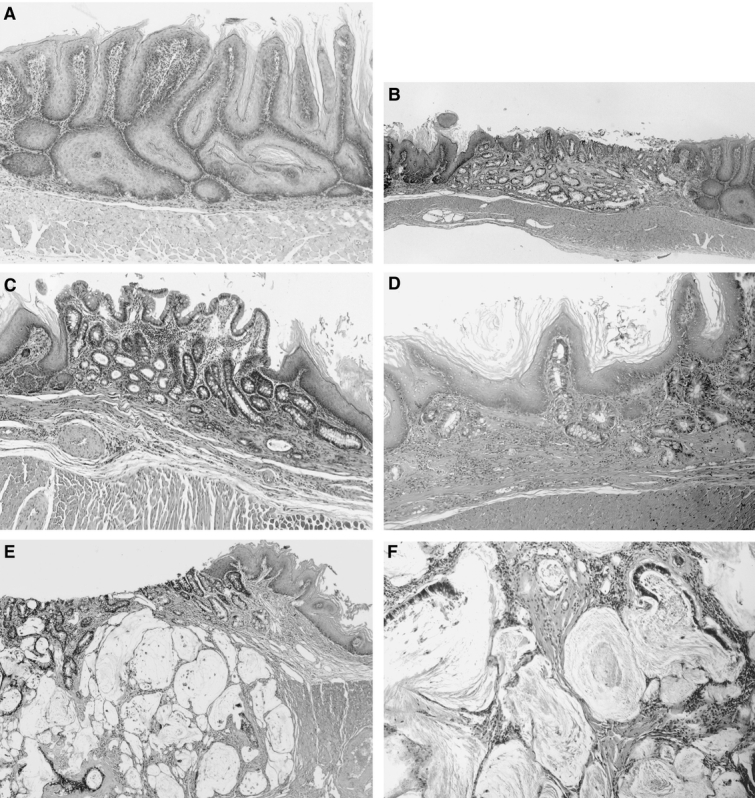
FIGURE 3. Photomicrographs showing esophageal histopathology. (Hematoxylin and eosin) A, Basal cell hyperplasia in the midesophagus of a rat from the DER20 group (×100). B, BE in a rat from the DER30 group. Columnar epithelium consists of absorptive cells with brush borders and goblet cells (×40). C, BE in a rat from the BD30 group (×100). D, Columnar cells under generating squamous epithelium are seen in a rat from the BD20 group (×100). E and F, Mucinous esophageal adenocarcinoma characterized by malignant glands associated with lakes of extracellular mucin in a rat from the DER50 group. The tumor is invading the adventitia (E, ×40; F, ×100). DER20, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 20 weeks; DER30, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 30 weeks; BD30, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 30th week, killed after 50 weeks; BD20, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 20th week, killed after 50 weeks; DER50, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 50 weeks.
Hepatobiliary Scintigraphy
Five animals in each group received an intravenous injection of 37 MBq of [99mTc]N-pyridoxyl-5-methyltryptophan (99mTc-PMT: Japan Mediphics, Japan) under diethyl ether anesthesia for serial hepatobiliary scanning in the supine position using a gamma camera. Pictures were taken every 2.5 minutes after radioisotope injection for 120 minutes.
Statistical Analysis
Data are presented as the mean ± SD. We used the χ2 test and Fisher exact test to compare categorical data. Differences in the means of continuous variables were tested by Student t test and checked by the Mann–Whitney U test. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Effective Animals
The effective number of animals was 30 in the DER50 group, 32 in the BD30, 29 in the BD20, 28 in the RY, 12 in the DER30, and 13 in the DER20. The mortality rate was 28% (56 of 200). Twenty-three rats died of respiratory failure before the scheduled of termination. This was probably the result of pulmonary aspiration secondary to reflux. Other causes of death were malnutrition (n = 12), peritonitis (n = 5), and unknown causes (n = 8). Eight rats died after the converting procedure as a result of malnutrition (n = 5) or unknown causes (n = 3). At the end of the experimental period, 144 rats were evaluated.
Hepatobiliary Scintigraphy
Among animals in the DER groups, 99mTc-PMT began to flow into the lower esophagus after 9.5 ± 2.1 minutes. Reflux into the lower two thirds of esophagus occurred after 23.5 ± 2.9 minutes and persisted during the entire 120-minute study period. No esophageal reflux of the 99mTc-PMT was observed during the 120-minute examination among animals in the BD groups or the RY group.
Macroscopic Findings
The esophageal wall of all animals in the DER groups was thickened, and the epithelial surface contained longitudinal folds extending along the lower two thirds of the esophagus (Fig. 4A). These findings represented gross evidence of severe esophagitis. In contrast, the esophagi of animals in the RY group had none of these findings, and surface was whitish, smooth, and glistening. There was a clearly defined anastomic border between the esophageal and the jejunal mucosa (Fig. 4B). Specimens from the DER50, DER30, and DER20 groups showed greater esophageal dilatation than specimens from the RY, BD30, or BD20 groups (P < 0.05; data not shown). Some nodular lesions in the lower esophagus were observed in all animals in the DER50 group (Fig. 4C). The nodular lesions were associated with carcinoma and basal cell hyperplasia. The esophagi of animals in the BD30 and BD20 groups were grossly normal, as were the esophagi of animals in the RY group, except for a small area of coarse thickening just proximal to the anastomosis (Fig. 4D). The incidence of coarse thickened area was 63% (20/32) and 31% (9/29) in the BD30 and BD20 groups, respectively. The coarse areas were associated with basal cell hyperplasia.
FIGURE 4. Macroscopic appearance of a resected esophagus of a rat from the DER30 group (A), from the RY group (B), from the DER50 group (C), and from the BD30 group (D). DER30, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 30 weeks; RY, total gastrectomy and Roux-en-Y reconstruction, killed after 50 weeks; DER50, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 50 weeks; BD30, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 30th week, killed after 50 weeks.
Histologic Findings
The results of the histologic examination of the specimens are shown in Table 1. All specimens from the DER50 and DER30 groups showed erosion and severe basal cell hyperplasia. In contrast, erosion and severe basal cell hyperplasia were not found in any of the animals in the BD30, BD20, or RY groups. The incidence of BE was 100%, 92%, and 94% in the animals in the DER50, BD30, and DER30 groups, respectively, and these percentages were significantly higher than in animals in the DER20 (54%), BD20 (62%), and RY groups (0%; P < 0.01; Fig. 5). The incidence of BE in the BD30 and DER30 groups and in the BD20 and DER20 groups were similar. Moreover, the mean length of columnar epithelium in the BD30 (2.7 ± 1.3 mm) and the DER30 group (2.3 ± 1.0 mm) and in the BD20 (1.9 ± 1.2 mm) and the DER20 group (1.1 ± 0.4 mm) also was similar (Fig. 6). The incidence of adenocarcinoma was significantly lower in the BD30 group and the BD20 group than in the DER50 group (P < 0.005; Fig. 7). Carcinoma was found in only one rat each in the BD20, DER30, and DER20 groups. No animal in the RY group had BE or carcinomas.
TABLE 1. Esophageal Histopathology in Rats With Duodenoesophageal Reflux
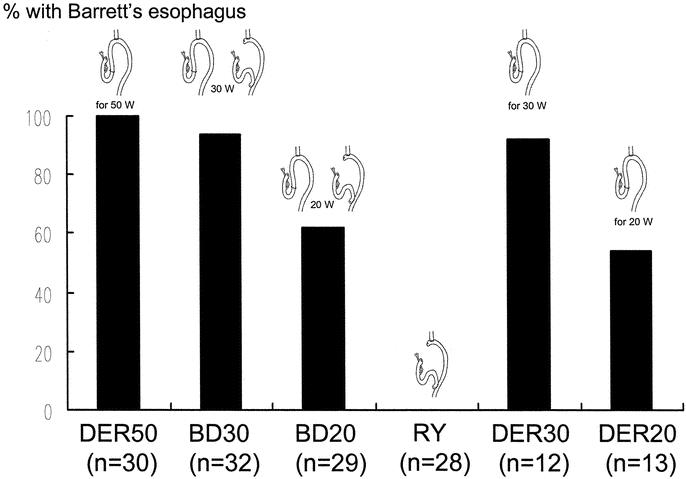
FIGURE 5. The incidence of BE. The DER50 group versus the BD20, DER20, and RY group: P < 0.001. The BD30 group versus the BD20, DER20, and RY group: P < 0.01. The BD20 or DER20 group versus the RY group: P < 0.001. DER50, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 50 weeks; BD30, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 30th week, killed after 50 weeks; BD20, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 20th week, killed after 50 weeks; RY, total gastrectomy and Roux-en-Y reconstruction, killed after 50 weeks; DER30, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 30 weeks; DER20, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 20 weeks.
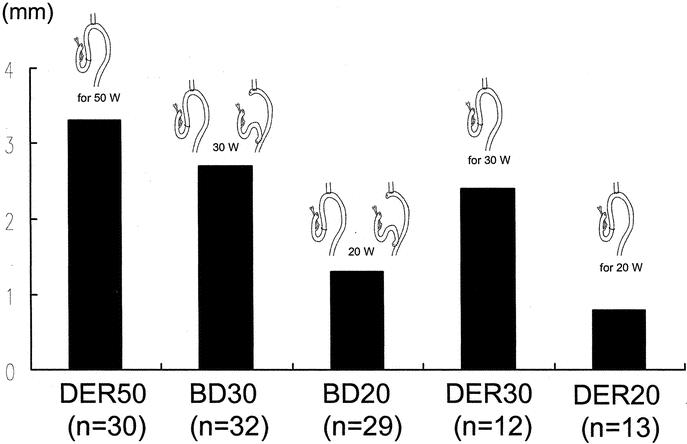
FIGURE 6. The mean length of BE. The DER50 or BD30 group versus the BD20 or DER20 group: P < 0.05. The DER30 group versus the DER20 group: P < 0.05. DER50, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 50 weeks; BD30, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 30th week, killed after 50 weeks; BD20, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 20th week, killed after 50 weeks; DER30, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 30 weeks; DER20, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 20 weeks.
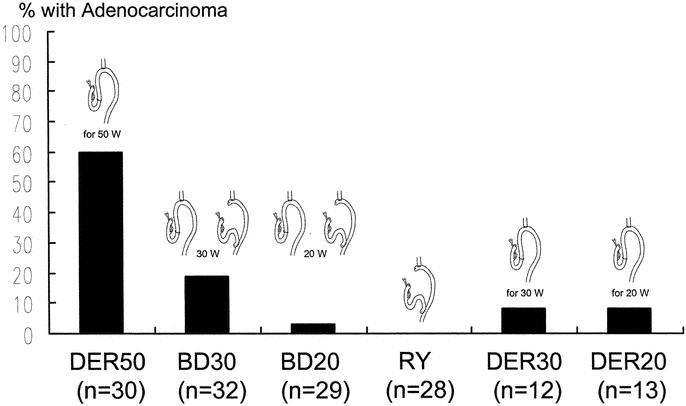
FIGURE 7. The incidence of adenocarcinoma. The DER50 group versus all other groups: P < 0.005. The BD30 group versus the RY group: P < 0.05. DER50, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 50 weeks; BD30, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 30th week, killed after 50 weeks; BD20, conversion from esophagojejunostomy to biliary diversion procedure to prevent duodenoesophageal reflux at the 20th week, killed after 50 weeks; RY, total gastrectomy and Roux-en-Y reconstruction, killed after 50 weeks; DER30, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 30 weeks; DER20, total gastrectomy and esophagojejunostomy to induce duodenoesophageal reflux, killed after 20 weeks.
DISCUSSION
Our previous study18,21 demonstrated that duodenal and duodenogastric contents induce not only erosive esophagitis and BE but also esophageal adenocarcinoma in rats. In contrast, gastric contents alone induced BE at low incidence and did not induce any esophageal adenocarcinomas.18 This finding shows that duodenal content, not gastric content, plays the major role in the genesis of BE and subsequently esophageal adenocarcinoma in rats. In the present study, we used a model in which the duodenal contents alone regurgitated into the esophagus and evaluated the effect of antireflux surgery (biliary diversion procedure) on esophageal metaplasia. Twenty, 30, and 50 weeks after the operation that caused DER, reflux esophagitis with erosion and basal cell hyperplasia surrounded areas of BE and esophageal adenocarcinoma. Erosive esophagitis was observed in all rats killed 20 weeks after DER. BE developed in more than half the rats killed 20 weeks after DER. Furthermore, more than 90% of rats with DER for 30 weeks had BE. In the incidence and the length of BE, there is no difference between the animals who underwent biliary diversion at 20 and 30 weeks and those that had DER for 20 and 30 weeks, respectively. However, the incidence of esophageal carcinoma was significantly lower in the rats that underwent the converting procedure than those that had DER for 50 weeks. These findings suggest that once BE is established, it is irreversible, even if the causal agent is eliminated. However, the conversion to biliary diversion prevented progression to esophageal adenocarcinoma in rats.
The animal model is reasonably well established and generally produces adenocarcinoma only in the presence of duodenal content.14,15,18,21 However, there has been controversy whether the columnar epithelium in this animal model is truly reflective of BE. In this study, diagnosis of BE was suspected from the presence of columnar-lined epithelium comprising absorptive cells with brush borders and goblet cells and confirmed based on mucin characteristics, by periodic acid-Schiff, and alcian blue staining same as previous study17,18,22 (not shown). Mucus glands similar to the pyloric glands were also observed and neither Paneth cells nor endocrine cells were observed. The incidence of BE and adenocarcinoma increased over time of DER exposure. The adenocarcinoma always occurred near the esophagojejunostoma and always within the BE. Conversely, the esophageal specimens in the control group with Roux-en-Y reconstruction showed no evidence of BE and adenocarcinoma. The morphologic changes and characteristics of disease progression associated with BE in this animal model were similar to those in humans. Therefore, we believe that the columnar epithelium in this model is reflective of BE.
Ireland et al16 showed in a carcinogen-treated rat model that when a partial and total gastric resection was added to an esophagoduodenostomy, the prevalence of adenocarcinomas increased, concluding gastric juice may protect from the development of esophageal adenocarcinoma. Conversely, we performed total gastrectomy and did not examine the effect of gastric juice on the carcinogenesis induced by reflux because we focused on the importance of duodenal content. Therefore, one limitation of our study is that our model may be not representative of typical human gastroesophageal reflux disease physiology because the responsible refluxuate was duodenal content alone but not mixture of gastric and duodenal contents.
In some,16,19,20 but not all14,15 previous studies, the mortality rate was slightly lower than that in our study. Other studies21,22 had similar mortality as in our study. The cause of the difference in mortality rates among these studies is unclear. However, it seemed likely that the mortality rate was not so high, considering longer exposure time of DER and observing time in this study compared with that in other studies.14–16,19,20,22 In this study, death before the scheduled termination came from mainly complications of reflux esophagitis, such as respiratory failure because of pulmonary aspiration pneumonia, and malnutrition. Actually, the intensity of inflammation and tissue destruction was severe in animals that had died of reflux disease. However, these rats had no adenocarcinoma.
Öberg et al35 reported in a rat model by performing esophagoduodenostomy that adenocarcinoma appeared to arise from the submucosa and did not involve the overlying mucosa and that there was no definite evidence of BE. Furthermore, the incidence of adenocarcinoma in the esophagoduodenostomy group was similar to that in the no reflux control Roux-en-Y group. They concluded that adenocarcinoma in the animal model were not reflux-induced and did not arise from the mucosa. The reason why our model was successful where Öberg's had failed is not clear. However, there were 2 differences between our study and Öberg's study. First, the strains of rats were different. In our study, we used F344 rats because of their lower incidence of spontaneous intestinal adenocarcinoma (0.056%)36 compared with that in the Sprague–Dawley rats (0.8%)37 used in Öberg's study. Furthermore, Tatematsu et al38 examined the incidence of gastric carcinoma in 5 different strains of rats treated with exogenous carcinogen. The incidence of carcinomas in F344 rats (6%) was significantly lower than that in Sprague–Dawley rats (60%). We speculate that the tumors related to the performance of an anastomosis between esophageal squamous and enteric columnar mucosa may easily occur in Sprague–Dawley rats. Second, the anastomosis to induce DER was different. In our esophagojejunostomy model, DER was severe to induce BE and carcinoma confirmed by hepatobiliary scintigraphy. However, in Öberg's esophagoduodenostomy model, a part of duodenal content might drain into anal side of duodenum without esophageal exposure. Therefore, DER may not be enough to contribute the reflux-induced esophageal carcinogenesis in BE.
The effect of antireflux surgery on regression of BE remains controversial in the clinical study. Moreover, the more important question as to whether antireflux surgery reduces the risk of carcinoma has not resolved yet either. Brand et al in 198023 described complete regression of BE after antireflux surgery in 4 of 10 patients. Skinner et al in 198339 reported that successful antireflux surgery leads to stabilization and possibly regression of the dysplasia in BE. Subsequently, a number of articles reported that BE did not regress completely in most cases after antireflux surgery.27,30,31,33,34 Sagar et al24 examined the effect of antireflux surgery on BE in a study involving 56 patients with histologically confirmed BE who had symptoms of gastroesophageal reflux refractory to medical therapy. Twenty-four patients had partial or complete regression of BE during a median follow-up of 5.5 years. Complete regression was observed in only 5 patients. However, 9 patients showed progression of the length of columnar mucosa, and adenocarcinoma developed in 1 patient who had persistent symptoms for 9 years after antireflux surgery. The length of BE remained unchanged in 23 patients. The authors concluded that BE might be reversible if causal agents are eliminated early in metaplasia but that patients with BE who undergo antireflux surgery rarely show complete regression. Recently, Bowers et al26 reported the greater incidence (47%) of regression of BE after successful antireflux surgery than that in previous studies. They suspected that this is a result of longer follow-up and the inclusion of patients with short-segment BE. None of the patients have developed high-grade dysplasia or esophageal carcinoma during surveillance endoscopy (377 total patient-years of follow-up). However, several studies have demonstrated that antireflux surgery is more effective in preventing the progression to dysplasia and cancer than medical therapy. McCallum et al27 studied 181 patients with BE prospectively to determine whether antireflux surgery decreases the incidence of esophageal dysplasia and adenocarcinoma more than medical therapy does. Adenocarcinoma did not develop in any of the 29 patients who underwent antireflux surgery, whereas cancer occurred in 2 of the 152 patients who received medical therapy, although the average observation period was longer in the former than in the latter group (62 vs. 49 months). In addition, the incidence of dysplasia was significantly lower in the group that underwent antireflux surgery (3.4%) than the group that received medical therapy (19.7%). Subsequently, Ortiz et al28 randomized 59 patients with BE to receive medical treatment (n = 27; median follow-up of 4 years) or antireflux surgery (n = 32; median follow-up of 5 years). A decrease in the length of BE was recorded in 8 patients in the surgical group but in only 2 in the medical group. However, none of the patients in either group achieved complete regression. Conversely, progression of BE was more frequent in the medical group (11 vs. 3). Dysplasia appeared in 6 patients in the medical group (mild in 5 and severe in 1), whereas severe dysplasia occurred in only 1 patient in surgical group who had a failed fundoplication, documented by 24-hour pH monitoring. The result of the latest study,31 which included 101 patients with a longer follow-up, confirmed those Ortiz et al28 have published previously. The metaplastic segment did not disappear in any case. High-grade dysplasia appeared in 2 of the 43 patients (5%) in the medical treatment group and in 2 of the 58 patients (3%) in the surgical treatment group. In the latter, both patients presented with clinical and pH-metric recurrence. There was no case of malignancy after successful antireflux surgery. Similarly, Katz et al29 reviewed records of 102 patients with BE who underwent endoscopic surveillance for 563 patient-years. In their study, none of the 15 patients who had undergone antireflux surgery developed dysplasia. These studies suggest that antireflux surgery for BE is superior to medical treatment in the prevention of esophageal carcinogenesis.
The present results, that antireflux surgery does not induce regression of BE but does reduce the incidence of adenocarcinoma, are similar to clinical observations. More recent work has demonstrated that antireflux surgery for patients with BE cannot only prevent malignant transformation but also can lead to regression of dysplastic change. Low et al30 followed 14 patients with BE who underwent successful antireflux surgery for a mean of 25.1 months. Ten patients showed partial regression of BE, and short segments of BE disappeared completely in 2 patients. No patients showed progression of dysplastic change, and 4 patients had regression of low-grade dysplasia to BE without dysplasia after antireflux surgery. Another study25 on the effect of antireflux surgery followed 97 patients with BE and found that low-grade dysplasia regressed to nondysplastic BE in 7 of 16 (44%) patients who had dysplasia before antireflux surgery, and intestinal metaplasia regressed completely in 9 (14%) patients. Low-grade dysplasia developed in 4 (6%) patients. No patient developed high-grade dysplasia or cancer in 410 patient-years of follow-up. Consequently, preponderance of evidence suggests that effective surgical treatment to eliminate duodenogastroesophageal reflux suppresses the progression from BE to dysplasia to carcinoma.
In contrast, several studies have found that some patients may still be at risk for the development of carcinoma even after antireflux surgery. Williamson et al32 followed 37 patients with BE after antireflux surgery and found that no patients had complete regression of BE, although 4 patients (11%) demonstrated endoscopic evidence of partial regression. Despite symptomatic and endoscopic improvement of reflux esophagitis after antireflux surgery, mild dysplasia developed in 4 patients, and carcinoma developed in 3 patients (8.1%). Csendes et al40 reported that low-grade dysplasia and adenocarcinoma developed in 15 and 4 patients, respectively, during 8 years of follow-up in their prospective study that included 152 patients with BE who underwent antireflux surgery. However, all of these patients had unsuccessful antireflux surgery. Furthermore, a recent nationwide population-based retrospective cohort study found that the risk of developing esophageal adenocarcinoma remained supranormal after antireflux surgery.34 The size of that study group was the largest to date, and almost all patients were followed for up to 32 years. However, the authors could not stratify patients according to whether BE was present. Moreover, McDonald et al41 reported the outcome of 113 patients with BE who underwent an antireflux procedure. Three patients developed esophageal adenocarcinoma 13, 25, and 39 months after the antireflux procedure. These 3 patients presented with adenocarcinoma in the early follow-up period, given that the median follow-up was 6.5 years and was as long as 18.2 years. The authors hypothesized that these 3 carcinomas were occult at the time when the antireflux procedure was performed, and that no new cancers developed during the late follow-up period. We also observed the development of esophageal adenocarcinomas in some rats even after the biliary diversion procedure. There are several possible explanations for this result. One possibility is that these carcinomas had already occurred by the time of conversion to biliary diversion. Another possible explanation is that genetic changes leading to the development of carcinoma had occurred before antireflux surgery, and that the carcinogenic process continued even though duodenal contents no longer refluxed.
Recent clinical analyses, especially using fiber-optic sensors to detect the presence of bilirubin (Bilitec 2000, Synectics, Sweden), suggest the importance of bile reflux as a cause of BE6,7 and other complications, such as stenosis, ulceration, and dysplasia,8,9 and ultimately adenocarcinoma.10,11 These findings demonstrate a relationship between duodenal contents reflux and carcinogenesis in BE. In 1987, DeMeester et al42 introduced a new operation for permanently controlling pathologic duodenogastric reflux called the duodenal switch procedure. The authors performed bile diversion by suprapapillary Roux-en-Y duodenojejunostomy for the treatment of alkaline reflux gastritis. Recently, Csendes et al43 have extended the indications for the duodenal switch procedure to include patients with BE caused by severe reflux esophagitis. The authors have prospectively evaluated this procedure in the treatment of 65 patients with BE. In their study, Bilitec analysis showed complete elimination of bilirubin reflux into the esophagus and endoscopic evidence of improvement in erosive esophagitis, and dysplastic changes disappeared in 3 of 7 patients. However, the authors did not study whether this procedure leads to regression of BE and prevents carcinoma.
Recently, a number of studies have investigated the molecular pathogenesis of carcinogenesis in BE. Genetic abnormality in BE may occur at the tumor suppressor gene level or the cellular cycle level during a relatively early stage, regardless of the presence or absence of dysplasia and cancer. For example, p53 tumor suppressor gene mutations have been reported in a patient with BE who had neither dysplasia nor cancer.44,45 Elevated levels of cyclo-oxygenase-2 (COX-2) expression, which is important in cell survival, have been detected in BE.46 Moreover, Buttar et al22 revealed that selective and nonselective COX-2 inhibitors can inhibit the development of adenocarcinoma induced by reflux in a rat model of BE. We hypothesize that these various genetic changes may also occur in the rats that developed BE in our study, and that continuous contact with duodenal contents is not necessary to maintain these changes. Therefore, once BE becomes established, it is irreversible even though DER is interrupted. However, such genetic changes need to be documented biochemically.
Bile acids alone do not appear to be carcinogenic because they did not act as a mutagen in a mutagenesis assay.47 However, a promoting effect of bile acids in carcinogenesis has been demonstrated in colon cancer in rats.48 Esophageal carcinogenesis caused by duodenal-content reflux in the absence of a carcinogen, however, cannot be fully explained by exposure to bile acids alone. Exposure to carcinogens, which act as initiators, during duodenal reflux is inferred. Bile acids may be a source of carcinogenic N-nitrosamides, such as N-nitrosotaurocholic acid and N-nitrosoglycocholic acids,49 and the esophageal mucosa is damaged by bile acids, resulting in esophagitis. Bile acids may make it easier for carcinogens to penetrate the esophageal mucosal barrier and reach the proliferative compartment of the esophageal mucosa. As a result, they become trapped within the cell, accumulate, and ultimately disrupt normal cellular function. Furthermore, at the molecular level, bile acids have been shown to activate protein kinase C and induce the transcription of the COX-2 gene.46,50 Therefore, bile acids under certain conditions may be carcinogenic. In the present study, elimination of duodenal-content reflux decreased the incidence of esophageal adenocarcinoma. This finding suggests that the initiation and promotion of a carcinogenic cascade by bile acids is necessary for BE to progress to esophageal adenocarcinoma in rats. However, the precise mechanism involved in bile-induced carcinogenesis requires further study.
In conclusion, BE and esophageal adenocarcinoma can be induced by duodenoesophageal reflux alone in rats, and the biliary diversion procedure does not lead to regression of BE once it has been established. Although care is required when extrapolating data from animal models to humans, we believe antireflux surgery to eliminate DER for BE may prevent the development of esophageal adenocarcinoma.
Footnotes
Reprints: Koichi Miwa, MD, Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, 13-1 Takaramachi, Kanazawa, 920-8641, Japan. E-mail: kmiwa@surg2.m.kanazawa-u.ac.jp.
REFERENCES
- 1.Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. [DOI] [PubMed] [Google Scholar]
- 2.Prach AT, MacDonald TA, Hopwood DA, et al. Increasing incidence of Barrett's oesophagus: education, enthusiasm, or epidemiology. Lancet. 1997;350:933. [DOI] [PubMed] [Google Scholar]
- 3.Cameron AJ, Lomboy CT, Pera M, et al. Adenocarcinoma of the esophagogastric junction and Barrett's esophagus. Gastroenterology. 1995;109:1541–1546. [DOI] [PubMed] [Google Scholar]
- 4.Spechler SJ, Goyal RK. Barrett's esophagus. N Engl J Med. 1986;315:362–371. [DOI] [PubMed] [Google Scholar]
- 5.Winter C Jr, Spurling TJ, Chobanian SJ, et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 6.Kauer WK, Peters JH, DeMeester TR, et al. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell MTP, Lawlor P, Byrne PJ, et al. Ambulatory oesophageal bile reflux monitoring in Barrett's oesophagus. Br J Surg. 1995;82:657–660. [DOI] [PubMed] [Google Scholar]
- 8.Vaezi MF, Richter JE. Synergism of acid and duodenogastroesophageal reflux in complicated Barrett's esophagus. Surgery. 1995;117:699–704. [DOI] [PubMed] [Google Scholar]
- 9.Fein M, Ireland AP, Ritter MP, et al. Duodenogastric reflux potentiates injurious effects of gastroesophageal reflux. J Gastrointest Surg. 1997;1:27–33. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski J, Hopwood D, Pringle R, et al. Increased expression of EGFR in Barrett's esophagus associated with alkaline reflux: a putative model for carcinogenesis. Am J Gastroenterol. 1993;56:1480–1483. [PubMed] [Google Scholar]
- 11.Stein HJ, Kauer WK, Feussner H, et al. Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and Nissen fundoplication. J Gastrointest Surg. 1998;2:333–341. [DOI] [PubMed] [Google Scholar]
- 12.Freedman J, Ye W, Naslund E, et al. Association between cholecystectomy and adenocarcinoma of the esophagus. Gastroenterology. 2001;121:548–553. [DOI] [PubMed] [Google Scholar]
- 13.Seto Y, Kobori O, Shimizu E, et al. The role of alkaline reflux in esophageal carcinogenesis induced by N-amyl-N-methylnitrosamine in rats. Int J Cancer. 1991;49:758–763. [DOI] [PubMed] [Google Scholar]
- 14.Attwood SEA, Smyrk TC, DeMeester TR, et al. Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery. 1992;111:503–510. [PubMed] [Google Scholar]
- 15.Pera M, Trastek VF, Carpenter HA, et al. Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg. 1993;55:1386–1393. [DOI] [PubMed] [Google Scholar]
- 16.Ireland AP, Peters JH, Smyrk TC, et al. Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg. 1996;224:358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miwa K, Segawa M, Takano Y, et al. Induction of oesophageal and forestomach carcinomas in rats by reflux of duodenal contents. Br J Cancer. 1994;70:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miwa K, Sahara H, Segawa M, et al. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269–274. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SR, Yang G, Curtis SK, et al. Development of esophageal metaplasia and adenocarcinoma in rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265–2270. [DOI] [PubMed] [Google Scholar]
- 20.Fein M, Peters JH, Chandrasoma P, et al. Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg. 1998;2:260–268. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Miwa K, Sahara H, et al. The sequential model of Barrett's esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens. Anticancer Res. 2002;22:39–44. [PubMed] [Google Scholar]
- 22.Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101–1112. [DOI] [PubMed] [Google Scholar]
- 23.Brand DL, Ylvisaker JT, Gelfand M, et al. Regression of columnar esophageal (Barrett's) epithelium after anti-reflux surgery. N Engl J Med. 1980;302:844–848. [DOI] [PubMed] [Google Scholar]
- 24.Sagar PM, Ackroyd R, Hosie KB, et al. Regression and progression of Barrett's oesophagus after antireflux surgery. Br J Surg. 1995;82:806–810. [DOI] [PubMed] [Google Scholar]
- 25.Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg. 2001;234:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowers SP, Mattar SG, Smith CD, et al. Clinical and histologic follow-up after antireflux surgery for Barrett's esophagus. J Gastrointest Surg. 2002;6:532–539. [DOI] [PubMed] [Google Scholar]
- 27.McCallum RW, Polepalle S, Davenport K, et al. Role of anti-reflux surgery against dysplasia in Barrett's esophagus [abstract]. Gastroenterology. 1991;100:A121. [Google Scholar]
- 28.Ortiz A, Martinez de Haro LF, Parrilla P, et al. Conservative treatment versus antireflux surgery in Barrett's esophagus: long-term results of a prospective study. Br J Surg. 1996;83:274–278. [PubMed] [Google Scholar]
- 29.Katz D, Rothstein R, Schned A, et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol. 1998;93:536–541. [DOI] [PubMed] [Google Scholar]
- 30.Low DE, Levine DS, Dail DH, et al. Histological and anatomic changes in Barrett's esophagus after antireflux surgery. Am J Gastroenterol. 1999;94:80–85. [DOI] [PubMed] [Google Scholar]
- 31.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett's esophagus. Ann Surg. 2003;237:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson WA, Ellis FH Jr, Gibb SP, et al. Effect of antireflux operation on Barrett's mucosa. Ann Thorac Surg. 1990;49:537–542. [DOI] [PubMed] [Google Scholar]
- 33.DeMeester SR, DeMeester TR. Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann Surg. 2000;231:303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye W, Chow W, Lagergren J, et al. Risk of adenocarcinoma of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286–1293. [DOI] [PubMed] [Google Scholar]
- 35.Öberg S, Lord RV, Peters JH, et al. Is adenocarcinoma following esophagoduodenostomy without carcinogen in the rat reflux-induced? J Surg Res. 2000;91:111–117. [DOI] [PubMed] [Google Scholar]
- 36.Goodman DG, Ward JM, Squire RA, et al. Neoplastic and nonneoplastic lesions in aging F344 rats. Toxicol Appl Phrmacol. 1979;48:237–248. [DOI] [PubMed] [Google Scholar]
- 37.Thompson SW, Huseby RA, Fox MA, et al. Spontaneous tumors in the Sprague-Dawley rat. J Natl Cancer Inst. 1961;27:1037–1057. [PubMed] [Google Scholar]
- 38.Tatematsu M, Aoki T, Inoue T, et al. Coefficient induction of pepsinogen 1-decreased pyloric glands and gastric cancers in five different strains of rats treated with N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis. 1988;9:495–498. [DOI] [PubMed] [Google Scholar]
- 39.Skinner DB, Walther BC, Riddell RH, et al. Barrett's esophagus: comparison of benign and malignant cases. Ann Surg. 1983;198:554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csendes A, Braghetto I, Burdiles P, et al. Long-term results of classic antireflux surgery in 152 patients with Barrett's esophagus: clinical, radiologic, endoscopic, manometric, and acid reflux test analysis before and late after operation. Surgery. 1998;123:645–657. [PubMed] [Google Scholar]
- 41.McDonald ML, Trastek VF, Allen MS, et al. Barrett's esophagus: does an antireflux procedure reduce the need for endoscopic surveillance? J Thorac Cardiovasc Surg. 1996;111:1135–1140. [DOI] [PubMed] [Google Scholar]
- 42.DeMeester TR, Fuchs KH, Ball CS, et al. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csendes A, Braghetto I, Burdiles P, et al. A new physiologic approach for the surgical treatment of patients with Barrett's esophagus. Ann Surg. 1997;226:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Younes M, Lebovitz RM, Lechago LV, et al. p53 protein accumulation in Barrett's metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993;105:1637–1642. [DOI] [PubMed] [Google Scholar]
- 45.Jones DR, Davidson AG, Summers CL, et al. Potential application of p53 as an intermediate biomarker in Barrett's esophagus. Ann Thorac Surg. 1994;57:598–603. [DOI] [PubMed] [Google Scholar]
- 46.Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. [DOI] [PubMed] [Google Scholar]
- 47.Silverman SJ, Andrews AW. Bile acids: Co-mutagenic activity in the salmonella-mammalian-microsome mutagenicity test. Brief communication. J Natl Cancer Inst. 1977;59:1557–1559. [DOI] [PubMed] [Google Scholar]
- 48.Reddy BS, Watanabe K, Weisburger JH, et al. Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res. 1976;37:3238–3242. [PubMed] [Google Scholar]
- 49.Busby WF, Shuker DEG, Charnley G, et al. Carcinogenicity in rats of the nitrosated bile acids conjugates N-nitrosoglycocholic acid and N-nitrosotaurocholic acid. Cancer Res. 1985;45:1367–1371. [PubMed] [Google Scholar]
- 50.Zhang F, Subbaramaiah K, Altorki N, et al. Dihydroxybile acids activate the transcription of cyclo-oxygenase-2. J Biol Chem. 1998;273:2424–2428. [DOI] [PubMed] [Google Scholar]