Abstract
Objective:
Highlight unresolved controversies in the management of Zollinger-Ellison syndrome (ZES).
Summary Background Data:
Recent studies have resolved some of the previous controversies including the surgical cure rate in patients with and without Multiple Endocrine Neoplasia-type1 (MEN1), the biological behavior of duodenal and pancreatic gastrinomas, role of imaging studies to localize tumor, and gastrectomy to manage acid output.
Methods:
Review of the literature based on computer searches in Index Medicus, Pubmed and Ovid.
Results:
Current controversies as identified in the literature include the role of endoscopic ultrasound (EUS), surgery in ZES patients with MEN1, pancreaticoduodenectomy (Whipple procedure), lymph node primary gastrinoma, parietal cell vagotomy, reoperation and surgery for metastatic tumor, and the use of minimally invasive surgical techniques to localize and remove gastrinoma.
Conclusions:
It is hoped that future studies will focus on these issues to improve the surgical management of ZES patients.
The role of surgery in the management of patients with Zollinger-Ellison syndrome is evolving. This review identifies surgical controversies that have been recently resolved and ones that require additional study.
In 1991,1 we identified unresolved surgical issues in the management of patients with ZES. Since 1991, there have been studies that have resolved some of these controversies. However, a number still exist. Resolved controversies1 include determination of percentages with and without MEN1 that are surgically cured (without Whipple resection) (Controversy 1 and 2, 1991),1 definition of the biologic behavior of duodenal versus pancreatic gastrinomas (Controversy 3, 1991),1 development of new imaging modalities (Controversy 4, 1991),1 and defining the role of gastric surgery (Controversy 5, 1991).1 However, controversies still exist in the role of surgery in both MEN1 (20% to 30%)2 and sporadic ZES (70% to 80%).2 Eight issues will be considered: lymph node primary gastrinoma; the role of endoscopic ultrasound (EUS); routine surgery in patients with ZES and MEN1; pancreaticoduodenectomy; routine lymphectomy; parietal cell vagotomy; reoperation; surgery in advanced, aggressive disease; and finally, laparoscopic or endoscopic resection of gastrinomas.
Recently Resolved or Partially Resolved Controversies
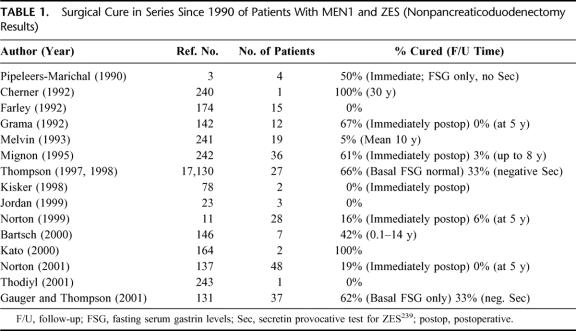
Surgical Cure Rate in Patients With ZES and MEN1 (Table 1)
TABLE 1. Surgical Cure in Series Since 1990 of Patients With MEN1 and ZES (Nonpancreaticoduodenectomy Results)
Before 1990, it was not appreciated that the majority of gastrinomas in patients with MEN1 and ZES were located in the duodenum.1,3,4 This contributed to the low cure rate1,5–10 and the controversy about routine surgical exploration for cure.1 Since 1990, our study published in 199911 and other studies (Table 1) have demonstrated that the surgical cure rate in these patients is very low (0% to 10%)11–15 without pancreaticoduodenectomy.
Some3,16,17 (Table 1) have reported cures; however, they did not include a negative secretin test and normal fasting gastrin levels measured postoperatively. Further, in most of these studies the follow-up was short. Patients develop late recurrences18,19 and the secretin test and gastrin levels are the most sensitive method to detect recurrence.18 Further, the role of pancreaticoduodenectomy for cure or improved survival is unclear.
Surgical Cure Rate in Patients With Sporadic ZES (No MEN1)
In patients with sporadic ZES, there was a disagreement on the disease-free rate in patients’ postresection.1 This occurred because most studies had small numbers of patients and follow-up was short and incomplete.1,20 In 1999, our study was published,11 which involved 123 patients with a mean follow-up of 8 ± 4 years. In 93% of patients, gastrinomas were found, including each of the last 81. The postoperative cure rate was 60%, 40% at 5 years, and 34% at 10 years.11 These results, coupled with no mortality and low morbidity (<15%) with surgery,12 strongly supported routine surgical exploration because it was both safe and produced long-term cures in some patients.21
Biologic Behavior of Duodenal and Pancreatic Gastrinomas (Table 2)
TABLE 2. Characteristics of Aggressive Gastrinomas and PETs
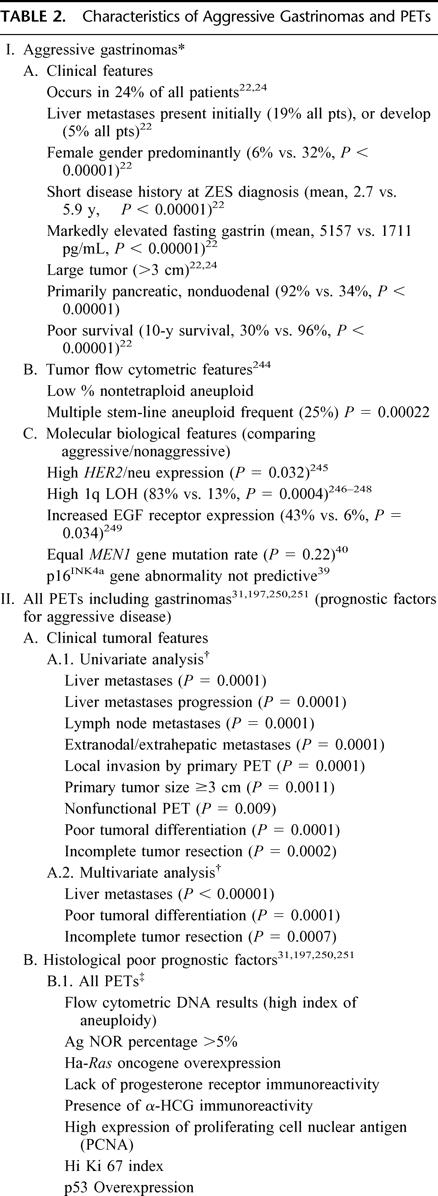
TABLE 2. Continued.
Studies have provided information on the biologic behavior of pancreatic and duodenal gastrinomas. Studies have shown that both locations are equally malignant (40% to 70% metastases), and the postoperative disease-free rate is similar.11,22,23 However, duodenal tumors are smaller, less likely to metastasize to liver, and have a better prognosis than pancreatic tumors.22–29
Recent studies22,24 support the hypothesis27 that there is an aggressive and nonaggressive form of gastrinoma. The aggressive form comprises 24% of patients. It is more common in woman and those without MEN1. It has a short disease duration, higher serum gastrin levels, large pancreatic tumors, liver metastases, and a long-term survival rate of 30% compared with 96% for the nonaggressive form22,24 (Table 2). This also applies to liver metastases.30–33 Of 19 liver gastrinomas, 26% demonstrated no growth, 32% had slow growth, and 42% had rapid growth. In patients with rapid growth, 62% died, whereas no other died.30
Studies have shown a number of clinical, laboratory, tumoral, flow cytometric, and molecular-biologic features which are predictors of aggressive growth (Table 2) The further definition of these features will likely have a significant impact on the surgical management of NET as additional studies are performed. Unfortunately, the molecular pathogenesis of gastrinomas is largely unknown.34–38 Recent studies demonstrate that alterations in 2 tumor suppressor genes, the MEN1 gene and p16INK4a, are frequent in PETs but do not predict aggressive behavior.34,39–41 Unfortunately at present, none of the abnormalities are sufficiently predictive to allow operative strategy to be affected.
Development of Additional Imaging Modalities That Might Increase Surgical Cure Rate
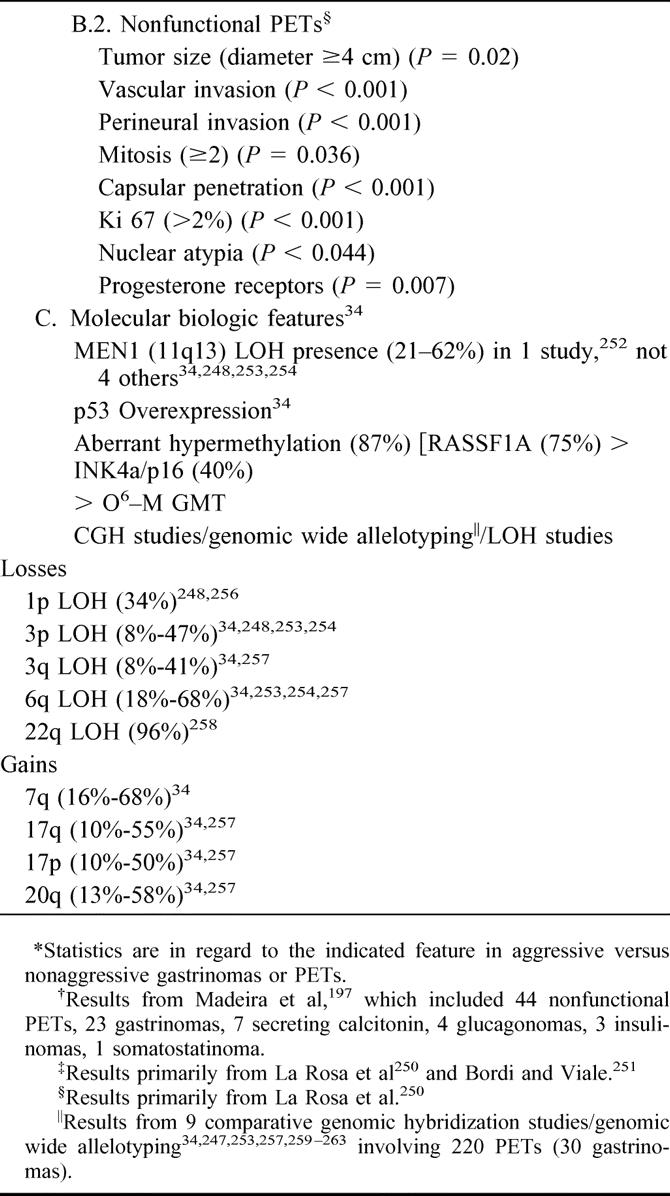
Although there have been improvements in MRI and CT scanning since 1991, these have not resulted in enhanced detection of small gastrinomas.42–47 Major advances have been in the use of somatostatin receptor scintigraphy (SRS) and, perhaps, EUS. Whereas the role of SRS is clear,14,44,47–49 that of EUS is controversial.45 Gastrinomas express somatostatin receptor that binds octreotide and images tumor.47,48,50–53 SPECT imaging is necessary for high sensitivity.50,54–57 SRS allows total body imaging and detection of distant metastases and primary gastrinomas in unusual intra-abdominal locations.58–62 It is more sensitive than all conventional imaging studies combined.43,47,50,51 It is also the most sensitive modality for detecting bony metastases.63,64 The addition of SRS identifies additional tumor that affects management.47,56,65–67 Unfortunately, SRS misses 50% of small duodenal gastrinomas.22,26,46,58,68,69 It gives no information on tumor size and exact location of tumor,46 knowledge of which alters surgical approach. Therefore, a CT or MRI42,70 is performed with SRS. In the patient seen in Figure 1, CT and MRI are negative, whereas SRS detected tumor in the left hepatic lobe. At surgery in addition to the liver tumor, a small (0.3 cm) duodenal gastrinoma and an adjacent positive lymph node were found, which were missed by SRS. Despite the value of SRS, it does not increase surgical cure rate. In 37 consecutive patients, the disease-free rate with SRS did not differ from that seen without SRS.18,46,71 What effect SRS has on surgical approach and results in patients with advanced gastrinomas is also unclear.
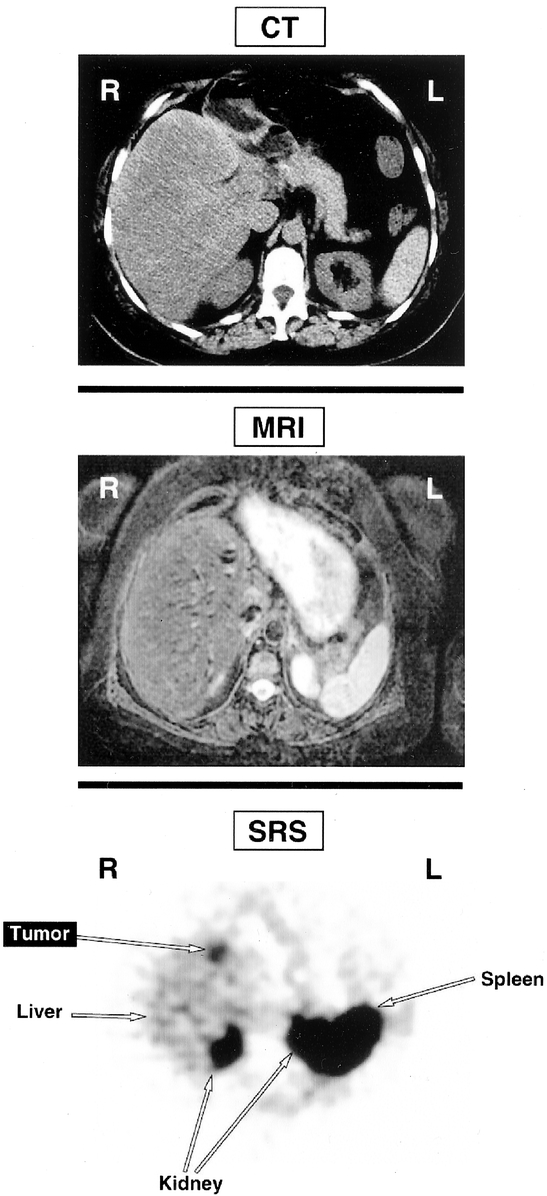
FIGURE 1. Comparison of CT scan, MRI scan, and SRS in a patient with ZES. Neither the CT scan (top) nor MRI (middle) localizes a gastrinoma. SRS however, showed a focus in the left lobe of the liver. At surgery the patient had two 1 cm left lobe liver metastases and a small duodenal (0.3 cm gastrinoma plus an adjacent lymph node. This result demonstrates the enhanced sensitivity of SRS but also shows that it frequently misses small tumors46,68
Recent studies have reported that helical CT scanning with contrast can detect small PETs and duodenal tumors.72–76 In one study,73 it was more sensitive than SRS and localized 4 of 4 duodenal gastrinomas. In our experience and that of others,68 similar results have not been found.46
In terms of intraoperative localization techniques, the major advance since 1990 is the establishment of routine duodenotomy as an essential part of the surgical approach.14,29 Duodenal gastrinomas are 3 to 10 times more common than pancreatic gastrinomas, small (<1.0 cm in diameter), may be multiple, and have (40% to 70%) lymph node metastases but rarely (5%) hepatic metastases.11–13,22,23,46,58,68,69,77–80 Numerous studies demonstrate that these small duodenal tumors are best detected by routine duodenotomy and that endoscopic transillumination of the duodenum can be helpful.12,13,29,58,77,79,81 Although it has been established that significantly more duodenal tumors are found using duodenotomy,14,25,82 it has not been clearly established that its use increases the surgical cure rate.
Role of Gastric Surgery in Management of ZES
In 1991, this was considered an unresolved issue (Controversy 7).1 Gastric surgery included the role of total gastrectomy for acid control, total or partial gastrectomy to treat gastric carcinoids which develop in hypergastrinemic states,83–86 and parietal cell vagotomy. Acid hypersecretion can be controlled in all patients with ZES with proton pump inhibitors.58,87–90 In one review of 116 patients88 with ZES, each had acid hypersecretion controlled by omeprazole for long periods. Numerous other studies have similar results with PPIs.58,89–91 A parenteral PPI, intravenous pantroprazole, is effective89,92,93 similar to parenteral omeprazole.94,95 Therefore, total gastrectomy for acid hypersecretory control is not indicated.
Hypergastrinemic states are associated with an increased risk of gastric carcinoids, some of which (10% to 20%) may be malignant.96,97 In most cases, these gastric carcinoids are small and can be treated endoscopically.96,98–100 However, some are large, multiple, and invasive and need surgery.96,98–100 In patients with sporadic ZES, the risk of gastric carcinoids is low (<2%).86,101–103 In one recent study86 involving 106 patients with sporadic ZES, none had a gastric carcinoid tumor. Even though gastric carcinoids have been reported in patients with sporadic ZES,96,103–105 this study86 concluded that they are rare. In contrast, gastric carcinoids develop in 13% to 37% of patients with ZES with MEN1.101–103 Although most of these (80% to 90%) are not invasive,96,97 aggressive cases can occur and require a total gastrectomy.106 The true frequency of aggressive gastric carcinoids in patients with MEN1/ZES is likely low (<10%).100 Nevertheless, total gastrectomy may be required in some and this may increase as patients age.100
Current Controversies
Need for EUS as a Routine Preoperative Tumor Localization Study
EUS is recommended for preoperative tumor localization and staging in ZES.44,45,68,107–116 Figure 2 demonstrates the ability of SRS to give regional and EUS specific localization. EUS has high sensitivity (Table 3) and the ability to perform cytologic confirmation of tumor but is operator-dependent, and false positives can occur.44,45,107–109,113,117 Further, cytologic confirmation for PET may not be needed because the diagnosis is made by hormonal and functional studies,118,119 and it does not provide information on distant metastases.
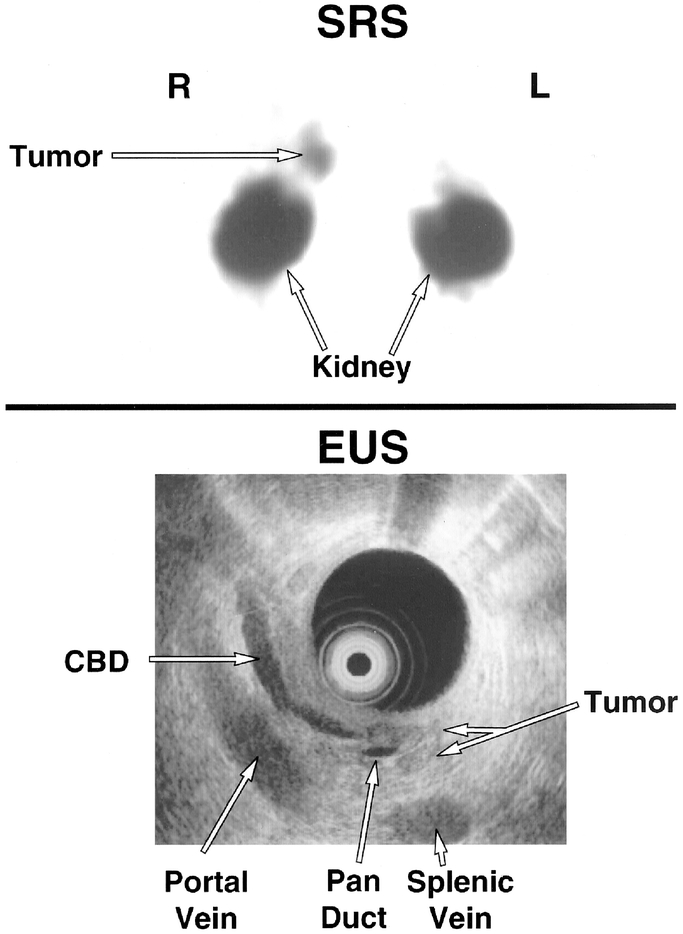
FIGURE 2. Results of SRS (top) and endoscopic ultrasound (EUS) localization of a gastrinoma. SRS localized a gastrinoma to the duodenum/pancreatic head area (arrow-labeled tumor). EUS localized a gastrinoma in the pancreatic head (labeled tumor) situated between the pancreatic duct (Pan duct) and common bile duct (CBD).
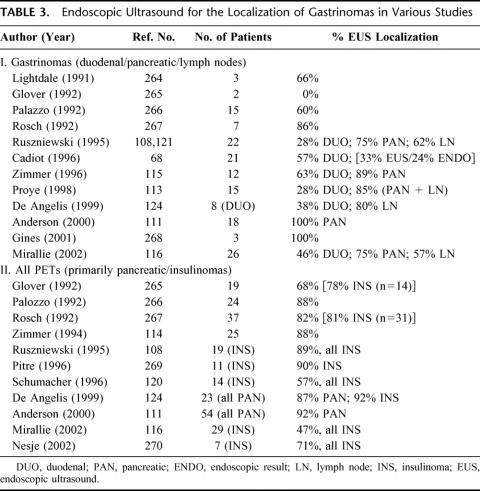
TABLE 3. Endoscopic Ultrasound for the Localization of Gastrinomas in Various Studies
An important issue in assessing EUS in gastrinomas is its sensitivity in different locations. Most studies suggest that EUS has a high sensitivity for detecting neuroendocrine tumors within the pancreas (Table 3). However, 2 recent series116,120 reported low sensitivities for pancreatic insulinoma (Table 3). Whether the differences in sensitivities are due to operator-dependence, patient populations, or instrumentation is unclear. In contrast to pancreatic PETs, the sensitivity of EUS is more variable in ZES with gastrinomas in different locations (Table 3). Whereas the sensitivity108,111,113,115,121,122 was 85% for detection of pancreatic gastrinomas, it was only 43%68,108,113,115,116,123,124 for detection of duodenal gastrinomas. Because gastrinomas are found 3 to 10 times more frequently in the duodenum than in the pancreas,11,58,68 this lack of sensitivity in localizing duodenal gastrinomas is a significant limitation. Furthermore, in an EUS study in patients with ZES,68 57% of duodenal gastrinomas localized by EUS were seen only on endoscopy, not by ultrasound. No other preoperative imaging study, including SRS, reliably localizes many duodenal gastrinomas,46 and therefore, it was hoped that EUS could do this. However, EUS adds little in this regard. Another important point is that pancreatic gastrinomas are generally larger than duodenal gastrinomas,22,24 and since the sensitivity for tumor detection by imaging modalities, including SRS, is dependent on tumor size,42,46,70,125–127 pancreatic gastrinomas are frequently detected by conventional imaging studies or SRS.45 Last, a recent study117 reports pancreatic nodules in 1% of all patients examined by EUS that can be easily mistaken for a PET. The benefit of EUS specifically in this context has not been well evaluated. Because of the factors reviewed above, in the case of ZES we would not recommend the use of EUS as a routine preoperative imaging study, especially in the 75% to 82% of patients with sporadic ZES. We would recommend SRS and CT scan with contrast as the routine initial studies, which will identify distant disease and most primaries. In patients with negative results from both of these tumor localization studies, either angiography with secretin stimulation or EUS should be considered as an additional preoperative study. Secretin stimulation with hepatic vein gastrin sampling during angiography has an advantage in that a positive response does not depend on tumor size128 and it has been shown to be a sensitive modality for localizing duodenal gastrinomas.128,129 However, it has the disadvantage that it only provides regional localization within an area, and the area it localizes to is the gastrinoma triangle, the location of most occult gastrinomas.128,129
In patients with MEN1 and ZES, it also has been proposed that EUS should be a routine preoperative study.130–132 Recent studies demonstrate that the majority (60% to 100%) of these patients have duodenal gastrinomas,3,11,130,131 but in addition, they usually possess pancreatic microadenomas or larger tumors detected on conventional imaging studies or SRS.101,133–135 In up to 30% of the patients, these larger pancreatic tumors are not gastrinoma.11,68,79,107,136 While EUS will frequently miss the small duodenal gastrinomas in MEN1 patients similar to sporadic cases, it will help define the exact location of additional pancreatic NETs as well as metastatic lymph nodes that frequently occur.11,68,132,137 Therefore, EUS may be particularly useful preoperatively in patients with MEN1 and ZES, especially patients who have had previous abdominal surgery, which can increase the difficulty of exploration during surgery.
Routine Surgical Exploration in Patients With ZES and MEN1
Recent studies report that cure of ZES in patients with MEN1 rarely occurs. Whether routine surgical exploration should be performed in a patient with ZES and MEN1 to possibly reduce the malignant spread and eventually increase survival still remains controversial.11,17,101,137–144 A number of groups recommend routine surgical exploration to decrease the probability of malignant spread.17,140,142,143,145 The operation includes distal pancreatectomy, intraoperative ultrasound and enucleation of tumors in the pancreatic head, duodenotomy, and removal of lymph nodes along the celiac trunk and hepatic ligament.17,143,144 In contrast, other groups recommend that surgical exploration only be performed when a tumor of 2 to 3 cm is imaged.11,101,138 There is not only disagreement about when surgical exploration should be performed but also what operation, with differences primarily over whether distal pancreatectomy should be done.101,130,137,140,146
This controversy has occurred for 3 principal reasons. First, no group has followed sufficient numbers of these patients for a long enough time in a controlled study. Second, these patients, even with metastatic disease, have a 15-year survival of 52%.137 Therefore, very long-term studies are needed to resolve treatment strategies. Finally, the natural history of patients with MEN1/ZES in the current era with satisfactory treatment of the parathyroid and pituitary disease is largely unknown.147 Even in patients dying of metastatic neuroendocrine tumors,101,148–150 it is unknown whether it is due to metastatic gastrinoma, another PET, development of a thymic carcinoid,151 or a gastric carcinoid.106,147,152 However, results over the last decade suggest that progression of the gastrinoma and/or other PETs is becoming one of the most important determinants of long-term survival.138,148,153,154 These findings support the role for surgery in selected patients. Second, a recent prospective study demonstrated that 23% of the PETs in MEN-1 patients had aggressive growth and hepatic metastases.155 Further, 38% of the patients with aggressive tumors died of tumor, which was significantly different from patients with nonaggressive PETs. These findings also support the role for surgery in selected patients. Third, when these patients were explored with imageable tumors of 2 to 3 cm, the majority (50% to 70%) had lymph node involvement; however, metastases to the liver were rare.11,140,145 This observation has led some to propose earlier exploration.11,140 Fourth, in a recent study involving 81 patients137 with MEN1/ZES, the 15-year survival was 100% in 25 patients with no surgical exploration with PETs <2.5 cm, 100% in 17 patients with a single PET <6 cm (with surgical exploration), and 89% in 31 patients with ≥2 tumors or >6 cm tumor (with surgery). In this study,137 these excellent survival rates were achieved without performing routine distal pancreatectomy, suggesting that it is not essential.
At present there are no reliable clinical, laboratory, or tumoral markers that allow prediction in an individual patient with ZES and MEN1 of the aggressiveness of the PET. The most important predictor of survival in patients with gastrinomas is the development of liver metastases, not lymph node metastases.22,138 Numerous studies have demonstrated that in gastrinomas, PETs, and carcinoids, primary tumor size is highly predictive of liver metastases.22,24,27,31,138,156 Therefore, at present, because patients with ZES/MEN1 are not cured, those with tumors ≤2 cm have a 100% 15-year survival, and those with larger tumors have an increased probability of developing liver metastases, we continue to recommend surgical exploration only for MEN1/ZES patients with an imageable tumor ≥2 cm and not to perform a routine distal pancreatectomy. Additional studies are needed to clearly define whether a more aggressive approach is indicated.
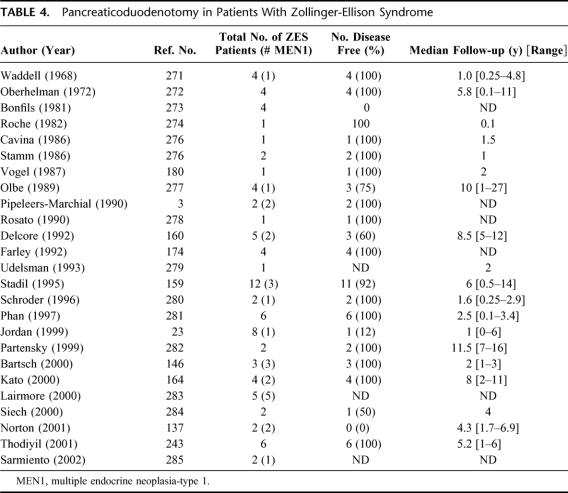
Should Pancreaticoduodenectomy Be More Frequently Used in ZES?
Most centers with considerable experience in management of patients with ZES do not recommend pancreaticoduodenectomy.11–13,15,17,19,101,137,143,157,158 However, a number of small series have reported the use of Whipple resection in patients with ZES with or without MEN1 (Table 4). In a high proportion of these cases, Whipple resection has resulted in cure in patients with ZES both with and without MEN1 (Table 4). This has resulted in the hypothesis that Whipple resection may provide a better chance of cure and increased survival, especially in patients with MEN1, where long-term cure with the standard operation is rarely achieved.19,146,159–161 In the largest series159 involving 12 patients with ZES, of whom 3 had MEN1, 92% of the patients were cured post-Whipple resection. Even though normal fasting gastrin levels persisted during a median follow-up of 6 years, suggesting cure, serial secretin tests were not done such that early recurrences may have not been detected.18 Further, Whipple resection may make reoperation more difficult, which is important in patients with ZES and MEN1 because they frequently develop additional large PETs. The development of hepatic metastases occurring after a Whipple resection cannot be treated with liver embolization118,162,163 because of the risk of ascending infection. Nevertheless, because of these results (Table 4) it has been recommended by a number of authorities that Whipple resection be performed in patients with a large pancreatic head or duodenal tumor that cannot be enucleated, with multiple duodenal tumors, with multiple lymph nodes with a duodenal or pancreatic head tumor, or if the patient is not cured after removal of a duodenal or pancreatic head gastrinoma with or without lymph node metastases by the standard operation (as assessed by intraoperative secretin or other methods).19,146,159–161,164
TABLE 4. Pancreaticoduodenotomy in Patients With Zollinger-Ellison Syndrome
At present, before more frequent use of Whipple resection can be recommended, a number of important issues need to be clarified. First, it should be established by a systematic study that Whipple resection in both sporadic ZES and patients with ZES with MEN1 leads to increased long-term cure, established by complete biochemical assessment (fasting gastrin levels and secretin test). Second, the long-term side effects of Whipple resection and their frequency need to be carefully assessed. This is an important point because this is largely unknown, and if significant, it could be a major determining factor against its use because these patients currently have an excellent quality and duration of life. Furthermore, the presence of lymph node metastases is not a justification for Whipple resection because they have not been shown to decrease survival.22,138 Third, ultimately it will need to be established that a Whipple resection extends survival in patients with ZES with and without MEN1. This will be difficult because for sporadic ZES the 10-year survival is 95%11 and in ZES/MEN1 it is 86%.11 Furthermore, it has not been established that these patients’ survival is determined by gastrinoma and not some other tumor such as thymic carcinoid,151 another PET, or some other tumor.151
Do Lymph Node Primary Gastrinomas Occur and Should Routine Removal of Duodenal/Pancreatic Head Lymph Nodes Be Part of the Standard Surgical Exploration?
The possible presence of lymph node primary gastrinomas in patients with ZES remains a controversial subject.13,23,165–173 There are numerous reports in the literature of patients with ZES (almost all with sporadic ZES) who had a lymph node(s) only resected and were cured by biochemical testing and imaging studies both short term and long term.10,13,23,27,68,165–171,174–185
Recently, our experience with 176 patients with ZES with or without MEN1 who underwent surgical exploration for the occurrence of a possible lymph node primary was analyzed (Fig. 3). 165 Twenty-six patients (15%) followed for a mean of 10 years fulfilled all the criteria for a lymph primary.165 Of the 138 sporadic ZES patients, 36 patients (26%) had only lymph nodes removed and 22 patients (16%) were disease-free immediately postresection and thus, had a possible lymph node primary (Fig. 3). During follow-up, 16 patients (12%) remained cured and, therefore, fit the diagnosis for a lymph node primary (Fig. 3). During this follow-up period, 6 patients relapsed and 2 had small duodenal primaries that were missed at the original exploration. These long-term cure results strongly support the conclusion that lymph node primary tumors exist.165 This possibility is further supported by 2 recent pathology studies172,173 that report neuroendocrine cells can be found in abdominal lymph nodes. Unfortunately, no clinical, laboratory or operative feature of the lymph node predicted which lymph node was a primary or a metastasis. This presents a potential problem for the surgeon. First, these findings do not determine whether a primary tumor is likely missed and whether a more aggressive resection like Whipple is indicated. Second, it provides an additional reason to support removal of peripancreatic lymph nodes and lymph nodes along the celiac trunk and hepatic ligament as part of the surgical exploration.17,69,144,165,166 In addition to recognizing possible lymph node primaries, the routine removal of lymph nodes may increase surgical cure rate in patients where an accompanying primary tumor is found, although at present this is unproven.
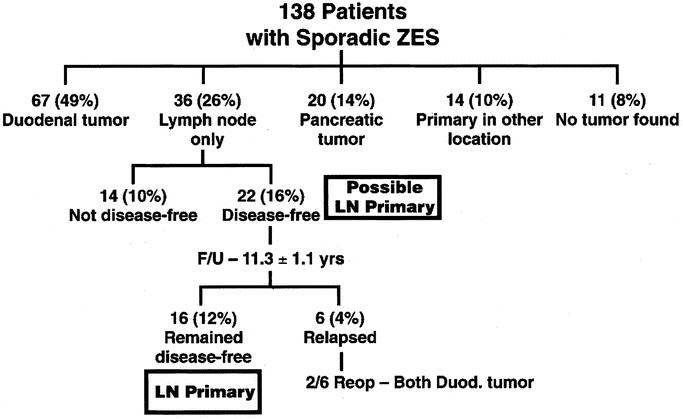
FIGURE 3. Algorithm showing patients with sporadic ZES based on the primary location and likely lymph node primary gastrinoma. Of the 138 patients, 36 had a lymph node only removed and 22 (16% of total) were disease free, suggesting a possible lymph node primary tumor. Subsequently, the patients were reevaluated with a yearly assessment for cure over a mean of 11.1 ± 1.3 years, 16 patients (12% of total) remained disease-free and are considered to have lymph node primary gastrinomas.165,166 Of the 6 patients who relapsed, 2 had duodenal primaries.
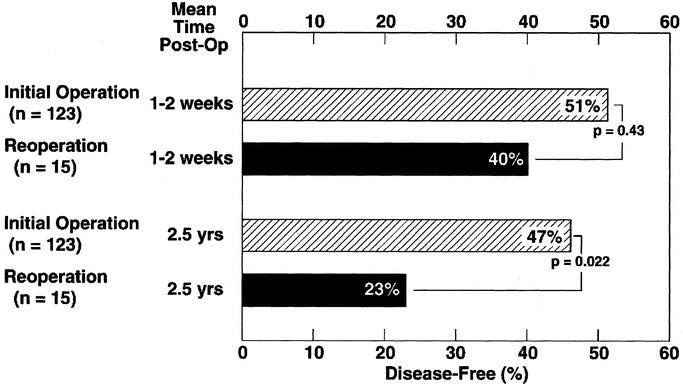
FIGURE 4. Comparison of the disease-free rate after initial surgical exploration and after reoperation for recurrent tumor. Plotted are the disease-free rates within 1 to 2 weeks of surgery and at a follow-up of at least 2.5 years. Results are plotted from data11 for initial surgery in 123 patients, and for reoperation195 in 15 patients
Role of Parietal Cell Vagotomy
Although at present parietal cell vagotomy is not frequently performed, some have advocated its routine use186,187 or its use in selected patients.188,189 Hence, its exact role remains controversial. In a study by Richardson et al,186 parietal cell vagotomy resulted in a 75% decrease in basal acid output. However, only 9% of the patients were able to stop all antisecretory drugs. Furthermore, parietal cell vagotomy188 increased the sensitivity to the antisecretory action of other drugs such as histamine H2-receptor antagonists. Richardson188 recommends it be routinely performed. However, the availability and effectiveness of PPIs led to them as the preferred treatment.87,90 In 1996, McArthur et al187 reported results from an 11-year follow-up study of Richardson's patients. This study187 demonstrated that parietal cell vagotomy continued to reduce acid secretion by 80% from preoperative values and that 32% of the patients required no drugs up to 16 years after the operation. An accompanying editorial188 pointed out that (1) 60% to 70% of patients with ZES are not cured long term and will need gastric antisecretory drugs11,188,190; and (2) the expense of medication is a significant deterrent in long-term compliance. The mean cost of omeprazole is $3276/y.188 There is increasing concern about the consequences of achlorhydria, which occurs in 35% of patients on omeprazole188,191 It may lead to vitamin B12 and iron malabsorption.191–194 Finally, some patients with ZES who are cured postresection189 continue to require gastric acid antisecretory drugs long term. The performance of routine parietal cell vagotomy may allow more than one third to stop PPIs187 or switch to histamine H2-antagonists, which are less expensive and potent. For these reasons, parietal cell vagotomy should be considered as a routine adjunct at the time of surgical exploration for cure. However, surgical exploration should not be performed solely to do a parietal cell vagotomy.
Place of Surgical Reexploration in Patients With ZES
Long-term studies show that most patients with sporadic11,190 and MEN1 ZES11,19,137,141 are not cured. Further, some will harbor aggressive tumors and may benefit from reexploration. At present, the indications for surgical reexploration, the type of operation such as a Whipple procedure, are largely undefined and controversial. Reoperations will be more important as ZES patients are living longer and localization methods such as SRS are improving. Only 1 study195 has dealt with this problem (Fig. 4). In this study195 of 120 patients with ZES undergoing surgical exploration (14% had MEN1/ZES), 78 patients (65%) had persistent disease postoperatively, and 17 patients undergoing 18 reoperations were analyzed. The indication used for reexploration195 was identification of imageable disease during follow-up. SRS was not used,195 which is an important factor in considering the applicability of this study to the present. Tumor was removed in all 17 patients at reexploration. The results of reexploration in the 15 patients with sporadic ZES are compared with initial exploration. There was no significant difference immediately postoperatively in the disease-free rate between the initial or second operation (51% versus 40%, Fig. 4, top); however, at 2.5 years postresection195 47% of the initial patients remained disease free, while only 23% following reoperations (Fig. 4, bottom). These results demonstrate the long-term cure rate was lower with reoperation. However, reoperation was thought to be indicated because some patients were still able to be rendered disease free.
This study's applicability to current management is unclear for a number of reasons. First, SRS is now available, widely used, and may identify recurrent disease earlier. Second, the 15-year survival rate postoperatively of these patients, even if not cured, is 95%11 and with ZES/MEN1 is 93%.137 Therefore, survival may not be altered by reexploration. Other variables such as tumor growth or molecular findings in the tumor (Table 2) may be used to identify a subset who may benefit from reexploration Last, should Whipple resection be considered in a subset of these patients at reexploration? How should this subset be selected? Will either secretin angiography or EUS be useful in selecting these patients? At present, all of these questions are unanswered and require study.
Role of Surgery in Treatment of Aggressive, Advanced Disease
The most important predictor of survival in patients with ZES and other gastrointestinal neuroendocrine tumors is hepatic metatases.22,24,31,118,138,156,196,197 Furthermore, the extent of the liver metastases is also important.24 Figure 5 shows survival data from 212 patients with ZES. Patients without any liver metastases had a 95% 20-year survival from diagnosis, whereas patients with diffuse metastases had a 10-year survival of only 15% (Fig. 5). Patients who had a single liver lobe metastasis or less than 5 discrete metastases in both liver lobes also had a decreased survival (60% at 15 years); however, it was significantly better than patients with diffuse metastases. Therefore, in ZES,14,137,198–200 and other malignant NETs,201–207 possible resection and cryoablation of hepatic metastases has been recommended. Only 5% to 15% of patients have metastases limited to 1 lobe or a discrete limited number (<5) in 2 lobes that would be fully resectable.24,137,196,198,200,201,206,208 Therefore, surgery for cure is only possible in a fraction of these patients. In various studies, this surgery in patients with advanced NETs is reported to occasionally result in cure,106,129,198,207 to have 5-year survival rates of 71% to 85%,106,129,198,201,202,207 and to result in increased survival.129,201,205 However, these results are difficult to evaluate for a number of reasons. First, there are no controlled trials where the groups are appropriately matched to control groups without cytoreductive surgery. Therefore, patients with resectable disease are not comparable to those with unresectable disease and differences in survival may be independent of surgery. Second, patients with functional NETs are often considered together with nonfunctional NETs in evaluating the value of cytoreductive surgery. Patients with advanced functional NETs in whom the symptoms of the hormone-excess state are not well controlled medically may benefit from enhanced symptom control postresection, whereas in patients with nonfunctional NETs or gastrinomas who are usually asymptomatic, the value of surgery is almost entirely assessed by its effect on survival. Third, because of the relatively slow growth of these malignant NETs compared with common malignancies such as adenocarcinomas, these studies need to be long term to demonstrate differences in survival with significant numbers of patients, which is difficult because of the rarity of ZES and other GI neuroendocrine tumors.
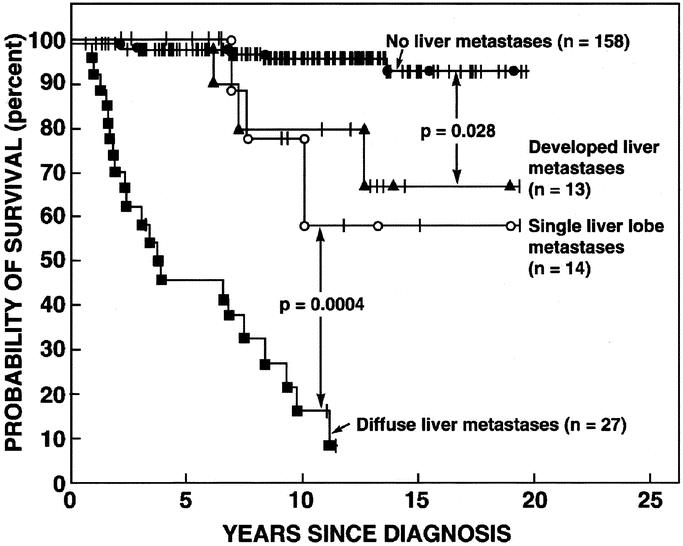
FIGURE 5. Effect of the extent of liver tumor on the survival of 212 patients. Disease-related survival is shown plotted in the form of Kaplan and Meier. Numbers in parenthesis refer to the number of patients in each group. Figure is drawn from the data in Yu et al.24
Because of these reservations, at present there are insufficient data to unequivocally determine in whom, if any patient, cytoreductive surgery or aggressive resection with advanced disease with ZES should be performed. Most of the surgical studies demonstrate these resections can be performed with acceptable morbidity, low mortality, and suggest they may prolong life. Because the medical treatment of advanced disease in patients with ZES, as well as most NETs,118,156 is generally unsatisfactory, our approach at present is to attempt surgical resection in any patient with advanced disease where all or at least 90% of the gross tumor is thought to be resectable based on imaging studies. This approach is used, recognizing that additional studies are needed to clearly establish its value in both patients with ZES and those with other advanced neuroendocrine tumors.
Role of Laparoscopic Surgery or Endoscopic Resection of Duodenal Gastrinomas in Patients With ZES
Recently endoscopic removal of duodenal gastrinomas has been reported,209–214 as well as use of the laparoscope to attempt to surgically resect primary gastrinomas215 or other PETs (especially insulinomas),215–223 as well as laparoscopic treatments of advanced disease in patients with metastatic PETs including gastrinomas.224,225
Endoscopic removal of duodenal gastrinomas or other duodenal neuroendocrine tumors is described using snare polypectomy with or without submucosal saline injection,209–213,226 or using endoscopic-assisted band ligation.227 EUS may be useful because it has been shown226 to allow assessment of the depth of invasion of the duodenal NET, determining whether it is confined to the submucosal layer and can be safely removed. In a few cases, endoscopic removal of a duodenal gastrinoma has resulted in cure.210,211,228 Nevertheless, in at least 1 case211 a duodenal perforation occurred. Our results from studies of duodenal tumors would suggest a number of important reservations in regard to this endoscopic approach. First, more than half of the duodenal gastrinomas are associated with adjacent metastatic lymph nodes,11,22,28,77 and endoscopic removal of the primary will not allow lymph node removal, which may contribute to cure. Second, almost half of duodenal gastrinomas are locally invasive beyond the submucosa28 and may not be easily removed by endoscopic means. Furthermore, aggressive endoscopic removal of these invasive tumors will likely lead to a significantly increased complication rate. Third, in patients with MEN1 the duodenal gastrinomas are invariably multiple, making their removal endoscopically difficult. Furthermore, no removal of the pancreatic macroadenomas that are frequently present can be carried out. For these reasons, we recommend against attempted endoscopic removal of duodenal gastrinomas.
Laparoscopic resection of pancreatic PETs, especially insulinomas, is being increasingly used.216–223,229–233 With insulinomas, especially in the distal pancreatic body or pancreatic tail, the laparoscopic approach using either enucleation or distal pancreatectomy has had a high success rate.219,220,223,232,234 At present, the experience with gastrinomas is very limited.215 In both of the patients with gastrinoma in one study,215 laparoscopic resection had to be converted to a laparotomy because of the extent of disease. The role for laparoscopic surgery for gastrinoma resection appears to be much more limited than its potential utility for insulinoma for a number of reasons. First, gastrinomas are 3 to 10 times more common in the duodenum than the pancreas. Furthermore, in the duodenum the gastrinomas are frequently small (<1.0 cm). Although there are reports of resection of duodenal carcinoids laparoscopically,235,236 the experience is very limited and it is unclear whether laparoscopy can successfully find and resect small duodenal gastrinomas. Second, laparoscopic resection of pancreatic PETs (primarily insulinomas) has generally been successful when the tumor was found on preoperative imaging and less successful if not seen on preoperative imaging.232 Many gastrinomas are not localized preoperatively, especially duodenal gastrinomas, and this will likely decrease the success rate. Third, many gastrinomas (>50% to 70%) are associated with adjacent lymph node metastases and are not solitary primaries, as in the case of insulinomas. This will make a laparoscopic approach more difficult, prolong its duration, and may limit its success. Fourth, laparoscopic resection of pancreatic body/tail PETs is generally successful; however, laparoscopic resection of PETs in the pancreatic head region has been more difficult because of the adjacent important structures.232 Greater than 75% of gastrinomas are in the pancreatic head region in the so-called gastrinoma triangle,9,14,58 complicating the laparoscopic approach. For the reasons outlined above, we recommend against a laparoscopic approach in patients with ZES and support the continued use of open surgery.
A laparoscopic approach is also being used to identify and treat patients with advanced malignant PETs including gastrinomas.224,225,237 Laparoscopic radiofrequency ablation of liver metastases of malignant PETs is reported to have low morbidity224,225 and to result in a decrease in tumor markers in 65% of patients with metastatic GI neuroendocrine tumors.224 While this may be a useful approach in patients with functional GI neuroendocrine tumors with poor medical control of the hormone-excess state, this is not the case with ZES where excellent treatment exists for the acid hypersecretion. Therefore, in the case of ZES, as with nonfunctional PETs, this approach will not be widely adopted if it is not shown to extend survival. A laparoscopic approach has been shown to be useful in staging the extent of malignant PETs and preventing unnecessary surgery.237 With the recent widespread use of SRS, which is highly sensitive for identifying hepatic metastases43,56,65,66,238 as well as helical CT,72–74,76 it is unclear whether the use of preoperative laparoscopy is warranted for this indication. At present, we do not recommend its routine preoperative use for staging gastrinomas or PETs.
CONCLUSIONS
Whereas a number of controversies involved in the surgical treatment of Zollinger-Ellison syndrome1 have been resolved over the last decade, a number of areas are identified in this article and discussed, where controversies remain unresolved. It is hoped by pointing out these specific areas and discussing them that surgeons who treat these patients will focus on some of these issues. Only by their systematic study will additional advances be made and these existing controversies resolved.
Footnotes
Reprints: Dr. Jeffrey A. Norton, Stanford University Medical Center, Department of Surgery, Rm. H-3591, 300 Pasteur Drive, Stanford, CA 94305-5641. E-mail: janorton@stanford.edu.
REFERENCES
- 1.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with the Zollinger-Ellison syndrome. World J Surg. 1991;15:151–159. [DOI] [PubMed] [Google Scholar]
- 2.Roy P, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. [DOI] [PubMed] [Google Scholar]
- 3.Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. [DOI] [PubMed] [Google Scholar]
- 4.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum: a cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg. 1989;209:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.vanHeerden JA, Smith SL, Miller LJ. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. Surgery. 1986;100:971–977. [PubMed] [Google Scholar]
- 6.Deveney CW, Deveney KS, Stark D, et al. Resection of gastrinomas. Ann Surg. 1983;198:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen RT, Gardner JD, Raufman JP, et al. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med. 1983;98:59–75. [DOI] [PubMed] [Google Scholar]
- 8.Malagelada JR, Edis AJ, Adson MA, et al. Medical and surgical options in the management of patients with gastrinoma. Gastroenterology. 1983;84:1524–1532. [PubMed] [Google Scholar]
- 9.Stabile BE, Morrow DJ, Passaro E Jr. The gastrinoma triangle: operative implications. Am J Surg. 1984;147:25–31. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JC, Lewis BG, Wiener I, et al. The role of surgery in the Zollinger-Ellison syndrome. Ann Surg. 1983;197:594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. [DOI] [PubMed] [Google Scholar]
- 12.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: results of a 10-year prospective study. Ann Surg. 1992;215:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard TJ, Zinner MJ, Stabile BE, et al. Gastrinoma excision for cure: a prospective analysis. Ann Surg. 1990;211:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1). Surg Oncol. 2003;12:145–151. [DOI] [PubMed] [Google Scholar]
- 15.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994;31:1–156. [DOI] [PubMed] [Google Scholar]
- 16.Thompson NW, Bondeson AG, Bondeson L, et al. The surgical treatment of gastrinoma in MEN I syndrome patients. Surgery. 1989;106:1081–1085. [PubMed] [Google Scholar]
- 17.Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease: results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495–500. [DOI] [PubMed] [Google Scholar]
- 18.Fishbeyn VA, Norton JA, Benya RV, et al. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993;119:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerstrom G, Hessman O, Skogseid B. Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN1. Langenbecks Arch Surg. 2002;386:558–569. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy DM. The place of surgery in the Zollinger-Ellison syndrome. N Engl J Med. 1980;302:1344–1347. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2003 (in press). [DOI] [PMC free article] [PubMed]
- 22.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. [DOI] [PubMed] [Google Scholar]
- 23.Jordan PH Jr. A personal experience with pancreatic and duodenal neuroendocrine tumors. J Am Coll Surg. 1999;189:470–482. [DOI] [PubMed] [Google Scholar]
- 24.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. [DOI] [PubMed] [Google Scholar]
- 25.Ruszniewski P, Podevin P, Cadiot G, et al. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas. 1993;8:295–304. [DOI] [PubMed] [Google Scholar]
- 26.Donow C, Pipeleers-Marichal M, Schroder S, et al. Surgical pathology of gastrinoma: site, size, multicentricity, association with multiple endocrine neoplasia type 1, and malignancy. Cancer. 1991;68:1329–1334. [DOI] [PubMed] [Google Scholar]
- 27.Stabile BE, Passaro E Jr. Benign and malignant gastrinoma. Am J Surg. 1985;49:144–150. [DOI] [PubMed] [Google Scholar]
- 28.Thom AK, Norton JA, Axiotis CA, et al. Location, incidence and malignant potential of duodenal gastrinomas. Surgery. 1991;110:1086–1093. [PubMed] [Google Scholar]
- 29.Norton JA, Alexander HR, Fraker DL, et al. Does the use of routine duodenotomy affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg. 2003 (in press). [DOI] [PMC free article] [PubMed]
- 30.Sutliff VE, Doppman JL, Gibril F, et al. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J Clin Oncol. 1997;15:2420–2431. [DOI] [PubMed] [Google Scholar]
- 31.Jensen RT. Natural history of digestive endocrine tumors. In: Mignon M, Colombel JF, eds. Recent Advances in Pathophysiology and Management of Inflammatory Bowel Diseases and Digestive Endocrine Tumors. Paris: John Libbey Eurotext; 1999:192–219. [Google Scholar]
- 32.Zayene A, Bonnaud G, Vuagnat A, et al. Survival factors in patients with endocrine liver metastases. Gut. 1997;41:A184. [Google Scholar]
- 33.Arnold R, Trautmann ME, Creutzfeldt W, et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corleto VD, Delle Fave G, Jensen RT. Molecular insights into gastrointestinal neuroendocrine tumors: importance and recent advances. Dig Liver Dis. 2002;34:668–680. [DOI] [PubMed] [Google Scholar]
- 35.Rindi G, Candusso ME, Solcia E. Molecular aspects of the endocrine tumours of the pancreas and the gastrointestinal tract. Ital J Gastroenterol Hepatol. 1999;31:S135–S138. [PubMed] [Google Scholar]
- 36.Gumbs AA, Moore PS, Falconi M, et al. Review of the clinical, histological, and molecular aspects of pancreatic endocrine neoplasms. J Surg Oncol. 2002;81:45–53. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RT. Carcinoid and pancreatic endocrine tumors: recent advances in molecular pathogenesis, localization, and treatment. Curr Opin Oncol. 2000;12:368–377. [DOI] [PubMed] [Google Scholar]
- 38.Weber HC, Jensen RT. Pancreatic endocrine tumors and carcinoid tumors: recent insights from genetic and molecular biologic studies. In: Dervenis CG, ed. Advances in Pancreatic Disease: Molecular Biology, Diagnosis and Treatment. New York: Georg Thieme Verlag; 1996:55–75. [Google Scholar]
- 39.Serrano J, Goebel SU, Peghini PL, et al. Alterations in the p16 INK4a/CDKN2A tumor suppressor gene in gastrinomas. J Clin Endocrinol Metab. 2000;85:4146–4156. [DOI] [PubMed] [Google Scholar]
- 40.Goebel SU, Heppner C, Burns AD, et al. Geneotype/phenotype correlations of MEN1 gene mutations in sporadic gastrinoma. J Clin Endocrinol Metab. 2000;85:116–123. [DOI] [PubMed] [Google Scholar]
- 41.Muscarella P, Melvin WS, Fisher WE, et al. Genetic alterations in gastrinomas and nonfunctioning pancreatic neuroendocrine tumors: an analysis of p16/MTS1 tumor suppressor gene inactivation. Cancer Res. 1998;58:237–240. [PubMed] [Google Scholar]
- 42.Orbuch M, Doppman JL, Strader DB, et al. Imaging for pancreatic endocrine tumor localization: recent advances. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:268–281. [Google Scholar]
- 43.Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med. 1996;125:26–34. [DOI] [PubMed] [Google Scholar]
- 44.Prinz RA. Localization of gastrinomas. Int J Pancreatol. 1996;19:79–91. [DOI] [PubMed] [Google Scholar]
- 45.Gibril F, Doppman JD, Jensen RT. Comparative analysis of tumor localization techniques for neuroendocrine tumors. Yale J Biol Med. 1997;70:481–500. [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander HR, Fraker DL, Norton JA, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998;228:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibril F, Jensen RT. Diagnostic use of radiolabeled somatostatin analogues in patients with gastroenteropancreatic endocrine tumors. Dig Liver Dis. 2003 (in press). [DOI] [PubMed]
- 48.Kwekkeboom D, Krenning EP, deJong M. Peptide receptor imaging and therapy. J Nucl Med. 2000;41:1704–1713. [PubMed] [Google Scholar]
- 49.Jensen RT, Gibril F. Somatostatin receptor scintigraphy in gastrinomas. Ital J Gastroenterol Hepatol. 1999;31(suppl. 2):S179–S185. [PubMed] [Google Scholar]
- 50.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. [DOI] [PubMed] [Google Scholar]
- 51.Krenning EP, Kwekkeboom DJ, Oei HY, et al. Somatostatin-receptor scintigraphy in gastroenteropancreatic tumors. Ann N Y Acad Sci. 1994;733:416–424. [DOI] [PubMed] [Google Scholar]
- 52.Reubi JC, Laissue J, Waser B, et al. Expression of somatostatin receptors in normal, inflamed, and neoplastic human gastrointestinal tissues. Ann N Y Acad Sci. 1994;733:122–137. [DOI] [PubMed] [Google Scholar]
- 53.Jensen RT. Peptide therapy: recent advances in the use of somatostatin and other peptide receptor agonists and antagonists. In: Lewis JH, Dubois A, eds. Current Clinical Topics in Gastrointestinal Pharmacology. Malden, MA: Blackwell Science; 1997:144–223. [Google Scholar]
- 54.Schillaci O, Scopinaro F, Danieli R, et al. Single photon emission computerized tomography increases the sensitivity of indium-111-pentetreotide scintigraphy in detecting abdominal carcinoids. Anticancer Res. 1997;17:1753–1756. [PubMed] [Google Scholar]
- 55.Schillaci O, Scopinaro F, Angeletti S, et al. SPECT improves accuracy of somatostatin receptor scintigraphy in abdominal carcinoid tumors. J Nucl Med. 1996;37:1452–1456. [PubMed] [Google Scholar]
- 56.Schillaci O, Spanu A, Scopinaro F, et al. Somatostatin receptor scintigraphy in liver metastasis detection from gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2003;44:359–368. [PubMed] [Google Scholar]
- 57.Schillaci O, Corleto VD, Annibale B, et al. Single photon emission computed tomography procedure improves accuracy of somatostatin receptor scintigraphy in gastro-entero pancreatic tumours. Ital J Gastroenterol Hepatol. 1999;31:S186–S189. [PubMed] [Google Scholar]
- 58.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B, eds. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001:291–344. [Google Scholar]
- 59.Soga J, Yakuwa Y. The gastrinoma/Zollinger-Ellison syndrome: statistical evaluation of a Japanese series of 359 cases. J Hep Bil Pancr Surg. 1998;5:77–85. [DOI] [PubMed] [Google Scholar]
- 60.Gibril F, Curtis LT, Termanini B, et al. Primary cardiac gastrinoma causing Zollinger-Ellison syndrome. Gastroenterology. 1997;112:567–574. [DOI] [PubMed] [Google Scholar]
- 61.Noda S, Norton JA, Jensen RT, et al. Surgical resection of intracardiac gastrinoma. Ann Thorac Surg. 1999;67:532–533. [DOI] [PubMed] [Google Scholar]
- 62.Abou-Saif A, Lei J, McDonald TJ, et al. A new cause of Zollinger-Ellison syndrome: non-small cell lung cancer. Gastroenterology. 2001;120:1271–1278. [DOI] [PubMed] [Google Scholar]
- 63.Gibril F, Doppman JL, Reynolds JC, et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J Clin Oncol. 1998;16:1040–1053. [DOI] [PubMed] [Google Scholar]
- 64.Lebtahi R, Cadiot G, Delahaye N, et al. Detection of bone metastases in patients with endocrine gastroenteropancreatic tumors: bone scintigraphy compared with somatostatin receptor scintigraphy. J Nucl Med. 1999;40:1602–1608. [PubMed] [Google Scholar]
- 65.Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112:335–347. [DOI] [PubMed] [Google Scholar]
- 66.Cadiot G, Bonnaud G, Lebtahi R, et al. Usefulness of somatostatin receptor scintigraphy in the management of patients with Zollinger-Ellison syndrome. Gut. 1997;41:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebtahi R, Cadiot G, Sarda L, et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853–858. [PubMed] [Google Scholar]
- 68.Cadiot G, Lebtahi R, Sarda L, et al. Preoperative detection of duodenal gastrinomas and peripancreatic lymph nodes by somatostatin receptor scintigraphy. Gastroenterology. 1996;111:845–854. [DOI] [PubMed] [Google Scholar]
- 69.Zogakis TG, Gibril F, Libutti SK, et al. Management and outcome of patients with sporadic gastrinomas arising in the duodenum. Ann Surg. 2003;238:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pisegna JR, Doppman JL, Norton JA, et al. Prospective comparative study of ability of MR imaging and other imaging modalities to localize tumors in patients with Zollinger-Ellison syndrome. Dig Dis Sci. 1993;38:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheppard BC, Norton JA, Doppman JL, et al. Management of islet cell tumors in patients with multiple endocrine neoplasia: a prospective study. Surgery. 1989;106:1108–1117. [PubMed] [Google Scholar]
- 72.Winter TC3, Freeny PC, Nghiem HV. Extrapancreatic gastrinoma localization: value of arterial-phase helical CT with water as an oral contrast agent. Am J Roentgenol. 1996;166:51–52. [DOI] [PubMed] [Google Scholar]
- 73.Panzuto F, Falconi M, Nasoni S, et al. Staging of digestive endocrine tumours using helical computed tomography and somatostatin receptor scintigraphy. Ann Oncol. 2003;14:586–591. [DOI] [PubMed] [Google Scholar]
- 74.Van Hoe L, Gryspeerdt S, Marchal G, et al. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. Am J Roentgenol. 1995;165:1437–1439. [DOI] [PubMed] [Google Scholar]
- 75.Blomley MJ, Strickland NH, Jackson JE. Case report: multiple duodenal gastrinomas demonstrated with spiral CT. Clin Radiol. 1996;51:811–813. [DOI] [PubMed] [Google Scholar]
- 76.Kay CL, Gordon L. Multiple duodenal gastrinomas demonstrated with spiral CT. Clin Radiol. 1997;52:481. [DOI] [PubMed] [Google Scholar]
- 77.Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993;17:455–462. [DOI] [PubMed] [Google Scholar]
- 78.Kisker O, Bastian D, Bartsch D, et al. Localization, malignant potential, and surgical management of gastrinomas. World J Surg. 1998;22:651–658. [DOI] [PubMed] [Google Scholar]
- 79.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frucht H, Norton JA, London JF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology. 1990;99:1622–1627. [DOI] [PubMed] [Google Scholar]
- 81.Jensen RT, Fraker DL. Zollinger-Ellison syndrome: advances in treatment of the gastric hypersecretion and the gastrinoma. J Am Med Assoc. 1994;271:1–7. [DOI] [PubMed] [Google Scholar]
- 82.Norton JA, Doppman JL, Collen MJ, et al. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg. 1986;204:468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hakanson R, Bottcher G, Sundler F, et al. Activation and hyperplasia of gastrin and enterochromaffin-like cells in the stomach. Digestion. 1986;1:23–41. [DOI] [PubMed] [Google Scholar]
- 84.Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39:61–79. [DOI] [PubMed] [Google Scholar]
- 85.Bordi C, D'Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol. 1995;19:S8–S19. [PubMed] [Google Scholar]
- 86.Peghini PL, Annibale B, Azzoni C, et al. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. [DOI] [PubMed] [Google Scholar]
- 87.Metz DC, Jensen RT. Advances in gastric antisecretory therapy in Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:240–257. [Google Scholar]
- 88.Metz DC, Strader DB, Orbuch M, et al. Use of omeprazole in Zollinger-Ellison: a prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7:597–610. [DOI] [PubMed] [Google Scholar]
- 89.Metz DC, Soffer E, Forsmark CE, et al. Maintenance oral pantoprazole therapy is effective for patients with Zollinger-Ellison syndrome and idiopathic hypersecretion. Am J Gastroenterol. 2003;98:301–307. [DOI] [PubMed] [Google Scholar]
- 90.Jensen RT. Use of omeprazole and other proton pump inhibitors in the Zollinger-Ellison syndrome. In: Olbe L, ed. Milestones in Drug Therapy. Basel, Switzerland: Birkhauser Verlag AG; 1999:205–221. [Google Scholar]
- 91.Metz DC, Pisegna JR, Ringham GL, et al. Prospective study of efficacy and safety of lansoprazole in Zollinger-Ellison syndrome. Dig Dis Sci. 1993;38:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lew EA, Pisegna JR, Starr JA, et al. Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 2000;118:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Metz DC, Forsmark C, Lew EA, et al. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Am J Gastroenterol. 2001;96:3274–3280. [DOI] [PubMed] [Google Scholar]
- 94.Vinayek R, Frucht H, London JF, et al. Intravenous omeprazole in patients with Zollinger-Ellison syndrome undergoing surgery. Gastroenterology. 1990;99:10–16. [DOI] [PubMed] [Google Scholar]
- 95.Vinayek R, Amantea MA, Maton PN, et al. Pharmacokinetics of oral and intravenous omeprazole in patients with the Zollinger-Ellison syndrome. Gastroenterology. 1991;101:138–147. [DOI] [PubMed] [Google Scholar]
- 96.Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. [DOI] [PubMed] [Google Scholar]
- 97.Rindi G, Azzoni C, La Rosa S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542. [DOI] [PubMed] [Google Scholar]
- 98.Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. [DOI] [PubMed] [Google Scholar]
- 99.Ahlman H, Kolby L, Lundell L, et al. Clinical management of gastric carcinoid tumors. Digestion. 1994;55:77–85. [DOI] [PubMed] [Google Scholar]
- 100.Jensen RT. MEN-1 carcinoids: diagnosis and therapy. In: Seventh International Workshop on Multiple Neoplasia. Gubbio, Italy: 1999:67–72. [Google Scholar]
- 101.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. [DOI] [PubMed] [Google Scholar]
- 102.Lehy T, Cadiot G, Mignon M, et al. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut. 1992;33:1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jensen RT. Gastrinoma as a model for prolonged hypergastrinemia in man. In: Walsh JH, ed. Gastrin. New York, NY: Raven Press Publishing Co; 1993:373–393. [Google Scholar]
- 104.Feurle GE. Argyrophil cell hyperplasia and a carcinoid tumour in the stomach of a patient with sporadic Zollinger-Ellison syndrome. Gut. 1994;35:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cadiot G, Vissuzaine C, Potet F, et al. Fundic argyrophil carcinoid tumor in a patient with sporadic-type Zollinger-Ellison syndrome. Dig Dis Sci. 1995;40:1275–1278. [DOI] [PubMed] [Google Scholar]
- 106.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003 (in press). [DOI] [PubMed]
- 107.Thompson NW, Czako PF, Fritts LL, et al. Role of endoscopic ultrasonography in the localization of insulinomas and gastrinomas. Surgery. 1994;116:1131–1138. [PubMed] [Google Scholar]
- 108.Ruszniewski P, Amouyal P, Amouyal G, et al. Localization of gastrinomas by endoscopic ultrasonography in patients with Zollinger-Ellison syndrome. Surgery. 1995;117:629–635. [DOI] [PubMed] [Google Scholar]
- 109.Doppman JL. Pancreatic endocrine tumors: the search goes on [editorial]. N Engl J Med. 1992;326:1770–1772. [DOI] [PubMed] [Google Scholar]
- 110.Kwekkeboom DJ, Bakker WH, Kam BL, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [(177)Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2003;30:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271–2277. [DOI] [PubMed] [Google Scholar]
- 112.Zimmer T, Ziegler K, Liehr RM, et al. Endosonography of neuroendocrine tumors of the stomach, duodenum, and pancreas. Ann N Y Acad Sci. 1994;733:425–436. [DOI] [PubMed] [Google Scholar]
- 113.Proye C, Malvaux P, Pattou F, et al. Noninvasive imaging of insulinomas and gastrinomas with endoscopic ultrasonography and somatostatin receptor scintigraphy. Surgery. 1998;124:1134–1144. [DOI] [PubMed] [Google Scholar]
- 114.Zimmer T, Ziegler K, Bader M, et al. Localisation of neuroendocrine tumours of the upper gastrointestinal tract. Gut. 1994;35:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmer T, Stolzel U, Bader M, et al. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996;39:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mirallie E, Pattou F, Malvaux P, et al. [Value of endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localization of insulinomas and gastrinomas: experience of 54 cases]. Gastroenterol Clin Biol. 2002;26:360–366. [PubMed] [Google Scholar]
- 117.Kann PH, Wirkus B, Keth A, et al. Pitfalls in endosonographic imaging of suspected insulinomas: pancreatic nodules of unknown dignity. Eur J Endocrinol. 2003;148:531–534. [DOI] [PubMed] [Google Scholar]
- 118.Alexander RA, Jensen RT. Pancreatic endocrine tumors. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001:1788–1813. [Google Scholar]
- 119.Jensen RT, Norton JA. Pancreatic endocrine tumors. In: Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, and Management. Philadelphia: W.B. Saunders; 2002:988–1016. [Google Scholar]
- 120.Schumacher B, Lubke HJ, Frieling T, et al. Prospective study on the detection of insulinomas by endoscopic ultrasonography. Endoscopy. 1996;28:273–276. [DOI] [PubMed] [Google Scholar]
- 121.Ruszniewski P, Amouyal P, Amouyal G, et al. Endocrine tumors of the pancreatic area: localization by endoscopic ultrasonography. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:258–267. [Google Scholar]
- 122.Chiti A, Briganti V, Fanti S, et al. Results and potential of somatostatin receptor imaging in gastroenteropancreatic tract tumours. Q J Nucl Med. 2000;44:42–49. [PubMed] [Google Scholar]
- 123.Eriksson B, Oberg K. PPomas and nonfunctioning endocrine pancreatic tumors: clinical presentation, diagnosis, and advances in management. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:208–222. [Google Scholar]
- 124.De Angelis C, Carucci P, Repici A, et al. Endosonography in decision making and management of gastrointestinal endocrine tumors. Eur J Ultrasound. 1999;10:139–150. [DOI] [PubMed] [Google Scholar]
- 125.Maton PN, Miller DL, Doppman JL, et al. Role of selective angiography in the management of Zollinger-Ellison syndrome. Gastroenterology. 1987;92:913–918. [DOI] [PubMed] [Google Scholar]
- 126.Wank SA, Doppman JL, Miller DL, et al. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:905–912. [DOI] [PubMed] [Google Scholar]
- 127.London JF, Shawker TH, Doppman JL, et al. Zollinger-Ellison syndrome: prospective assessment of abdominal US in the localization of gastrinomas. Radiology. 1991;178:763–767. [DOI] [PubMed] [Google Scholar]
- 128.Strader DB, Doppman JL, Orbuch M, et al. Functional localization of pancreatic endocrine tumors. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S Karger; 1995:282–297. [Google Scholar]
- 129.Thom AK, Norton JA, Doppman JL, et al. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992;112:1002–1008. [PubMed] [Google Scholar]
- 130.Thompson NW. Multiple endocrine neoplasia type I: surgical therapy. Cancer Treat Res. 1997;89:407–419. [DOI] [PubMed] [Google Scholar]
- 131.Gauger PG, Thompson NW. Early surgical intervention and strategy in patients with multiple endocrine neoplasia type 1. Best Pract Res Clin Endocrinol Metab. 2001;15:213–223. [DOI] [PubMed] [Google Scholar]
- 132.Wamsteker EJ, Gauger PG, Thompson NW, et al. EUS detection of pancreatic endocrine tumors in asymptomatic patients with type 1 multiple endocrine neoplasia. Gastrointest Endosc. 2003;58:531–535. [DOI] [PubMed] [Google Scholar]
- 133.Thompson NW, Lloyd RV, Nishiyama RH, et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg. 1984;8:561–574. [DOI] [PubMed] [Google Scholar]
- 134.Komminoth P, Heitz PU, Kloppel G. Pathology of MEN-1: morphology, clinicopathologic correlations and tumour development. J Intern Med. 1998;243:455–464. [DOI] [PubMed] [Google Scholar]
- 135.Kloppel G, Willemar S, Stamm B, et al. Pancreatic lesions and hormonal profile of pancreatic tumors in multiple endocrine neoplasia type I: an immunocytochemical study of nine patients. Cancer. 1986;57:1824–1832. [DOI] [PubMed] [Google Scholar]
- 136.Fraker DL, Norton JA, Alexander HR, et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cadiot G, Vuagnat A, Doukhan I, et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Gastroenterology. 1999;116:286–293. [DOI] [PubMed] [Google Scholar]
- 139.Wells SA Jr. Surgery for the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:689–690. [DOI] [PubMed] [Google Scholar]
- 140.Skogseid B, Oberg K, Eriksson B, et al. Surgery for asymptomatic pancreatic lesion in multiple endocrine neoplasia type 1. World J Surg. 1996;20:872–877. [DOI] [PubMed] [Google Scholar]
- 141.MacFarlane MP, Fraker DL, Alexander HR, et al. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-type 1. Surgery. 1995;118:973–980. [DOI] [PubMed] [Google Scholar]
- 142.Grama D, Eriksson B, Martensson H, et al. Clinical characteristics, treatment and survival in patients with pancreatic tumors causing hormonal syndromes. World J Surg. 1992;16:632–639. [DOI] [PubMed] [Google Scholar]
- 143.Thompson NW. Management of pancreatic endocrine tumors in patients with multiple endocrine neoplasia type 1. Surg Oncol Clin North Am. 1998;7:881–891. [PubMed] [Google Scholar]
- 144.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. [DOI] [PubMed] [Google Scholar]
- 145.Lowney JK, Frisella MM, Lairmore TC, et al. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery. 1999;125:1043–1049. [DOI] [PubMed] [Google Scholar]
- 146.Bartsch DK, Langer P, Wild A, et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery. 2000;128:958–966. [DOI] [PubMed] [Google Scholar]
- 147.Gibril F, Schumann M, Pace A, Jensen RT. Zollinger-Ellison syndrome and multiple endocrine neoplasia-type 1 (MEN1): a prospective NIH study of 107 patients and comparison with 1009 patients from the literature. Medicine. 2003 (in press). [DOI] [PubMed]
- 148.Doherty GM, Olson JA, Frisella MM, et al. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22:581–587. [DOI] [PubMed] [Google Scholar]
- 149.Shepherd JJ. The natural history of multiple endocrine neoplasia type I: highly uncommon or highly unrecognized. Arch Surg. 1991;126:935–952. [DOI] [PubMed] [Google Scholar]
- 150.Shepherd JJ, Challis DR, Davies PF, et al. Multiple endocrine neoplasm, type 1: gastrinomas, pancreatic neoplasms, microcarcinoids, the Zollinger-Ellison syndrome, lymph nodes, and hepatic metastases. Arch Surg. 1993;128:1133–1142. [DOI] [PubMed] [Google Scholar]
- 151.Gibril F, Chen Y-J, Schrump DS, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2003;88:1066–1081. [DOI] [PubMed] [Google Scholar]
- 152.Bordi C, Falchetti A, Azzoni C, et al. Aggressive forms of gastric neuroendocrine tumors in multiple endocrine neoplasia type I. Am J Surg Pathol. 1997;21:1075–1082. [DOI] [PubMed] [Google Scholar]
- 153.Dean PG, vanHeerden JA, Farley DR, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. [DOI] [PubMed] [Google Scholar]
- 154.Wilkinson S, Teh BT, Davey KR, et al. Cause of death in multiple endocrine neoplasia type 1. Arch Surg. 1993;128:683–690. [DOI] [PubMed] [Google Scholar]
- 155.Gibril F, Venzon DJ, Ojeaburu JV, et al. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86:5282–5293. [DOI] [PubMed] [Google Scholar]
- 156.Jensen RT, Doherty GM. Carcinoid tumors and the carcinoid syndrome. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001:1813–1833. [Google Scholar]
- 157.Chanson P, Cadiot G, Murat A. Management of patients and subjects at risk for multiple endocrine neoplasia type 1: MEN1. Horm Res. 1997;47:211–220. [DOI] [PubMed] [Google Scholar]
- 158.Norton JA. Surgical treatment of islet cell tumors with special emphasis on operative ultrasound. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:309–332. [Google Scholar]
- 159.Stadil F. Treatment of gastrinomas with pancreaticoduodenectomy. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:333–341. [Google Scholar]
- 160.Delcore R, Friesen SR. Role of pancreatoduodenectomy in the management of primary duodenal wall gastrinomas in patients with Zollinger-Ellison syndrome. Surgery. 1992;112:1016–1022. [PubMed] [Google Scholar]
- 161.Imamura M, Kanda M, Takahashi K, et al. Clinicopathological characteristics of duodenal microgastrinomas. World J Surg. 1992;16:703–709. [DOI] [PubMed] [Google Scholar]
- 162.Ruszniewski P, Malka D. Hepatic arterial chemoembolization in the management of advanced digestive endocrine tumors. Digestion. 2000;62:79–83. [DOI] [PubMed] [Google Scholar]
- 163.Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors: a prospective phase II study in 24 patients. Cancer. 1993;71:2624–2630. [DOI] [PubMed] [Google Scholar]
- 164.Kato M, Imamura M, Hosotani R, et al. Curative resection of microgastrinomas based on the intraoperative secretin test. World J Surg. 2000;24:1425–1430. [DOI] [PubMed] [Google Scholar]
- 165.Norton JA, Alexander HA, Fraker DL, et al. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: a prospective study. Ann Surg. 2003;237:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arnold WS, Fraker DL, Alexander HR, et al. Apparent lymph node primary gastrinoma. Surgery. 1994;116:1123–1130. [PubMed] [Google Scholar]
- 167.Kitagawa M, Hayakawa T, Kondo T, et al. Gastrinoma in a mesenteric lymph node. Am J Gastroenterol. 1989;84:660–662. [PubMed] [Google Scholar]
- 168.Bhagavan BS, Slavin RE, Goldberg J, et al. Ectopic gastrinamas and the Zollinger-Ellison syndrome. Hum Pathol. 1986;17:584–592. [DOI] [PubMed] [Google Scholar]
- 169.Delcore R Jr, Cheung LY, Friesen SR. Outcome of lymph node involvement in patients with Zollinger-Ellison syndrome. Ann Surg. 1988;206:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thompson NW, Vinik AI, Eckhauser FE, et al. Extrapancreatic gastrinomas. Surgery. 1985;98:1113–1120. [PubMed] [Google Scholar]
- 171.Friesen SR. Are aberrant nodal gastrinomas pathogenetically similar to lateral aberrant thyroid nodules. Surgery. 1990;107:236–238. [PubMed] [Google Scholar]
- 172.Herrmann ME, Ciesla MC, Chejfec G, et al. Primary nodal gastrinomas: an immunohistochemical study in support of a theory. Arch Pathol Lab Med. 2000;124:832–835. [DOI] [PubMed] [Google Scholar]
- 173.Perrier ND, Batts KP, Thompson GB, et al. An immunohistochemical survey for neuroendocrine cells in regional pancreatic lymph nodes: a plausible explanation for primary nodal gastrinomas? Surgery. 1995;118:957–965. [DOI] [PubMed] [Google Scholar]
- 174.Farley DR, Van Heerden JA, Grant CS, et al. The Zollinger-Ellison syndrome: a collective surgical experience. Ann Surg. 1992;215:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fox PS, Hofmann JW, Decosse JJ, et al. The influence of total gastrectomy on survival in malignant Zollinger-Ellison tumors. Ann Surg. 1974;180:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaplan EL, Horvath K, Udekwu A, et al. Gastrinomas: a 42-year experience. World J Surg. 1990;14:365–375. [DOI] [PubMed] [Google Scholar]
- 177.Mee AS, Bornman PC, Marks IN. Conservative surgery in the Zollinger-Ellison syndrome. Br J Surg. 1984;71:423–424. [DOI] [PubMed] [Google Scholar]
- 178.Richardson CT, Feldman M, McClelland RN, et al. Effect of vagotomy in Zollinger-Ellison syndrome. Gastroenterology. 1979;77:682–686. [PubMed] [Google Scholar]
- 179.Sircus W. Vagotomy in Zollinger-Ellison syndrome. Gastroenterology. 1979;79:607–608. [Google Scholar]
- 180.Vogel SB, Wolfe MM, McGuigan JE, et al. Localization and resection of gastrinomas in Zollinger-Ellison syndrome. Ann Surg. 1987;205:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wilson SD. The role of surgery in children with the Zollinger-Ellison syndrome. Surgery. 1982;92:682–692. [PubMed] [Google Scholar]
- 182.Wolfe MM, Alexander RW, McGuigan JE. Extrapancreatic, extraintestinal gastrinoma: effective treatment by surgery. N Engl J Med. 1982;306:1533–1536. [DOI] [PubMed] [Google Scholar]
- 183.Schwartz DL, White JJ, Saulsbury F, et al. Gastrin response to calcium infusion: an aid to the improved diagnosis of Zollinger-Ellison syndrome in children. Pediatrics. 1974;54:599–602. [PubMed] [Google Scholar]
- 184.Bornman PC, Marks IN, Mee AS, et al. Favourable response to conservative surgery for extra-pancreatic gastrinoma with lymph node metastases. Br J Surg. 1987;74:198–201. [DOI] [PubMed] [Google Scholar]
- 185.Stremple JF. Gastrinomas: gastrin-producing tumors. Surg Clin North Am. 1975;55:303–323. [DOI] [PubMed] [Google Scholar]
- 186.Richardson CT, Peters MN, Feldman M, et al. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists: a prospective study. Gastroenterology. 1985;89:357–367. [DOI] [PubMed] [Google Scholar]
- 187.McArthur KE, Richardson CT, Barnett CC, et al. Laparotomy and proximal gastric vagotomy in Zollinger-Ellison syndrome: results of a 16-year prospective study. Am J Gastroenterol. 1996;91:1104–1111. [PubMed] [Google Scholar]
- 188.Jensen RT. Should the 1996 citation for Zollinger-Ellison syndrome read: “acid-reducing surgery in, aggressive resections out”? Am J Gastroenterol. 1996;91:1067–1070. [PubMed] [Google Scholar]
- 189.Pisegna JR, Norton JA, Slimak GG, et al. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology. 1992;102:767–778. [DOI] [PubMed] [Google Scholar]
- 190.Hirschowitz BI. Clinical course of nonsurgically treated Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:360–371. [Google Scholar]
- 191.Stewart CA, Termanini B, Sutliff VE, et al. Assessment of the risk of iron malabsorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther. 1998;12:83–98. [DOI] [PubMed] [Google Scholar]
- 192.Dutta SK. Editorial: vitamin B12 malabsorption and omeprazole therapy. J Am Coll Nutr. 1994;13:544–545. [DOI] [PubMed] [Google Scholar]
- 193.Koop H, Bachem MG. Serum iron, ferritin, and vitamin B12 during prolonged omeprazole therapy. J Clin Gastroenterol. 1992;14:288–292. [DOI] [PubMed] [Google Scholar]
- 194.Termanini B, Gibril F, Sutliff VE III, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–430. [DOI] [PubMed] [Google Scholar]
- 195.Jaskowiak NT, Fraker DL, Alexander HR, et al. Is reoperation for gastrinoma excision in Zollinger-Ellison syndrome (ZES) indicated? Surgery. 1996;120:1057–1063. [DOI] [PubMed] [Google Scholar]
- 196.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, et al, eds. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press; 1993:931–978. [Google Scholar]
- 197.Madeira I, Terris B, Voss M, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Norton JA, Sugarbaker PH, Doppman JL, et al. Aggressive resection of metastatic disease in selected patients with malignant gastrinoma. Ann Surg. 1986;203:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Norton JA, Kirlen MA, Li M, et al. Morbidity and mortality of aggressive resections in patients with advanced neuroendocrine tumors. Arch Surg. 2003 (in press). [DOI] [PubMed]
- 200.Carty SE, Jensen RT, Norton JA. Prospective study of aggressive resection of metastatic pancreatic endocrine tumors. Surgery. 1992;112:1024–1031. [PubMed] [Google Scholar]
- 201.Nagorney DM, Que FG. Cytoreductive hepatic surgery for metastatic gastrointestinal neuroendocrine tumors. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:416–430. [Google Scholar]
- 202.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–43. [DOI] [PubMed] [Google Scholar]
- 203.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. [DOI] [PubMed] [Google Scholar]
- 204.Pederzoli P, Falconi M, Bonora A, et al. Cytoreductive surgery in advanced endocrine tumours of the pancreas. Ital J Gastroenterol Hepatol. 1999;31:S207–S212. [PubMed] [Google Scholar]
- 205.Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–93. [DOI] [PubMed] [Google Scholar]
- 206.McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091–1096. [PubMed] [Google Scholar]
- 207.Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375–382. [DOI] [PubMed] [Google Scholar]
- 208.Fraker DL, Jensen RT. Pancreatic endocrine tumors. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Raven; 1997:1678–1704. [Google Scholar]
- 209.Otten MH, Birkenhager JC, van Blankenstein M. Zollinger-Ellison syndrome treated by endoscopic removal of a duodenal gastrinoma. Neth J Med. 1978;21:248–251. [PubMed] [Google Scholar]
- 210.Gilhool WJ. Endoscopic diagnosis and removal of a duodenal wall gastrinoma. Am J Gastroenterol. 1984;79:679–683. [PubMed] [Google Scholar]
- 211.Straus E, Raufman JP, Samuel S, et al. Endoscopic cure of the Zollinger-Ellison syndrome. Gastrointest Endosc. 1992;38:709–711. [DOI] [PubMed] [Google Scholar]
- 212.Il’ichev VA, Gorchakov KV, Popov EA, et al. [Endoscopic removal of isolated gastrinoma of the duodenum after Billroth II gastric resection]. Khirurgiia (Mosk). 1992;20–23. [PubMed] [Google Scholar]
- 213.Rolny P, Ekman R, Sjolund K, et al. Endoscopic diagnosis and treatment of duodenal gastrinoma. Gastrointest Endosc. 1990;36:511–513. [DOI] [PubMed] [Google Scholar]
- 214.Straus E, Raufman JP. The Brooklyn gastrinomas. Mt Sinai J Med. 1992;59:125–128. [PubMed] [Google Scholar]
- 215.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051–1054. [DOI] [PubMed] [Google Scholar]
- 216.Van Nieuwenhove Y, Delvaux G. Laparoscopic management of neuroendocrine tumours of the pancreas. Acta Chir Belg. 1999;99:249–252. [PubMed] [Google Scholar]
- 217.Fernandez-Cruz L, Saenz A, Astudillo E, et al. Outcome of laparoscopic pancreatic surgery: endocrine and nonendocrine tumors. World J Surg. 2002;26:1057–1065. [DOI] [PubMed] [Google Scholar]
- 218.Fernandez-Cruz L, Herrera M, Saenz A, et al. Laparoscopic pancreatic surgery in patients with neuroendocrine tumours: indications and limits. Best Pract Res Clin Endocrinol Metab. 2001;15:161–175. [DOI] [PubMed] [Google Scholar]
- 219.Furihata M, Tagaya N, Kubota K. Laparoscopic enucleation of insulinoma in the pancreas: case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2001;11:279–283. [DOI] [PubMed] [Google Scholar]
- 220.Tagaya N, Kasama K, Suzuki N, et al. Laparoscopic resection of the pancreas and review of the literature. Surg Endosc. 2003;17:201–206. [DOI] [PubMed] [Google Scholar]
- 221.Gramatica L Jr, Herrera MF, Mercado-Luna A, et al. Videolaparoscopic resection of insulinomas: experience in two institutions. World J Surg. 2002;26:1297–1300. [DOI] [PubMed] [Google Scholar]
- 222.Spitz JD, Lilly MC, Tetik C, et al. Ultrasound-guided laparoscopic resection of pancreatic islet cell tumors. Surg Laparosc Endosc Percutan Tech. 2000;10:168–173. [DOI] [PubMed] [Google Scholar]
- 223.Berends FJ, Cuesta MA, Kazemier G, et al. Laparoscopic detection and resection of insulinomas. Surgery. 2000;128:386–391. [DOI] [PubMed] [Google Scholar]
- 224.Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985–990. [DOI] [PubMed] [Google Scholar]
- 225.Siperstein A, Garland A, Engle K, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors: technical considerations. Surg Endosc. 2000;14:400–405. [DOI] [PubMed] [Google Scholar]
- 226.Nishimori I, Moritz M, Sano S, et al. Endosonography-guided endoscopic resection of duodenal carcinoid tumor. Endoscopy. 1997;29:214–217. [DOI] [PubMed] [Google Scholar]
- 227.Korc M, Williams JA, Goldfine ID. Stimulation of the glucose transport system in isolated mouse pancreatic acini by cholecystokinin and analogues. J Biol Chem. 1979;254:7624–7629. [PubMed] [Google Scholar]
- 228.Halvorsen SW, Nathanson NM. In vivo regulation of muscarinic acetylcholine receptor number and function in embryonic chick heart. J Biol Chem. 1981;256:7941–7948. [PubMed] [Google Scholar]
- 229.Huard GS, Stephens RL, Friesen SR. Zollinger-Ellison syndrome: streptozotocin therapy for metastatic gastrinomas. J Kans Med Soc. 1981;82:216–221. [PubMed] [Google Scholar]
- 230.Amikura K, Alexander HR, Norton JA, et al. The role of surgery in the management of ACTH-producing islet cell tumors of the pancreas. Surgery. 1995;118:1125–1130. [DOI] [PubMed] [Google Scholar]
- 231.Underwood RA, Soper NJ. Current status of laparoscopic surgery of the pancreas. J Hepatobil Pancreat Surg. 1999;6:154–164. [DOI] [PubMed] [Google Scholar]
- 232.Iihara M, Obara T. Minimally invasive endocrine surgery: laparoscopic resection of insulinomas. Biomed Pharmacother. 2002;56:227s–230s. [DOI] [PubMed] [Google Scholar]
- 233.Iihara M, Kanbe M, Okamoto T, et al. Laparoscopic ultrasonography for resection of insulinomas. Surgery. 2001;130:1086–1091. [DOI] [PubMed] [Google Scholar]
- 234.Young JM, Hiley R, Burgen AS. Homologues of bonzihylcholine mustard. J Pharm Pharmacol. 1972;24:950–954. [DOI] [PubMed] [Google Scholar]
- 235.Larose L, Leclerc L, Asselin J, et al. Muscarinic cholinergic induced secretin subsensitivity in rat isolated pancreatic acini: effects on amylase release, cyclic adenosine monophosphate and inositol phosphate formation. Pancreas. 1989;4:71–78. [DOI] [PubMed] [Google Scholar]
- 236.Blanc P, Porcheron J, Pages A, et al. [Laparoscopic excision of a duodenal neuroendocrine tumor]. Ann Chir. 2000;125:176–178. [DOI] [PubMed] [Google Scholar]
- 237.Hochwald SN, Weiser MR, Colleoni R, et al. Laparoscopy predicts metastatic disease and spares laparotomy in selected patients with pancreatic nonfunctioning islet cell tumors. Ann Surg Oncol. 2001;8:249–253. [DOI] [PubMed] [Google Scholar]
- 238.Termanini B, Gibril F, Doppman JL, et al. Distinguishing small hepatic hemangiomas from vascular liver metastases in patients with gastrinoma: use of a somatostatin-receptor scintigraphic agent. Radiology. 1997;202:151–158. [DOI] [PubMed] [Google Scholar]
- 239.Frucht H, Howard JM, Slaff JI, et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome: a prospective study. Ann Intern Med. 1989;111:713–722. [DOI] [PubMed] [Google Scholar]
- 240.Cherner JA, Sawyers JL. Benefit of resection of metastatic gastrinoma in multiple endocrine neoplasia type I. Gastroenterology. 1992;102:1049–1053. [DOI] [PubMed] [Google Scholar]
- 241.Melvin WS, Johnson JA, Sparks J, et al. Long-term prognosis of Zollinger-Ellison syndrome in multiple endocrine neoplasia. Surgery. 1993;114:1183–1188. [PubMed] [Google Scholar]
- 242.Mignon M, Cadiot G, Rigaud D, et al. Management of islet cell tumors in patients with multiple endocrine neoplasia type 1. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:342–359. [Google Scholar]
- 243.Thodiyil PA, El-Masry NS, Williamson RC. Achieving eugastrinaemia in Zollinger-Ellison syndrome: resection or enucleation? Dig Surg. 2001;18:118–123. [DOI] [PubMed] [Google Scholar]
- 244.Metz DC, Kuchnio M, Fraker DL, et al. Flow cytometry and Zollinger-Ellison syndrome: relationship to clinical course. Gastroenterology. 1993;105:799–813. [DOI] [PubMed] [Google Scholar]
- 245.Goebel SU, Iwamoto M, Raffeld M, et al. HER-2/neu expression and gene amplification in gastrinomas: correlations with tumor biology, growth, and aggressiveness. Cancer Res. 2002;62:3702–3710. [PubMed] [Google Scholar]
- 246.Chen Y-J, Vortmeyer A, Zhuang ZP, et al. Loss of heterozygosity of chromosome 1q in gastrinomas: occurrence and prognostic significance. Cancer Res. 2003;63:817–823. [PubMed] [Google Scholar]
- 247.Yu F, Jensen RT, Lubensky IA, et al. Survey of genetic alterations in gastrinomas. Cancer Res. 2000;60:5536–5542. [PubMed] [Google Scholar]
- 248.Guo SS, Wu AY, Sawicki MP. Deletion of chromosome 1, but not mutation of MEN-1, predicts prognosis in sporadic pancreatic endocrine tumors. World J Surg. 2002;26:843–847. [DOI] [PubMed] [Google Scholar]
- 249.Peghini PL, Iwamoto M, Raffeld M, et al. Overexpression of epidermal growth factor and hepatocyte growth factor receptors in a proportion of gastrinomas correlates with aggressive growth and lower curability. Clin Cancer Res. 2002;8:2273–2285. [PubMed] [Google Scholar]
- 250.La Rosa S, Sessa F, Capella C, et al. Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch. 1996;429:323–333. [DOI] [PubMed] [Google Scholar]
- 251.Bordi C, Viale G. Analysis of cell proliferation and tumor antigens of prognostic significance in pancreatic endocrine tumors. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management: Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:45–59. [Google Scholar]
- 252.Hessman O, Lindberg D, Einarsson A, et al. Genetic alterations on 3p, 11q13, and 18q in nonfamilial and MEN1-associated pancreatic endocrine tumors. Genes Chromosomes Cancer. 1999;26:258–264. [PubMed] [Google Scholar]
- 253.Speel EJ, Richter J, Moch H, et al. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao J, Moch H, Scheidweiler AF, et al. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer. 2001;32:364–372. [DOI] [PubMed] [Google Scholar]
- 255.House MG, Herman JG, Guo MZ, et al. Aberrant hypermethylation of tumor suppressor genes in pancreatic endocrine neoplasms. Ann Surg. 2003;238:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ebrahimi SA, Wang EH, Wu A, et al. Deletion of chromosome 1 predicts prognosis in pancreatic endocrine tumors. Cancer Res. 1999;59:311–315. [PubMed] [Google Scholar]
- 257.Stumpf E, Aalto Y, Hoog A, et al. Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer. 2000;29:83–87. [DOI] [PubMed] [Google Scholar]
- 258.Wild A, Langer P, Celik I, et al. Chromosome 22q in pancreatic endocrine tumors: identification of a homozygous deletion and potential prognostic associations of allelic deletions. Eur J Endocrinol. 2002;147:507–513. [DOI] [PubMed] [Google Scholar]
- 259.Tönnies H, Toliat MR, Ramel C, et al. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut. 2001;48:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Terris B, Meddeb M, Marchio A, et al. Comparative genomic hybridization analysis of sporadic neuroendocrine tumors of the digestive system. Genes Chromosomes Cancer. 1998;22:50–56. [DOI] [PubMed] [Google Scholar]
- 261.Speel EJ, Scheidweiler AF, Zhao J, et al. Genetic evidence for early divergence of small functioning and nonfunctioning endocrine pancreatic tumors: gain of 9Q34 is an early event in insulinomas. Cancer Res. 2001;61:5186–5192. [PubMed] [Google Scholar]
- 262.Chung DC, Brown SB, Graeme-Cook F, et al. Localization of putative tumor suppressor loci by genome-wide allelotyping in human pancreatic endocrine tumors. Cancer Res. 1998;58:3706–3711. [PubMed] [Google Scholar]
- 263.Rigaud G, Missiaglia E, Moore PS, et al. High resolution allelotype of nonfunctional pancreatic endocrine tumors: identification of two molecular subgroups with clinical implications. Cancer Res. 2001;61:285–292. [PubMed] [Google Scholar]
- 264.Lightdale CJ, Botet JF, Woodruff JM, et al. Localization of endocrine tumors of the pancreas with endoscopic ultrasonography. Cancer. 1991;68:1815–1820. [DOI] [PubMed] [Google Scholar]
- 265.Glover JR, Shorvon PJ, Lees WR. Endoscopic ultrasound for localisation of islet cell tumours. Gut. 1992;33:108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Palazzo L, Roseau G, Salmeron M. Endoscopic ultrasonography in the preoperative localization of pancreatic endocrine tumors. Endoscopy. 1992;24:350–353. [DOI] [PubMed] [Google Scholar]
- 267.Rosch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721–1726. [DOI] [PubMed] [Google Scholar]
- 268.Gines A, Vazquez-Sequeiros E, Soria MT, et al. Usefulness of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of functioning neuroendocrine tumors. Gastrointest Endosc. 2002;56:291–296. [DOI] [PubMed] [Google Scholar]
- 269.Pitre J, Soubrane O, Palazzo L, et al. Endoscopic ultrasonography for the preoperative localization of insulinomas. Pancreas. 1996;13:55–60. [DOI] [PubMed] [Google Scholar]
- 270.Nesje LB, Varhaug JE, Husebye ES, et al. Endoscopic ultrasonography for preoperative diagnosis and localization of insulinomas. Scand J Gastroenterol. 2002;37:732–737. [DOI] [PubMed] [Google Scholar]
- 271.Waddell WR, Coppinger WR, Loughry RW. Pancreaticoduodenectomy for Zollinger-Ellison syndrome. Ann Surg. 1968;168:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Oberhelman HA Jr. Excisional therapy for ulcerogenic tumors of the duodenum: long- term results. Arch Surg. 1972;104:447–453. [DOI] [PubMed] [Google Scholar]
- 273.Bonfils S, Landor JH, Mignon M, et al. Results of surgical management in 92 consecutive patients with Zollinger-Ellison syndrome. Ann Surg. 1981;194:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Roche A, Raisonnier A, Gillon-Savouret MC. Pancreatic venous sampling and arteriography in localizing insulinomas and gastrinomas: procedure and results in 55 cases. Radiology. 1982;145:621–627. [DOI] [PubMed] [Google Scholar]
- 275.Cavina E, Pagliai EF, Goletti O, et al. Pylorus preserving pancreatoduodenectomy for gastrinoma: a case report. Ital J Surg Sci. 1986;16:291–295. [PubMed] [Google Scholar]
- 276.Stamm B, Hedinger CE, Saremaslani P. Duodenal and ampullary carcinoid tumors: a report of 12 cases with pathological characteristics, polypeptide content and relation to MEN 1 syndrome and von Recklinghausen's disease (neurofibromatosis). Virchows Arch A Pathol Anat Histopathol. 1986;408:475–489. [DOI] [PubMed] [Google Scholar]
- 277.Lind T, Olbe L. Long term follow up of patients with Zollinger-Ellison syndrome (ZES). Acta Chir Scand. 1989;155:383–388. [PubMed] [Google Scholar]
- 278.Rosato FE, Bonn F, Shapiro M, et al. Selective arterial stimulation of secretin in localization of gastrinomas. Surg Gynecol Obstet. 1990;171:196–200. [PubMed] [Google Scholar]
- 279.Udelsman R, Yeo CJ, Hruban RH, et al. Pancreaticoduodenectomy for selected pancreatic endocrine tumors. Surg Gynecol Obstet. 1993;177:269–278. [PubMed] [Google Scholar]
- 280.Schroder W, Holscher AH, Beckurts T, et al. Duodenal microgastrinomas associated with Zollinger-Ellison syndrome. Hepatogastroenterology. 1996;43:1465–1469. [PubMed] [Google Scholar]
- 281.Phan GQ, Yeo CJ, Cameron JL, et al. Pancreaticoduodenectomy for selected periampullary neuroendocrine tumors: fifty patients. Surgery. 1997;122:989–997. [DOI] [PubMed] [Google Scholar]
- 282.Partensky C, Berger F, Owono P, et al. Cephalic duodenopancreatectomy for endocrine tumor of the ampulla of Vater and of the minor papilla. Gastroenterol Clin Biol. 1999;23:832–836. [PubMed] [Google Scholar]
- 283.Lairmore TC, Chen VY, DeBenedetti MK, et al. Duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2000;231:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Siech M, Mattfeldt T, Schlosser W, et al. Duodenum-preserving pancreatic head resection in patients with benign and borderline tumors of the pancreatic head. Langenbecks Arch Surg. 2000;385:229–233. [DOI] [PubMed] [Google Scholar]
- 285.Sarmiento JM, Farnell MB, Que FG, et al. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg. 2002;26:1267–1271. [DOI] [PubMed] [Google Scholar]