Abstract
Objective:
To determine the evidence-based value of prophylactic drainage in gastrointestinal (GI) surgery.
Methods:
An electronic search of the Medline database from 1966 to 2004 was performed to identify articles comparing prophylactic drainage with no drainage in GI surgery. The studies were reviewed and classified according to their quality of evidence using the grading system proposed by the Oxford Centre for Evidence-based Medicine. Seventeen randomized controlled trials (RCTs) were found for hepato-pancreatico-biliary surgery, none for upper GI tract, and 13 for lower GI tract surgery. If sufficient RCTs were identified, we performed a meta-analysis to characterize the drain effect using the random-effects model.
Results:
There is evidence of level 1a that drains do not reduce complications after hepatic, colonic, or rectal resection with primary anastomosis and appendectomy for any stage of appendicitis. Drains were even harmful after hepatic resection in chronic liver disease and appendectomy. In the absence of RCTs, there is a consensus (evidence level 5) about the necessity of prophylactic drainage after esophageal resection and total gastrectomy due to the potential fatal outcome in case of anastomotic and gastric leakage.
Conclusion:
Many GI operations can be performed safely without prophylactic drainage. Drains should be omitted after hepatic, colonic, or rectal resection with primary anastomosis and appendectomy for any stage of appendicitis (recommendation grade A), whereas prophylactic drainage remains indicated after esophageal resection and total gastrectomy (recommendation grade D). For many other GI procedures, especially involving the upper GI tract, there is a further demand for well-designed RCTs to clarify the value of prophylactic drainage.
Many surgeons consider prophylactic drainage as a method to reduce and detect postoperative complications. On the other hand, there is growing evidence that routine drainage may not be useful or may even be harmful after many GI procedures. There is grade A recommendation that drains do not reduce complications after hepatic, colonic, or rectal resection with primary anastomosis and appendectomy for any stage of appendicitis. The routine use of drains should be revisited.
Drainage of body cavities has been practiced in medicine for a long time. Historical reports of drainage of chest empyema and ascites go back to the Hippocratic era.1 During the last 2 centuries, surgeons also used drains for prophylactic purposes. Prophylactic drains have been employed to remove intraperitoneal collections such as ascites, blood, bile, chyle, and pancreatic or intestinal juice. These collections might become potentially infected or are, in the case of bile and pancreatic juice, toxic for adjacent tissue. Another potential function of prophylactic drains is their signal function to detect early complications, such as postoperative hemorrhage and leakage of enteric suture lines.2 Therefore, prophylactic drainage has gained wide acceptance as a useful method to prevent complications after gastrointestinal (GI) surgery.
Sims was the first surgeon who used prophylactic drains after gynecologic operations in the last quarter of the 19th century.1 Since that time, surgeons have routinely used prophylactic drainage of the peritoneal cavity after abdominal surgery. Theodor Billroth was convinced that prophylactic drainage of the peritoneal cavity saved many lives after GI surgery.3 Other contemporaries believed that drainage of the peritoneal cavity is impossible and, therefore, prophylactic drainage is useless.4,5
During the last 3 decades, surgeons have made efforts to investigate the value of prophylactic drainage after abdominal surgery in controlled randomized clinical trials (RCTs). Despite evidence-based data questioning prophylactic drainage in many instances, most surgeons around the world continued to use them on a routine basis. Therefore, we reviewed the literature by using the novel approach of evidence-based medicine, and classified the studies according to their quality of evidence to identify the value of prophylactic drainage after several GI procedures. Additionally, we performed 3 meta-analyses of RCTs to characterize the drain effect after hepatic resection, colorectal surgery, and appendectomy for gangrenous and perforated appendicitis.
METHODS
Literature Search
An electronic search of the Medline database from 1966 to February 2004 was performed to identify relevant articles that compare prophylactic drainage with no drainage in GI surgery. The following search terms were used: “surgical drainage,” “intraperitoneal drainage,” “prophylactic drainage,” and “abdominal drainage” in various combinations and in combination with the corresponding GI organ system and procedures such as “liver,” “liver resection,” and “hepatic resection.” The search was extended to the GI organs “liver,” “gallbladder,” “pancreas,” “esophagus,” “stomach,” “small intestine,” “appendix,” “colon,” and “rectum.” Abdominal surgery for trauma was excluded from our analysis. Outcome variables such as “mortality,” “morbidity,” “complication,” “leakage,” “wound infection,” “collections,” “pulmonary complications,” “reoperation,” and “hospital stay” were applied to the search terms mentioned above. In addition to the Medline search, we used manual cross-referencing to identify further studies.
Study Review and Quality Grading
Data were extracted from original articles by 2 independent reviewers (HP and ND) regarding the methods and results of studies. Discrepancy between the reviewers was resolved by consensus. We included only published full-length papers. Although we identified studies by an English-written title and abstract, the review and meta-analysis were not language-restricted. All studies were classified according to their level of evidence by using the classification proposed by the Oxford Centre for Evidence-based Medicine (Table 1). 6,7 In addition to a narrow confidence interval, sample size calculation, reported method of randomization, and precise definition of exclusion/inclusion were further criteria for a RCT to be classified as 1b; otherwise, RCTs were ranked as 2b. Based on the level of evidence, grades of recommendations (A, B, C, D) were given (Table 1). Studies were compared for the following end points: mortality, overall complication rates, leakage rates, infection rates (wound, intra-abdominal collections, abscess), pulmonary complication rates, reoperation rates, and hospital stay.
TABLE 1. Levels of Evidence and Grade of Recommendation Proposed by the Oxford Centre for Evidence-based Medicine [6,7]
Meta-analysis
Inclusion criteria for meta-analysis were RCTs of any sample size, which compared a prophylactic drain group and a no-drain group with respect to various dichotomous end points (eg, wound infection, pulmonary complication, etc). Meta-analysis was performed either if no meta-analysis has been published yet or if new RCTs were published after a previous meta-analysis. We characterized the drain effect by odds ratio (OR) such that values less than 1 favor drains, whereas values larger than 1 favor no drains. Our goal was to calculate a 95% confidence interval (CI) for the OR and hence to test for a significant drain effect at the 5% level by using the data of all RCTs. For this, we used for all meta-analysis the random-effects model described by Whitehead and Whitehead,8 which models the logarithm of OR. Thus, we considered the “true drain effect” to differ from study to study and estimated an “average drain effect.” This statistic model assumes a normal distribution for the estimates of the logarithm of the OR. The weights of each study in the meta-analysis are also provided. Heterogeneity between studies was evaluated by using the χ2-based Q statistic proposed by Cochran.9 When no events were observed for certain end points in some groups, we added 0.25 events to each group and each study for the corresponding end point to avoid computational problems. P values smaller than 0.05 were considered as significant, which corresponds to a 95% CI of the OR that excludes the value 1 on a log scale.
RESULTS
Liver Resection
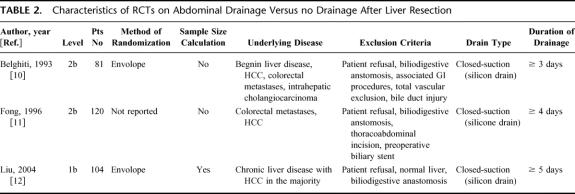
Intraperitoneal drainage after elective liver resection is still routinely used in many hospitals worldwide. We identified 4 studies (3 RCTs of level 1b12 and 2b10,11 and 1 large retrospective cohort study of level 2b13) that examined the prophylactic value of intraperitoneal drainage after hepatic resection (Table 2).
TABLE 2. Characteristics of RCTs on Abdominal Drainage Versus no Drainage After Liver Resection
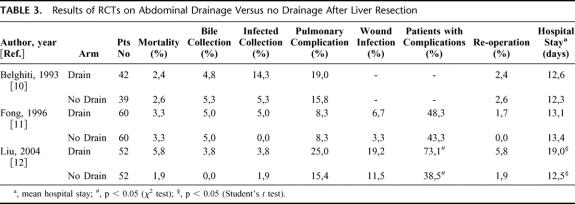
The study population of the French and American RCT was heterogeneous in regard to the underlying disease,10,11 whereas the Asian RCT included only patients with chronic liver disease (Table 2).12 In each RCT, patients were randomized to closed-suction drainage or to no drainage after elective liver resection. Each trial, including the large level 2b cohort study, demonstrated increased rates of infected collections in drained patients when compared with nondrained patients (Table 3). The reported biloma rate of approximately 5% in the RCTs was either equal to or even higher in drained patients compared with nondrained patients. This implies that drainage does not prevent the occurrence of bile collections. Despite operative drainage, 5 of 60 (8%) patients had to be drained percutaneously in the American RCT, whereas 11 of 60 patients (18%) in the nondrained group needed percutaneous drainage.11 However, the univariate analysis revealed that operative drainage status did not correlate with the need of percutaneous drainage in this study where the majority of cases (70%) were extended procedures such as hemi-hepatectomies or more. The recently published RCT on abdominal drainage in chronic liver disease demonstrated higher rates of postoperative wound, septic, and overall complications in drained patients, resulting in a significantly longer hospital stay.12 The multivariate analysis revealed that abdominal drainage was an independent risk factor for postoperative morbidity in this study. Drains also failed to detect significant postoperative complications such as bile leak and hemorrhage that needed surgical or radiologic interventions.12
TABLE 3. Results of RCTs on Abdominal Drainage Versus no Drainage After Liver Resection
Although 1 RCT included only patients with chronic liver disease,12 we performed a meta-analysis including all 3 RCTs because the other 2 RCTs also included patients with chronic liver disease.10,11 The meta-analysis was performed with pooled data from 154 drained and 150 nondrained patients. The analysis revealed a slight advantage for nondrained patients with respect to infected intra-abdominal collections (OR 2.83; CI 0.82–9.71); however, this advantage did not reach statistical significance. Drainage status had no influence on the outcome of bile collections (OR 1.15; CI 0.36–3.68) or pulmonary complications (OR 1.40; CI 0.73–2.68) (Fig. 1).
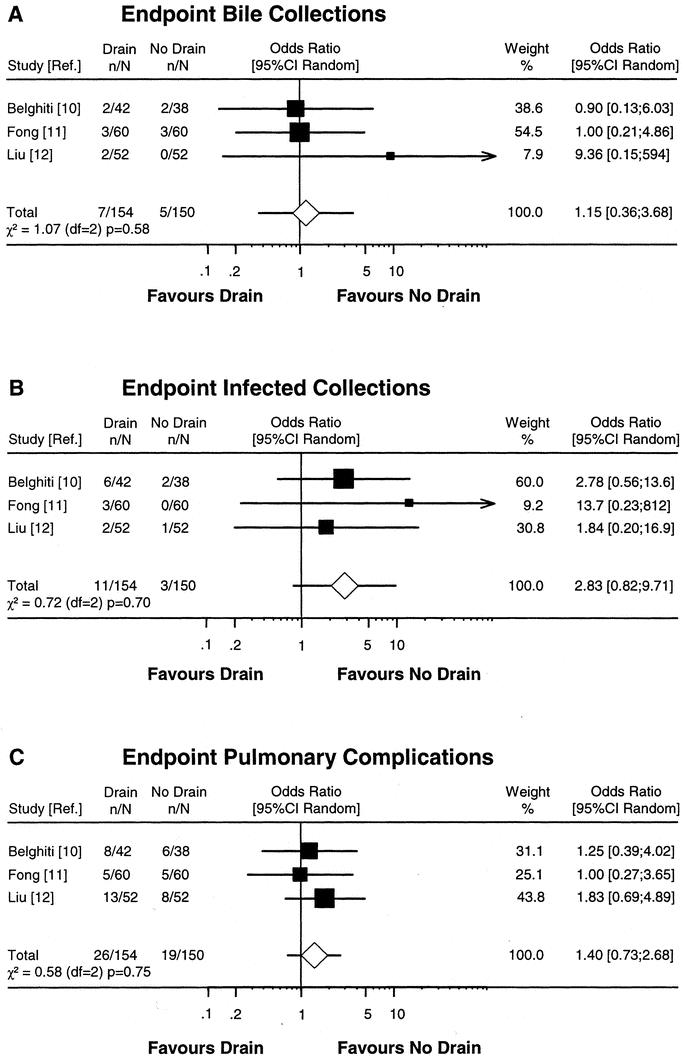
FIGURE 1. Meta-analysis of RCTs comparing prophylactic drain versus no drain after liver resection. Estimates of drain effects of each study are presented on a log scale along with the 95% CI. The weight of each study is reflected by the size of square. Studies were analyzed according to the complication end points bile collections (A), infected collections (B), and pulmonary complications (C). The open diamond represents the global estimate of the drain effect along with the 95% CI (random-effects model). Test of heterogeneity is provided by the χ2-based Q statistic.
Cholecystectomy
Numerous RCTs were performed on prophylactic drainage after open cholecystectomy.14–24 All trials, including the meta-analysis by Lewis et al,17 failed to demonstrate a reduction of postoperative complications by routine drainage. Regarding the laparoscopic approach, only 2 RCTs were identified, and both studies were primarily focused on the effect of intraperitoneal gas drains.25,26 Gas drains are designated to remove remnant gas after laparoscopy that might be responsible for postoperative nausea and pain. In 1 recent study, 4 of 34 drained patients (11.7%) developed complications after laparoscopic cholecystectomy, whereas only 2 of 33 nondrained patients (6.1%) had complications; however, this difference was not statistically significant.26 Gas drains had no significant effect on postoperative nausea and pain in this study. The other RCT demonstrated a benefit of gas drains by reducing postoperative shoulder tip pain, but data on complications after laparoscopic cholecystectomy were not reported.25
Pancreatic Resection
We identified 1 RCT (level 1b)28 and 1 cohort study (level 2b)27 that compared prophylactic drainage versus no drainage after pancreatic resection for pancreatic cancer. Both studies arose from a specialized institution (Memorial Sloan-Kettering Cancer Center, New York, NY). The retrospective cohort study of 89 consecutive pancratico-duodenectomies failed to demonstrate any benefit of prophylactic drainage.27 In the RCT, 88 patients were randomized to closed-suction drainage and 91 to no drainage after pancreatic resection.28 Each patient had pancreatic cancer with a tumor predominantly located in the head of the pancreas. In this RCT, intraperitoneal drainage status had no influence on the overall complication rate. Interestingly, the subgroup analysis showed that drained patients had a significantly higher incidence of intra-abdominal collections and fistulas (19 vs. 8 patients, P < 0.02). Thirteen patients were drained percutaneously due to intra-abdominal collections, and 8 of them belonged to the intraperitoneal drainage group.
Esophageal, Gastric, and Duodenal Surgery
In contrast to hepato-pancreatico-biliary (HPB) and lower GI tract surgery, neither prospective studies on prophylactic drainage versus no drainage after esophageal and gastric surgery could be identified by our literature search. We found only 1 nonrandomized prospective cohort study (level 2b) that examined the role of prophylactic drains after surgery for perforated duodenal ulcer.29 In this study, 119 patients underwent surgery with omental patch technique for perforated duodenal ulcer. Based on the surgeon's decision, 75 patients received a prophylactic drain, whereas 44 patients had no intraperitoneal drains after surgery. Drainage neither reduced the incidence of intra-abdominal fluid collections including abscesses nor the duration of hospital stay. Furthermore, there were a significant number of drain-related complications such as drain tract infections (10.7%) and acute intestinal obstruction (2.7%).
Colorectal Surgery
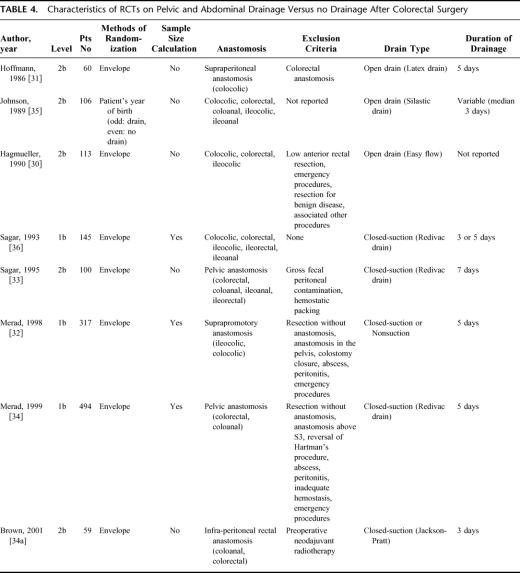
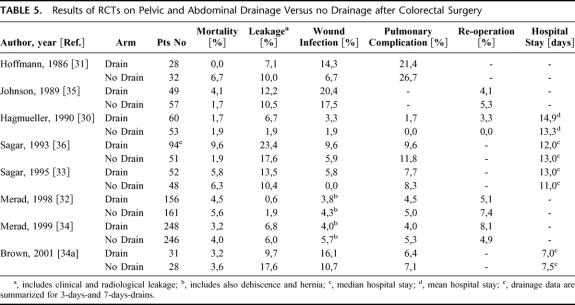
Currently, 8 RCTs on abdominal/pelvic drainage versus no drainage after colorectal surgery have been published (Tables 4 and 5). Three RCTs were classified as level 1b studies, whereas the other 5 RCTs had a lower evidence level (2b). Six of 8 RCTs included specific population of patients based on the level of the anastomosis, such as supraperitoneal,30,31 suprapromotory,32 or pelvic anastomosis,33,34,34a whereas 2 RCTs were composed of a heterogeneous population.35,36 The majority of trials30–32,34,31–35 studied only elective surgery, whereas 2 other also included emergency procedures.33,36 Emergency procedures were in the minority and were equally represented in the drained and nondrained group in both studies. All studies revealed a similar pattern of postoperative complications for drained and nondrained patients. Some studies noted higher wound infection rates in drained patients;31,33,36 however, the 2 largest multicenter series published recently showed comparable wound infection rates in drained and nondrained patients.32,34 Anastomotic leakage detected clinically or radiologically ranged from 1% to 23%, and was not statistically significant between drainage and no drainage. Differences in mortality among the groups were unrelated to the anastomosis in the majority.
TABLE 4. Characteristics of RCTs on Pelvic and Abdominal Drainage Versus no Drainage After Colorectal Surgery
TABLE 5. Results of RCTs on Pelvic and Abdominal Drainage Versus no Drainage after Colorectal Surgery
Despite some heterogeneity in anastomotic population, drain type, and duration of drainage (Table 4) we performed a new meta-analysis including all 8 RCTs (level 1b and 2b) with pooled data from 717 drained and 673 nondrained patients (Fig. 2). The analysis confirmed the results of a previously published meta-analysis of 4 RCTs with pooled data from 223 drained and 188 nondrained patients.37 A slight advantage for nondrained patients in respect to clinical leakage (OR 1.38; CI 0.77–2.49) and wound infections (OR 1.41; CI 0.87–2.29) was documented, although this advantage was not statistically significant. Pulmonary complications were comparable in both groups (OR 0.83; CI 0.52–1.32). Moreover, the meta-analysis by Urbach et al showed that in only 1 of 20 clinical leakages pus or feces emerged through the drain,37 indicating that drains have a low sensitivity (5%) to detect clinical leakage.
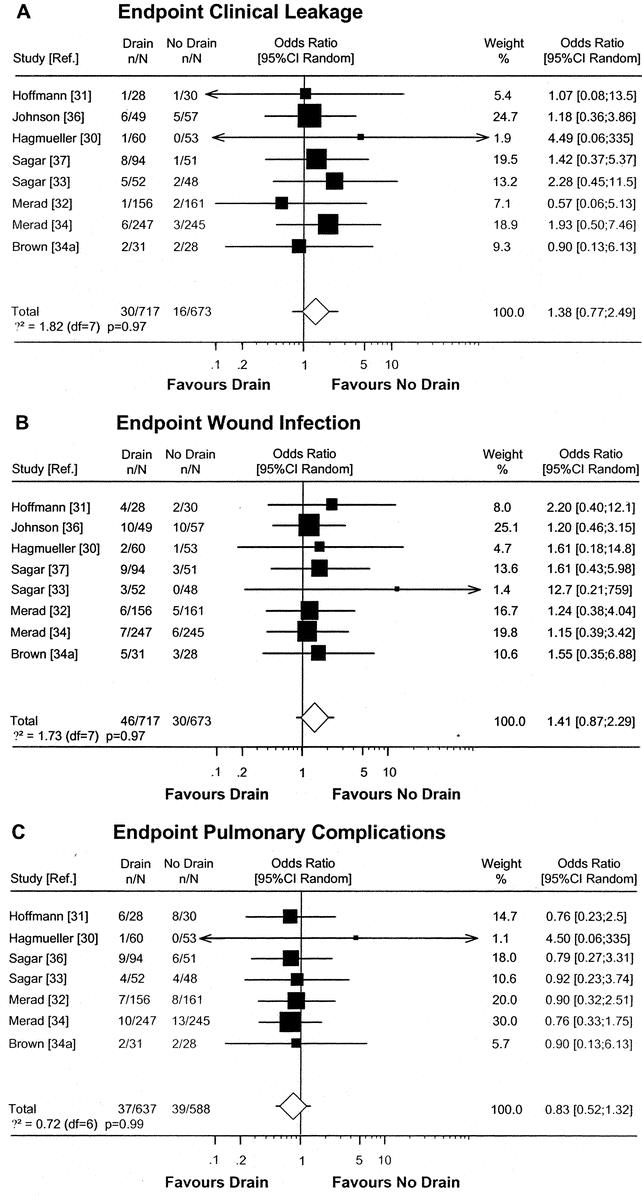
FIGURE 2. Meta-analysis of RCTs comparing prophylactic drain versus no drain after colorectal resection. Estimates of drain effects of each study are presented on a log scale along with the 95% CI. The weight of each study is reflected by the size of square. Studies were analyzed according to the complication end points clinical leakage (A), wound infections (B), and pulmonary complications (C). The open diamond represents the global estimate of the drain effect along with the 95% CI (random-effects model). Test of heterogeneity is provided by χ2-based Q statistic.
In light of the anastomotic heterogeneity among the 7 RCTs, the question arises whether the value of prophylactic drains is different for pelvic and suprapromotoric anastomosis. Although we did not perform a meta-analysis on special anastomotic populations, the RCTs on pelvic anastomosis33,34,34a and suprapromotoric anastomosis31,32 demonstrated no difference in complications between drained and nondrained patients regarding both anastomotic levels (Table 5).
Appendectomy
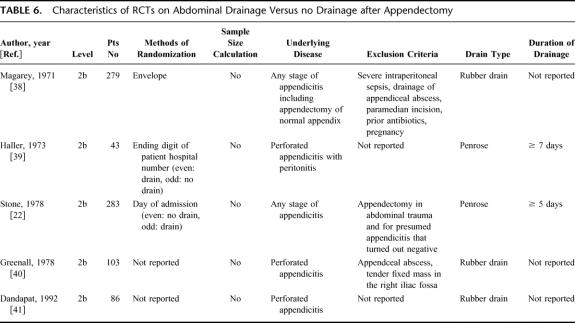
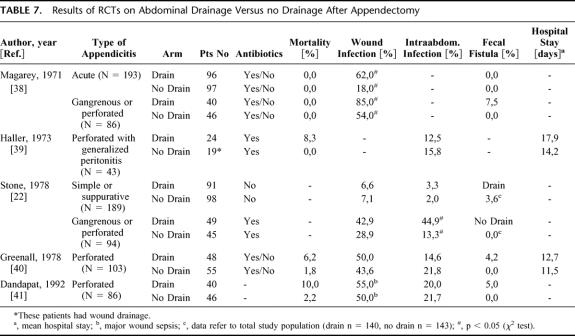
Appendectomy is the most common GI operation, usually performed for acute appendicitis. The stage of appendicitis can range from a simple acute type to a severe gangrenous or perforated form. Two RCTs investigated the value of prophylactic drainage after open appendectomy for acute/simple appendicitis.22,38 Although both arms (drainage, no drainage) of the trials had a relatively large sample size (>90 patients each group), the studies were performed without a power and sample size calculation and were therefore ranked as level 2b (Table 6). One study reported a significantly higher wound infection rate in drained patients with acute/simple appendicitis,38 whereas the other study found similar wound and intra-abdominal infection rates in drained and nondrained patients (Table 7). 22
TABLE 6. Characteristics of RCTs on Abdominal Drainage Versus no Drainage after Appendectomy
TABLE 7. Results of RCTs on Abdominal Drainage Versus no Drainage After Appendectomy
The value of prophylactic drainage after appendectomy might be different in the gangrenous and perforated form. Five RCTs on prophylactic drainage for gangrenous and perforated appendicitis were identified (Table 6). Because of the same reasons mentioned above, the level of evidence was classified as 2b in each RCT. The results showed higher wound infection rates in drained patients (range 43–85%) than in nondrained patients (range 29–54%). The pattern of intra-abdominal infections was not uniform among the studies, as 2 studies reported slightly higher intra-abdominal infection rates in nondrained patients,39,40 1 study a higher rate in drained patients,22 and another a similar rate in both groups.41 Interestingly, the development of fecal fistulas was only observed in drained patients with a rate ranging from 4.2 to 7.5%.
We performed a meta-analysis including series with gangrenous or perforated appendicitis only. Four RCTs (all level 2b) were included in the meta-analysis with the end point wound infection, whereas data from 3 RCTs were available for the end points intra-abdominal infection and fecal fistula (Fig. 3). The study by Haller et al was excluded from the meta-analysis because the entire study population was composed of pediatric patients, and the no-drainage group had additional wound drainage.39 The analysis calculated an OR for wound infections of 1.75 (CI 0.96–3.19). The OR for fecal fistulas of 12.4 (CI 1.14–135) favors the no-drainage group, whereas the OR for the end point intra-abdominal infection of 1.43 (CI 0.39–5.29) favors neither group.
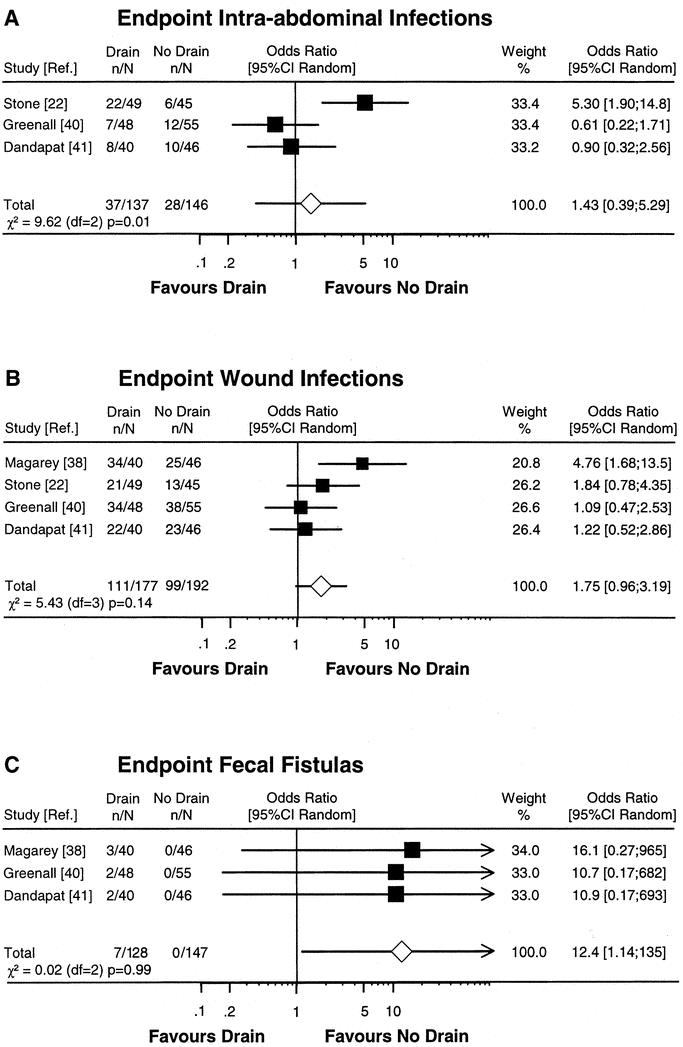
FIGURE 3. Meta-analysis of RCTs comparing prophylactic drain versus no drain after appendectomy for gangrenous or perforated appendicitis. Estimates of drain effects of each study are presented on a log scale along with the 95% CI. The weight of each study is reflected by the size of square. Studies were analyzed according to the complication end points intra-abdominal infections (A), wound infections (B), and fecal fistulas (C). The open diamond represents the global estimate of the drain effect along with the 95% CI (random-effects model). Test of heterogeneity is provided by χ2-based Q statistic.
DISCUSSION
Only well-designed RCTs with adequate sample sizes can provide convincing answers on the value of prophylactic drainage to reduce and detect postoperative complications after GI procedures. In the absence of a definitive review study in this area, we conducted an exhaustive evidence-based analyses based on the methodology from the Center of Evidence-based Medicine to provide recommendations on the use of prophylactic drainage in GI surgery (Table 1).6,7 The data demonstrate that in many instances prophylactic drains are useless or may even add to the morbidity or cost of a procedure. The discussion will be presented separately for HPB surgery and upper and lower GI tract surgery.
HPB Surgery
Currently, hepatic resection can be performed with mortality rates below 5% and acceptable complication rates of 16% to 31%.42,44,45 Subphrenic collections and biliary fistulas/bilomas are still the most common intra-abdominal complications after liver resection,43,44 and most surgeons around the world still use prophylactic drains to prevent or detect these complications at an early stage. Interestingly, the available evidence contradicts this practice, as 3 RCTs from a French, an American, and an Asian HPB center failed to show any advantage for prophylactic drainage.10–12 For example, 1 major finding of these RCTs and our meta-analysis was that prophylactic drains did not prevent the occurrence of bile collections. Thus, even if drains are initially productive, they seem unable to evacuate bilomas completely. The important drawback of ascending infection associated with drains may also occur in the setting of liver resection; a trend toward increased rates of infected collections was observed in the present analysis. Similar to colorectal surgery, drains also failed to detect bile leakage or hemorrhage after liver resection.12 In the current era of modern interventional radiology, symptomatic collections can be safely and successfully managed percutaneously in most cases. Thus, from this analysis we conclude that the routine use of prophylactic drains after elective liver surgery in both normal and diseased liver is no longer jusitified (recommendation grade A). Moreover, it seems that patients with chronic liver disease are particularly prone to increased complications related to the drain.12
Cholecystectomy is the second most commonly performed operation in GI surgery after appendectomy. The limited data on the value of prophylactic drains for laparoscopic cholecystectomy is possibly related to the previous evidence that such approach was not justified in the era of open cholecystectomy (level 1a evidence).17 Only 2 RCTs were performed after laparoscopic cholecystectomy with a different focus on removing residual gas and decreasing postoperative pain.25,26 Here also, gas drains failed to provide any advantage. We conclude that routine prophylactic drainage is not indicated after open and laparoscopic cholecystectomy (recommendation grade A).
Prophylactic drainage after pancreatic resection was considered for a long time by many experts as mandatory to detect and mainly control potential leaks, particularly from the pancreato-jejunostomy anastomosis. This rule possibly explains the paucity of data available on prophylactic drainage in pancreas surgery. This belief was recently challenged by a RCT from a specialized institution with a highly selected study population where prophylactic drainage failed to demonstrate any benefit.29 However, the conclusion of this trial can be applied only to pancreatic resection for pancreatic cancer and cannot be accepted for pancreatic resections for other diseases such as chronic pancreatitis. Additional well-designed RCTs from other institutions are needed to provide convincing information on the value of prophylactic drainage after pancreatic resection.
Upper GI Tract Surgery
In contrast to HPB and lower GI tract surgery, very few studies have focused on prophylactic drainage in upper GI tract surgery. Esophageal resections are usually extended procedures that are associated with significant mortality and morbidity rates, often related to anastomotic leakage. In contrast to the lower GI tract, intrathoracic leaks are poorly surrounded by tissue, and thus the majority of leaks are apparent and better detectable by a drain.46 Therefore, there is a consensus that esophageal resections have to be drained and failure to do so would likely be considered as malpractice in most countries (level 5 evidence). Thus, the analysis concludes that prophylactic intrathoracic drains should be used routinely in esophageal surgery (recommendation grade D).
The fact that there are no studies on the value of prophylactic drainage after gastric surgery was surprising because gastric surgery constitutes a significant part of GI surgery. Prophylactic drainage after total gastrectomy is a common practice in many institutions. Drains are normally placed in relation to the esophago-jejunostomy. Similarly to esophageal resections, this practice is justified due to potentially life-threatening mediastinitis in the case of disruption of the transhiatal anastomosis that occurs in 3% to 11% of cases (recommendation grade D).47 For distal gastrectomies and gastric bypass surgery, the value of prophylactic drainage remains unclear. Therefore, evidence-based recommendations cannot be given for these procedures. This illustrates that the field of gastric surgery needs well-designed RCTs on prophylactic drainage.
Duodenal surgery with omental patch technique for perforated duodenal ulcer appears to be safe without prophylactic drainage,29 and routine drainage cannot be recommended after this procedure (recommendation grade B).
Lower GI Tract Surgery
The value of prophylactic drainage in colorectal surgery has been better investigated than in other GI procedures. The available RCTs and our own meta-analysis point out that the use of routine prophylactic drainage provides no benefit after uncomplicated major colon and rectal surgeries.30–36 There was a trend favoring a no-drainage policy regarding wound infection and the incidence of clinically apparent anastomotic leakage. Similarly to liver resection, the studies underscore the low sensitivity of drains in detecting leakage and bleeding, questioning the putative warning function of a prophylactic drain.12,40 The large variability of drainage duration among the RCTs (3–7 days) may indicate the need for future RCTs that are focused on drainage duration, especially on short-term drainage (24–48 hours), which has not been investigated yet. From this analysis, we conclude there is no evidence that justifies routine drainage of colon and rectal anastomosis after uncomplicated surgery (recommendation grade A).
Similar conclusions can be drawn in appendectomy for appendicitis. Neither simple/acute nor the gangrenous or perforated appendicitis benefit from the routine use of prophylactic drainage. Even considering the heterogeneity in the antibiotic regimens used among the studies, drainage did not reduce postoperative complications and even appeared harmful in respect to the development of fecal fistula. We conclude from the analysis that prophylactic drains should be avoided in any stage of appendicitis (recommendation grade A).
In conclusion, the current review shows that many GI operations can be performed safely without prophylactic drainage and that new guidelines on the use of prophylactic drainage are necessary in many centers. The current review illustrates also that there is further need for RCTs on prophylactic drainage for many GI procedures, especially upper GI tract surgery.
Note: A recently published RCT (level 2b) has demonstrated that prophylactic drainage failed to have any benefit after subtotal or total gastrectomy with extended lymph node dissection as compared to no drainage.48 The authors of this RCT do not recommend prophylactic drainage on a routine basis after gastric cancer surgery (recommendation grade B). This study which is the first RCT on prophylactic drainage in gastric surgery was published after the publication of this manuscript was in process.
Discussion
Dr. Johnson: When I first started gastric surgery in the 1960s, I was told that if I drained my first 100 gastrectomies, I would be wise enough to drain my next 100! There was an understandable fear of abdominal sepsis in the days when there was very little antibiotic cover and no scanning to detect intra-abdominal collections. The hardest thing in surgery is to stop something that has been done for years, and you must be congratulated on looking at the evidence for drainage. However, it is disappointing that there is so little good data in an area that lends itself to randomized controlled trials.
You mentioned that drains were put in for 3 reasons—to drain residual contamination, to drain an early leak, or to provide a track for late leaking. Did you look at the time that drains were left in, because there is a great difference between 24 hours and 10 days? Surgeons tend to use drains when they are unhappy about the surgical technique—Were there differences in technique between the different trials used in meta-analysis? Thirdly, was there evidence about whether the drains were actually functioning? Closed drains tend to block, and open drains tend to contaminate the wound. Perhaps the main value of this article is to counter those lawyers who, advised by old-fashioned surgeons, blame lack of drains for complications. Thank you very much for this contribution.
Dr. Petrowsky: Thank you very much, Dr. Johnson, for your nice comments and insight into this study. The duration that drains were left in place varied from study to study ranging between 3 and 7 days. We found only 1 study that compared drainage left in place for 3 days versus 7 days versus no drainage following colorectal surgery. In this study, drains were found to be of no use, regardless of the duration of drainage. Thus, how the duration of drainage influences outcome remains open. Your second question is whether different surgical techniques among studies may have influenced the meta-analysis. Although we carefully selected studies, which met similar criteria, there is an inherent degree of heterogeneity among the trials. To address this issue in a statistically correct manner, we used the random-effects model, which considers “between-study” variability, rather than the fixed-effects model, which assumes homogeneous treatments and homogeneous study populations. Your third question is whether closed drainage remained functional, as you point out that such drainage tends to get blocked easily. This information was not reported in detail in most studies. Although we did not present the topic of open versus closed drain in our paper, our insight into this topic indicates that closed drains are superior, as open drainage without suction is associated with higher infection rates.
Dr. Adam: I have been very impressed by your work, and I first would like to thank you for the tremendous work that this represents. I would like to say that this is probably very useful for what our president has called general surgery. I personally belong to a school that drains routinely all the patients we have operated on. I think that your data has reminded me of all the studies, and possibly we'll change our practice a little bit.
Dr. Bozetti: I would like to come back to the point made by Dr. Johnson. I understand that you looked at what the literature has to offer, but in my practice the most important point is not “to drain or not to drain,” but how long to maintain a drain. If there is a possibility to distinguish this, that would be quite interesting. Furthermore, I wonder if it is correct to put together colon and rectal surgery. I think it would be more correct to try to distinguish rectal surgery and especially surgery involving a supraperitoneal anastomosis from those that need pelvic anastomosis.
Dr. Petrowsky: Dr Bozetti, thank you for your comments. As discussed above, your first question on the optimal length a drain should be left in place would be an excellent topic for a next trial; however, as mentioned above, such data will be of interest only if a “no drain” group is available. Next, is there a bias in lumping rectal and colon resections together? We did not show the slide presenting the different levels of anastomosis, but in Fig. 2 in the manuscript, we observed similar drain effects among trials in colorectal surgery, indicating a high degree of homogeneity. Additionally, when we looked separately at studies of rectal and colon surgery, we found that drains failed to reduce complications following either supraperitoneal or pelvic anastomoses. Thus, we conclude that drains may not be used; because of no help, why should we use them?
Dr. Gouma: Dr. Petrowsky I also enjoyed your paper very much, and I agree with most of the conclusions. I had the opportunity to see the manuscript beforehand and I also looked in more detail at the part you did not discuss today, for example, drainage after cholecystectomy. In that part, the evidence is based on studies written more than 20 years ago only analyzing cholecystectomy, and they should not be extrapolated for laparoscopic cholecystectomy.
Another controversy concerns pancreatic surgery. The conclusion of drainage after pancreatic surgery is mainly based on 1 randomized study from Sloan-Kettering in New York. You should realize that in that particular study, mainly patients with pancreatic carcinoma are included. All pancreatic surgeons know that patients with pancreatic cancer with a pancreatic duct of 2 cm and a hard/firm pancreas do not suffer from leakage and, indeed, you do not have to drain that particular group of patients. But again, you cannot extrapolate these data for patients with periampullary tumors or neuro-endocrine tumors of the pancreas with a soft pancreas and nondilated ducts.
So, we still should be cautious with conclusions from 1 trial of a particular subset of patients extrapolating to the entire group of patients undergoing pancreatic surgery. But again, I enjoyed the presentation and excellent analysis of these studies.
Dr. Petrowsky: Dr Gouma, thank you for your questions. It is true that most data on cholecystectomy were gathered in trials published more than 20 years ago, and thus apply only to the open approach. The rationale for the lack of data in the laparoscopic area is probably related to the feeling by most that drains should not be used, as they failed to provide a benefit after open surgery. We know only about 2 randomized trials in laparoscopic cholecystectomy, but these trials were primarily focused on gas drains to prevent postoperative pain. One of these trials reported also on postoperative complications that were not significantly different for drained and nondrained patients. Regarding pancreas surgery, we fully agree with you on being cautious with the conclusion. The only available trial was from a highly specialized institution, Sloan-Kettering in New York, and was limited to patients with pancreatic cancer. It is clear that further trials are needed to reach any conclusion. Of note, the credit for no drainage should go to Dr. Jeekel, who first published in 1992 a series of 22 patients who underwent a safe Whipple procedure without drainage.
Dr. Bismuth: In some patients, in my experience, I get the feeling that I have to drain and I do, and in some patients I do not have to drain and I do not. In your study, your answer is yes or no for all patients. Which study do we have to perform to answer to the question, For a given operation, which patient do you have to drain?
Dr. Clavien: Thanks, Dr. Bismuth, and each other discussant for this lively debate. This discussion has clearly reached his goal in that evidence-based medicine is not a cookbook, as stated by Dr Petrowsky, but rather should serve as a guideline for standardized procedures, and as a basis to debate on specific cases. Of course, a surgeon who is dealing with a situation which does not tone with the evidence-based data should go for her/his own decision. What should be the next study? I can think about many. I would favor a trial testing the length of drainage (eg, no vs. 24 hour vs. 3–5 days of drainage) in a situation where a drain may still appear worthwhile, for example, following gastric, pancreas, or rectal surgery. I would like also to take the opportunity to underscore the need for us to better understand the methodology used in the complex field of evidence-based medicine. While we need to accept a change in our practice, we have to remain highly critical in interpreting the data. These studies should be performed from the beginning in close collaboration with a statistician who is familiar with such an approach, for example, meta-analysis.
Finally, I would like to welcome the last point from Dr. Johnson, in that data from evidence-based medicine might help a surgeon facing a legal issue, but I would add that such data should be used with great caution to demonstrate a mistake, as our judgment remains the mainstay of our art to treat a specific patient.
Footnotes
Reprints: Pierre-Alain Clavien, MD, PhD, FACS, FRCS, Department of Visceral and Transplant Surgery, University Hospital Raemistrasse 100, CH-8091 Zürich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Robinson JO. Surgical drainage: a historical perspective. Br J Surg. 1986;73:422–426. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty HH, Simmons RL. The biology and practice of surgical drains. Curr Probl Surg. 1992;29:559–730. [DOI] [PubMed] [Google Scholar]
- 3.Billroth T. Clinical Surgery. Translated by Dent CT. London: The New Sydenham Society; 1881. [Google Scholar]
- 4.Delbert P. Recherches experimentelle sur la lavage au peritoneum. Ann Gynekol Obstet. 1889;32:165–197. [Google Scholar]
- 5.Von Ott. Die drainage nach laparotomyie –experimentelle untersuchung. Medicinsky Westnick, No. 1978. Zentralbl Gynaek 1879;3:86–87.
- 6.http://www.cebm.net (Accessed 2004).
- 7.Meakins JL. Innovations in surgery: the rules of evidence. Am J Surg. 2002;183:399–405. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. [DOI] [PubMed] [Google Scholar]
- 9.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 10.Belghiti J, Kabbej M, Sauvanet A, et al. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. [DOI] [PubMed] [Google Scholar]
- 12.Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver disease. Ann Surg. 2004;239:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burt BM, Brown K, Jarnagin W, et al. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441–445. [DOI] [PubMed] [Google Scholar]
- 14.Budd DC, Cochran RC, Fouty WJ. Cholecystectomy with and without drainage: a randomized prospective study of 300 patients. Am J Surg. 1982;143:307–309. [DOI] [PubMed] [Google Scholar]
- 15.Edlund G, Gedda S, van der Linden W. Intraperitoneal drains and nasogastric tubes in elective cholecystectomy: a controlled clinical trial. Am J Surg. 1979;137:775–779. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AB, Bates T, Fiddian RV. A controlled trial of drainage after cholecystectomy. Br J Surg. 1976;63:278–282. [DOI] [PubMed] [Google Scholar]
- 17.Lewis RT, Goodall RG, Marien B, et al. Simple elective cholecystectomy: to drain or not. Am J Surg. 1990;159:241–245. [DOI] [PubMed] [Google Scholar]
- 18.Maull KI, Daugherty ME, Shearer GR, et al. Cholecystectomy: To drain or not to drain. A randomized prospective study of 200 patients. J Surg Res. 1978;24:259–263. [DOI] [PubMed] [Google Scholar]
- 19.Monson JR, Guillou PJ, Keane FB, et al. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery. 1991;109:740–746. [PubMed] [Google Scholar]
- 20.Playforth MJ, Sauven P, Evans M, et al. Suction drainage of the gallbladder bed does not prevent complications after cholecystectomy: a random control clinical trial. Br J Surg. 1985;72:269–271. [DOI] [PubMed] [Google Scholar]
- 21.Ragoonanan C, Crosby DL, Morgan WP, et al. Peritoneal drainage following cholecystectomy: a controlled trial. Ann R Coll Surg Engl. 1983;65:403. [PMC free article] [PubMed] [Google Scholar]
- 22.Stone HH, Hooper CA, Millikan WJ. Abdominal drainage following appendectomy and cholecystectomy. Ann Surg. 1978;187:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trowbridge PE. A randomized study of cholecystectomy with and without drainage. Surg Gynecol Obstet. 1982;155:171–176. [PubMed] [Google Scholar]
- 24.Truedson H. Cholecystectomy with and without intraperitoneal drain. Acta Chir Scand. 1983;149:393–399. [PubMed] [Google Scholar]
- 25.Jorgensen JO, Gilles RB, Hunt DR, et al. A simple and effective way to reduce postoperative pain after laparoscopic cholecystectomy. Aust N Z J Surg. 1995;65:466–469. [DOI] [PubMed] [Google Scholar]
- 26.Nursal TZ, Yildirim S, Tarim A, et al. Effect of drainage on postoperative nausea, vomiting, and pain after laparoscopic cholecystectomy. Langenbecks Arch Surg. 2003;388:95–100. [DOI] [PubMed] [Google Scholar]
- 27.Heslin MJ, Harrison LE, Brooks AD, et al. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373–378. [DOI] [PubMed] [Google Scholar]
- 28.Conlon KC, Labow D, Leung D, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai D, Sharma A, Kanungo R, et al. Role of abdominal drains in perforated duodenal ulcer patients: a prospective controlled study. Aust N Z J Surg. 1999;69:210–213. [DOI] [PubMed] [Google Scholar]
- 30.Hagmüller E, Lorenz D, Werthmann K, et al. Uses and risks of drainage following elective colon resection. A prospective, randomized and controlled clinical study. Chirurg. 1990;61:266–271. [PubMed] [Google Scholar]
- 31.Hoffmann J, Shokouh-Amiri MH, Damm P, et al. A prospective, controlled study of prophylactic drainage after colonic anastomoses. Dis Colon Rectum. 1987;30:449–452. [DOI] [PubMed] [Google Scholar]
- 32.Merad F, Yahchouchi E, Hay J-M, et al. Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis. A multicenter study controlled by randomization. Arch Surg 1998;133:309–314. [DOI] [PubMed] [Google Scholar]
- 33.Sagar PM, Hartley MN, MacFie J, et al. Randomized trial of pelvic drainage after rectal resection. Dis Colon Rectum. 1995;38:254–258. [DOI] [PubMed] [Google Scholar]
- 34.Merad F, Hay J-M, Fingerhut A, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery. 1999;125:529–535. [PubMed] [Google Scholar]
- 34a.Brown SR, Seon-Choen F, Eu KW, et al. A prospective randomised study of drains in infra-peritoneal rectal anastomoses. Tech Coloproctol. 2001;5:89–92. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CD, Lamont PM, Orr N, et al. Is a drain necessary after colonic anastomosis? J R Soc Med. 1989;82:661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagar PM, Couse N, Kerin M, et al. Randomized trial of drainage of colorectal anastomosis. Br J Surg. 1993;80:769–771. [DOI] [PubMed] [Google Scholar]
- 37.Urbach DR, Kennedy ED, Cohen MM. Colon and rectal anastomoses do not require routine drainage. Ann Surg. 1999;229:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magarey CJ, Chant ADB, Rickford CRK, et al. Peritoneal drainage and systemic antibiotics after appendectomy. Lancet. 1971;2:179–182. [DOI] [PubMed] [Google Scholar]
- 39.Haller JA, Shaker IJ, Donahoo JS, et al. Peritoneal drainage versus non-drainage for generalized peritonitis from ruptured appendicitis in children: a prospective study. Ann Surg. 1973;177:595–600. [PMC free article] [PubMed] [Google Scholar]
- 40.Greenall MJ, Evans M, Pollock AV. Should you drain a perforated appendix? Br J Surg. 1978;65:880–882. [DOI] [PubMed] [Google Scholar]
- 41.Danadapat MC, Panda C. A perforated appendix: should we drain? J Indian Med Assoc. 1992;90:147–148. [PubMed] [Google Scholar]
- 42.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. [DOI] [PubMed] [Google Scholar]
- 44.Scheele J, Stangl R, Altendorf-Hofmann A. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 45.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whooley BP, Law S, Alexandrou A, et al. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg. 2001;181:198–203. [DOI] [PubMed] [Google Scholar]
- 47.Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157–1168. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Lee J, Hyung WJ, et al. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg. 2004;8:727–732. [DOI] [PubMed] [Google Scholar]