Abstract
Calnexin is a ubiquitously expressed type I membrane protein which is exclusively localized in the endoplasmic reticulum (ER). In mammalian cells, calnexin functions as a chaperone molecule and plays a key role in glycoprotein folding and quality control within the ER by interacting with folding intermediates via their monoglucosylated glycans. In order to gain more insight into the physiological roles of calnexin, we have generated calnexin gene-deficient mice. Despite its profound involvement in protein folding, calnexin is not essential for mammalian-cell viability in vivo: calnexin gene knockout mice were carried to full term, although 50% died within 48 h and the majority of the remaining mice had to be sacrificed within 4 weeks, with only a very few mice surviving to 3 months. Calnexin gene-deficient mice were smaller than their littermates and showed very obvious motor disorders, associated with a dramatic loss of large myelinated nerve fibers. Thus, the critical contribution of calnexin to mammalian physiology is tissue specific.
Protein folding in living cells is a complex and error-prone process. The molecular chaperones ensure that only correctly folded proteins leave the endoplasmic reticulum (ER), and the lectin calnexin is a major component of this system (7, 9, 23). Mammalian calnexin is a nonglycosylated type I membrane protein which consists of a large luminal region, a transmembrane segment, and a cytoplasmic tail which carries an ER retention motif (16). Together with a number of other associated folding factors such as oxidoreductases, calnexin and calreticulin (the soluble luminal homologue of calnexin) promote correct folding of newly synthesized glycoproteins (6, 23). Folding substrates associate with calnexin and calreticulin through their monoglucosylated N-linked glycans and enter cycles of re-deglucosylation which regulate association with and dissociation from the lectin-like chaperones (6). Upon successful folding, the protein exits the calnexin/calreticulin cycle and enters the secretory pathway. It is still debated whether calnexin and calreticulin are pure lectins or if protein-protein interactions also contribute to substrate binding (10).
Calnexin and calreticulin have the same substrate specificity, and therefore their functions are largely overlapping. However, closer analysis of the two chaperones has revealed considerable differences in their specificities not only for different folding intermediates but also for distinct domains of the same glycoprotein (5). In addition, some proteins, such as vesicular stomatitis virus G protein (4) or the acetylcholine receptor ɛ chain (AChRɛ), interact with calnexin exclusively (12), while other glycoproteins such as coagulation factor V interact only with calreticulin (18). Calnexin contains a transmembrane domain, which has also been implicated in the binding of nascent proteins and therefore could fulfil functions distinct from those of luminal chaperones such as calreticulin (14).
Calnexin homologues (but no calreticulin homologue) have been identified in the budding yeast Saccharomyces cerevisiae and the fission yeast Schizosaccharomyces pombe (23). The S. pombe calnexin (cnx1) shows much higher homology to mammalian calnexin than the S. cerevisiae calnexin (CNE1). Disruption of the cnx1 gene leads to a lethal phenotype, demonstrating that cnx1p fulfils essential functions, probably by playing a major role in protein quality control in the yeast ER (11, 17). Inactivation of CNE1 does not interfere with the viability of yeast cells or with the secretion levels of endogenous proteins (17).
A mammalian cell line deficient in CNX gene expression has also been identified. This cell line (CEM-NKR) was originally derived as a subclone of the human T-lymphoblastoid cell line CEM that had lost its susceptibility to natural killer cell (NK)-mediated lysis (8). It was subsequently demonstrated that CEM NKR fails to express calnexin (22). Although calnexin clearly associates with major histocompatibility complex class 1 (MHC I) heavy chains, no reduction in the expression levels of MHC I on the surface and no reduction in the transport rate to the cell membrane was detected (19, 21). Recently, calreticulin-deficient mice have been generated (15). The loss of both calreticulin alleles results in prenatal lethality, with the embryos dying at 12 to 18 days of gestation. Calreticulin gene-deficient embryos suffer from a failure to absorb the umbilical hernia (omphalocele) and show severe misdevelopment of the heart, which is the most likely cause of death (15). To gain further information about the role of calnexin in mammalian development in vivo, we generated mice congenitally deficient in the expression of the calnexin gene. Homozygous calnexin gene-deficient mice are carried to full term. However, about 50% die within 2 days after birth, and the remainder develop severe motor disorders, which lead to premature death.
MATERIALS AND METHODS
Construct design.
A murine 129/Sv genomic DNA library cloned into λ phage EMBL 3A was screened using a cDNA probe corresponding to the murine calnexin gene. One genomic clone that contained an 8-kb insert was characterized further and used to construct the targeting vector diagrammed in Fig. 1a. A 6.4-kb ApaI-KpnI fragment was subcloned into pSP72. A 1.2-kb fragment containing 137 bp of exon 4 and exon 5 was replaced by a positive and a negative selection marker, the neomycin resistance gene (neo) and the herpes simplex virus thymidine kinase gene (HSV-tk). The selectable markers were flanked by loxP sites, which allows excision of these markers upon expression of the Cre recombinase.
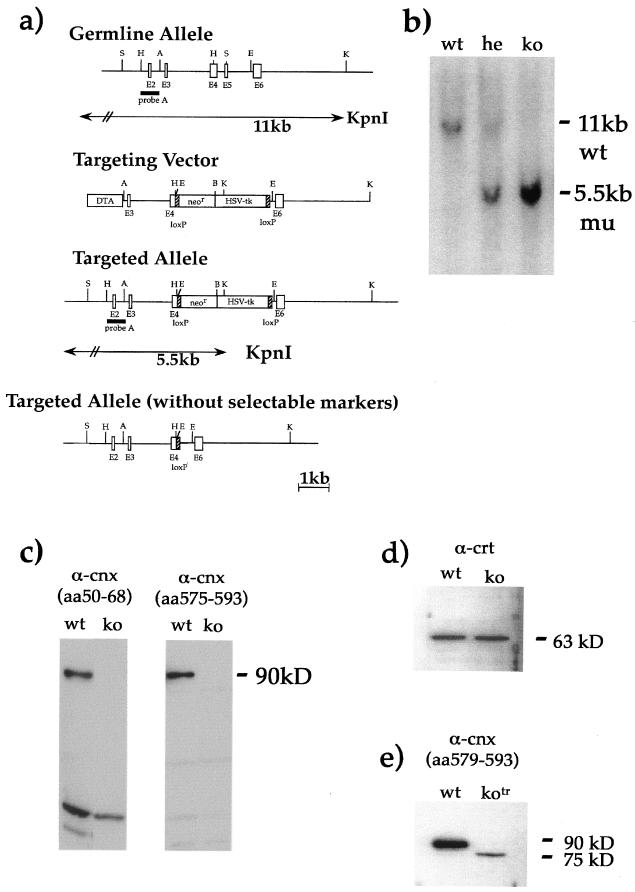
FIG. 1.
Generation and screening of calnexin gene-deficient mice. (a) Map of calnexin germ line configuration, the targeting vector, the targeted allele with selectable markers, and the targeted allele after removal of the selectable markers. Restriction enzyme sites are indicated by single letters as follows: A, ApaI; B, BamHI; E, EcoRI; H, HindIII; K, KpnI; S, SmaI. (b) Southern blot screening of representative mouse tail biopsy specimens showed correct recombination at the 5′ calnexin locus. wt, wild type; he, heterozygous; ko, knockout. (c) Western blot analysis with antibodies (α) against the N terminus and C terminus of calnexin confirmed the absence of calnexin protein in calnexin gene-deficient mice. Additional, low-molecular-weight bands were observed on the Western blots. These are most probably nonspecific, since they were variably observed in different experiments. (d) Calrecticulin levels are unaltered in the absence of calnexin. (e) A second, independent calnexin gene-deficient mouse strain, with selectable markers deleted, expressed a truncated protein 15 kDa smaller than full-length calnexin because exons 4 to 6 were missing. The deletion included regions required for the glucose-binding pocket; the ER-targeting region was still expressed.
Transfection of ES cells and screening of homologous recombinants.
GK129 embryonic stem (ES) cells (107/0.8 ml) were electroporated with 25 μg of AscI-digested targeting vector. Electroporated cells were allowed to recover for 20 min at room temperature and then plated onto mitomycin C-treated STO feeder layers at a density of 2 × 106/10-cm dish. ES cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with nonessential amino acids, l-glutamine, penicillin-streptomycin, 2-mercaptoethanol, 1,000 U of leukemia inhibitory factor (ESGRO)/ml, and 17% fetal calf serum (all from GIBCO-BRL). Cells were maintained on selection medium for 8 days, with selection medium replacement every day. G418-resistant colonies were picked, trypsinized, and seeded onto mitotically inactivated STO feeder layers in 96-well plates. After a further 3 days, cells were split into two 96-well plates, one plate for subsequent freezing and storage, the other for DNA preparation. DNA was analyzed after Asp718 digestion by Southern blot hybridization.
Generation of calnexin mutant mice.
Correctly targeted cells were injected into C57BL/6 blastocysts. Injected blastocysts were cultured overnight and then implanted into 2.5-day-pseudopregnant females. Chimeric mice were bred to C57BL/6 mice to yield mice heterozygous for the mutant allele. Heterozygous mice were interbred in an attempt to generate homozygous mutant mice. A second calnexin gene-deficient mouse strain was generated by crossing mice heterozygous for targeted disruption of the calnexin gene with Zp3-Cre transgenic mice, which express the Cre recombinase under the promoter specific for the zona pellucida protein Zp3 (13). The majority of offspring from such breeding pairs had the selectable markers excised from their genomes. All animals were maintained under barrier conditions and in accordance with Home Office regulations.
Mice were genotyped by PCR analysis of tail DNA using the following primers: a 5′ primer located upstream of the first exon (5′-CCGGACTCTAGGTCCGCCAA-3′), a 3′ primer located in the first exon (5′-TCTGGTTTGTTTGGCCCACTCTCCG-3′), and a 3′ primer located in the neomycin resistance gene (5′-AATTCGCCAATGACAAGACGCT-3′).
Preparation of histological samples.
Detailed histopathological analysis was carried out on knockout, heterozygous, and control mice. This included a skeletal survey by Faxitron X-ray analysis, external examination, internal examination of the macroscopic anatomy, pathology of all major organs, and microscopic examination of organs including the heart, lungs, trachea, tongue, skin, liver, kidneys, pancreas, gonads, skeletal muscle, brain and spinal cord, sciatic nerve, adrenal glands, and thymus. Hematoxylin-and-eosin-stained sections were examined for all organs, and in addition Luxol fast blue/cresyl violet stain preparations were made of cerebrum, cerebellum, and spinal cord sections. All organs were weighed prior to sampling for microscopic analysis. In addition to this sampling, samples of the sciatic nerve were taken for electron microscopic analysis, and nerve fiber quantitation was undertaken for the sciatic-nerve electron microscopic preparations.
RESULTS
Generation of calnexin gene-deficient mice.
The murine calnexin gene was inactivated by targeted disruption of the coding sequence. To this aim, a targeting vector was designed to replace 137 bp of the fourth exon, the fifth exon, and the intervening intron with the neomycin and HSV-tk selectable markers flanked by loxP sites (Fig. 1a). Correct gene targeting in ES cells and transmission of the mutated allele to the progeny were verified by Southern blot analysis (Fig. 1b). Western blot analysis with antibodies against the N terminus (amino acids 50 to 68) and the C terminus (amino acids 575 to 593) confirmed the loss of calnexin protein in calnexin gene-deficient mice (Fig. 1c). Several additional bands of lower molecular weight were detected on the Western blots with both calnexin-specific antibodies. These bands are most probably nonspecific, since they were variably observed in different experiments, they were seen in both wild-type and cnx−/− mice, and they were not consistently associated with either of the two different antibodies. Moreover, no difference in calreticulin levels between wild-type and calnexin gene-deficient mice was distinguishable (Fig. 1d), suggesting that calreticulin is not overexpressed in the absence of calnexin. In addition, we have generated a second calnexin gene-deficient mouse strain, which expresses a truncated calnexin protein as a result of deletion of the selectable markers (Fig. 1e). The truncated calnexin protein shows a loss of exons 4, 5, and 6 (residues 307 to 724) and is about 15 kDa smaller than the full-length calnexin protein. The deleted region includes cysteine residues 161 and 195, which form a disulfide bond, and amino acids Y165 and K167, which are involved in formation of the glucose-binding pocket. Because the deletion did not include the ER-targeting region, the truncated protein could still locate to the ER. Mice expressing the truncated form of calnexin in the absence of any selectable markers were phenotypically identical to calnexin gene-deficient mice, which express no calnexin. All the work presented in this paper was performed exclusively on the calnexin gene-deficient mice expressing no calnexin.
Matings of heterozygous calnexin gene-deficient mice produced numbers of +/+, +/−, and −/− pups (66, 126, and 61, respectively) with a typical Mendelian distribution of 1.04:1.99:0.96, indicating that disruption of calnexin did not result in embryonic lethality. Immediately after birth, calnexin gene-deficient pups were indistinguishable from their wild-type and heterozygous littermates in size, weight, and external morphology. However, within the first 48 h after birth, approximately half (28 of 61) of the calnexin gene knockout mice died (Table 1). Directly after birth, the respiration and feeding of calnexin gene-deficient mice appeared normal; nonetheless, about 50% of cnx−/− newborn mice died, and the cause of the death remained to be determined.
TABLE 1.
Numbers of wild-type, heterozygous, and knockout micea
| Time | No. of mice
|
||
|---|---|---|---|
| WT | HE | KO | |
| At birth | 66 | 126 | 61 |
| 2 days | 66 | 126 | 33 |
| 4 wk | 66 | 126 | 10 |
| 3 mo | 66 | 126 | 3 |
At birth we saw a Mendelian distribution of wild type, heterozygous, and homozygous mice. However, this ratio became dramatically skewed, because half the knockout mice died within 48 h and by the age of 3 months fewer than 5% of calnexin gene-deficient mice were alive. WT, wild type; HE, heterozygous; KO, knockout.
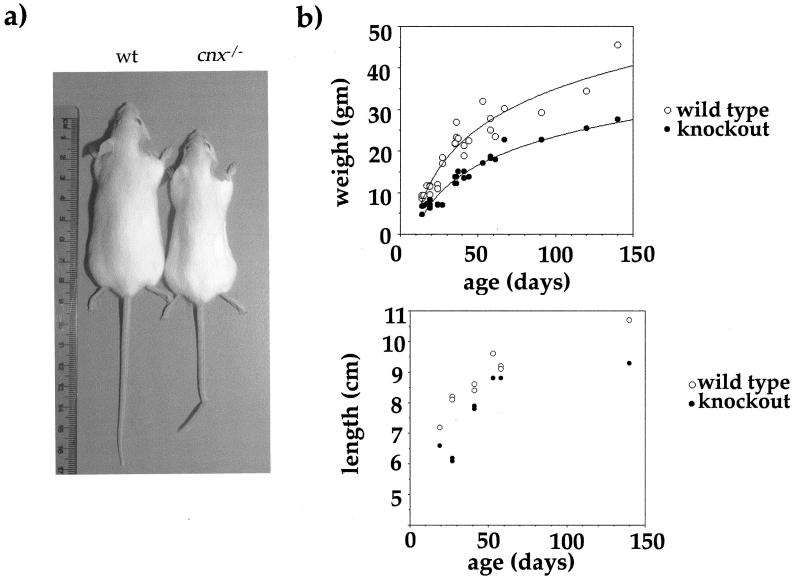
The majority of calnexin gene knockout mice either died or had to be sacrificed within 4 weeks. Only a very few mice survived to 3 months. Calnexin gene knockout mice showed obvious motor abnormalities. They had an unstable gait and were less active in walking, with marked truncal ataxia when they moved. At 8 to 10 days after birth, calnexin gene-deficient mice were significantly smaller than their littermates—they appeared thin and were one-third lighter (36.3%)—but only about 11% smaller than their age- and sex-matched littermate controls (Fig. 2b). With progression of the severity of the phenotype, calnexin gene knockout mice stopped moving and feeding (even provision of moist food in the cage did not prevent weight loss), and to prevent distress, mice were sacrificed.
FIG. 2.
Macroscopic analysis of calnexin gene-deficient mice. (a) Calnexin gene knockout mice are significantly thinner and smaller than their littermates. (b) (Top) Weights of 27 wild-type and 27 calnexin gene-deficient mice, aged 15 to 140 days. At any given time point, calnexin gene-deficient mice are much lighter (about one-third) than their littermates. (Bottom) In addition, calnexin gene knockout mice are shorter than their littermate controls. Shown are lengths of 15 cnx−/− mice and their littermates, also aged 15 to 140 days. Calnexin gene knockouts are about 10% shorter than control mice. Although the length difference is significant, it is not as profound as the inability to gain weight.
Characterization of the calnexin gene knockout phenotype by use of the SHIRPA protocol.
Because of the evident phenotype, a behavioral and functional analysis of calnexin gene knockout mice was performed according to the primary stage of the SHIRPA protocol, excluding temperature measurement (20). The SHIRPA protocol provides guidelines which, by use of standardized evaluation methods, allow a better comparative analysis of any immediately detectable abnormalities and does not rely on an exclusively descriptive assessment. Results with the SHIRPA protocol are summarized in Table 2. All tested reflexes in calnexin gene knockout mice were normally developed, and mice showed no evident visual deficiencies, as assessed by the visual approach and visual placing methods, or hearing deficiencies, as assessed by the Preyer and startle responses. However, calnexin gene-deficient mice suffered truncal ataxia, the hind legs were abnormally splayed when walking, and an intention tremor of the forelimb was detected. In addition, calnexin gene knockout mice showed behavioral abnormalities, with negative geotaxis and retropulsion. Negative geotaxis describes the observation that cnx−/− mice, when placed facing downward on a vertical grid, walk down, without turning around, in contrast to wild-type mice, which turn around when placed on a vertical grid and walk upward. Retropulsion refers to the fact that calnexin gene-deficient mice start walking backward when placed in a new environment and may then start walking forward, unlike wild-type mice, which immediately (after a short freeze period) start walking forward. Retropulsion may be seen in normal mice placed on a smooth, unfamiliar surface, but calnexin gene-deficient mice showed this as a characteristic reaction to all surfaces and during periods of undisturbed observation. In sum, calnexin gene knockout mice show reduced viability associated with severe behavioral abnormalities.
TABLE 2.
Selected results of SHIRPA analysisa
| Parameter | Result for:
|
|
|---|---|---|
| Wild-type mice | Calnexin gene knockout mice | |
| Visual placing | Normal | Normal |
| Startle response | Normal | Normal |
| Righting reflex | Normal | Normal |
| Sensory system | Normal | Normal |
| Tremor | None | Marked |
| Gait | Normal | Fluid but abnormal |
| Abnormal behavior | None | Retroplusion, negative geotaxis |
Results show that calnexin gene-deficient mice have normal visual and hearing abilities, normal sensory reflexes, and a normal righting reflex but show a clearly unsteady gait, tremor, and abnormal behavior such as retropulsion and negative geotaxis.
Histopathological analysis of calnexin gene-deficient mice.
A skeletal survey and macroscopic and microscopic histopathological analysis of all organs and of the central and peripheral nervous systems (including the cerebellum, inferior olive, and stratonigral regions) showed no obvious changes in the calnexin gene knockout mice. Anterior horn cells were not found to be reduced when nuclei were counted in serial sections (2-mm-thick block section of whole spinal cord). Hematoxylin-and-eosin-stained sections of the quadriceps and muscle of the posterior compartment of the hindlimb showed no evidence of fibrosis or muscle fiber degeneration.
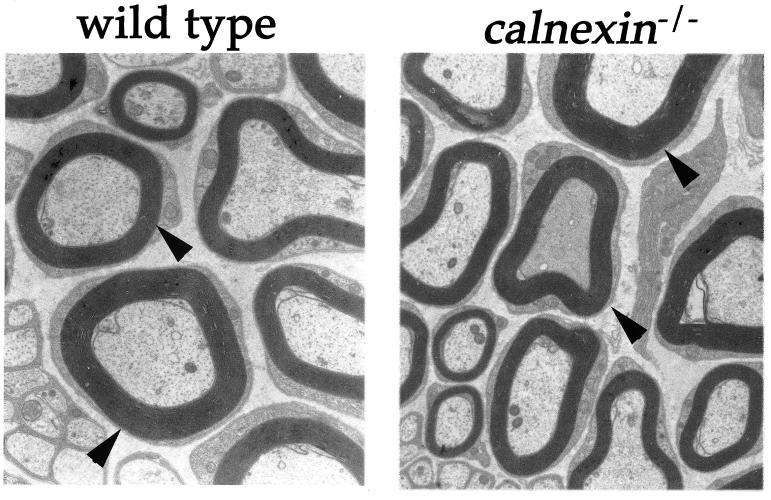
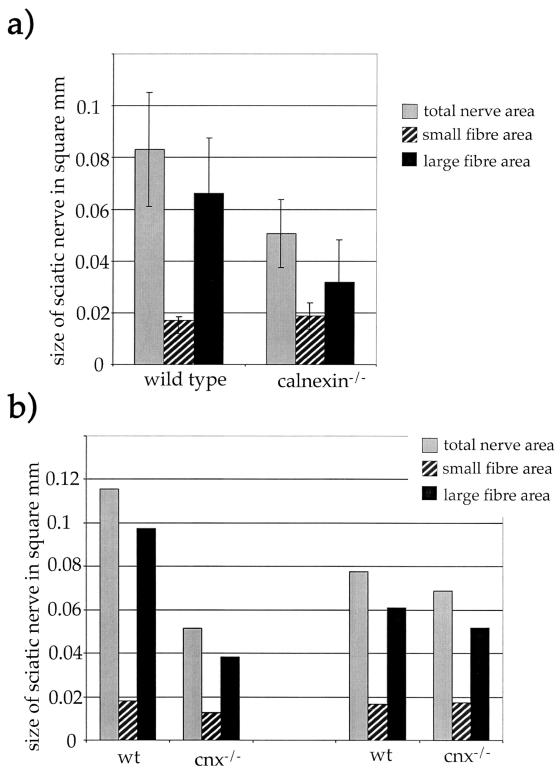
Since no obvious gross abnormalities would explain the observed ataxic phenotype of calnexin gene-deficient mice, an analysis of candidate molecules, whose dysregulation might result in this phenotype, was undertaken. One obvious candidate is the AChR, both because calnexin is involved in correct folding and assembly of AChR subunits (3, 12) and because AChRɛ knockout mice die prematurely and are much smaller than their littermates (24), a phenotype similar to that observed for calnexin gene knockout mice. Despite this, calnexin gene knockout mice displayed no alterations in the surface expression and clustering of the AChR receptor in skeletal muscle (data not shown). In addition, we could not detect any ongoing muscle fiber degradation as assessed by expression of the neural cell adhesion molecule and utrophin and of dystrophin, which are up-regulated and down-regulated in denervated muscle, respectively (data not shown). Biopsy specimens of the main sciatic-nerve trunk, taken about 0.5 cm from the trifurcation, showed no evidence of demyelination or remyelination; myelin distribution was normal in both wild-type and calnexin gene-deficient mice (Fig. 3). However, decreased numbers of large to medium myelinated fibers within the sciatic nerve were observed (Table 3). Of note, there was no significant change in the small-fiber area. The loss of large fibers correlated with the severity of the phenotype. Mice with pronounced symptoms (e.g., severe ataxia and a heavily shaking body) showed a more-prominent loss of large myelinated fibers than calnexin gene knockout mice with only a mild phenotype (Table 3). The profound loss of large to medium myelinated fibers resulted in a decrease in the size of the sciatic nerve, as shown in Fig. 4a. The decreased number of large myelinated fibers correlates with the reduction in size of the sciatic nerve; in addition, this reduction is more profound in severely affected calnexin gene-deficient mice, which have the lowest number of large myelinated fibers (Fig. 4b).
FIG. 3.
Electron microscopy on sciatic nerve fibers from wild-type and cnx−/− mice. The left panel shows a sciatic nerve fiber from a wild-type control mouse at a 22,725-fold magnification, while the right panel shows a comparable section from a calnexin gene knockout mouse. Arrowheads indicate normal myelination in both wild-type and calnexin gene-deficient mice.
TABLE 3.
Total numbers of large and small nerve fibers in the sciatic nerves of wild-type and calnexin gene-deficient micea
| Nerve fibers | No. in:
|
||
|---|---|---|---|
| Wild-type mice | Calnexin gene knockout mice
|
||
| Mild phenotype | Severe phenotypeb | ||
| Total | 1,981 ± 452 | 1,703 ± 152 | 1,370 ± 181 |
| Large | 1,085 ± 423 | 685 ± 94 | 350 ± 148 |
| Small | 896 ± 94 | 1,018 ± 120 | 1,020 ± 325 |
n = 3. This table shows that the total number of nerve fibers in cnx−/− mice is reduced to a level of about 70% of that in the wild type and that this loss is caused by a profound reduction in the number of large fibers (to 30% of the wild-type level) while the number of small fibers is slightly increased.
E.g., truncal ataxia.
FIG. 4.
Loss of large myelinated nerve fibers correlates with severity of phenotype. (a) Average sizes of the sciatic nerve, small-fiber area, and large-fiber area. The large error bars result from age and sex variations. (b) Average sizes of the sciatic nerve, small-fiber area, and large-fiber area for two sets of one wild-type (wt) and one calnexin gene-deficient mouse at the age of 60 days. (Left) The knockout mouse showed a severe phenotype. (Right) The knockout mouse still showed symptoms of loss of calnexin, but they were less prominent, suggesting that progression of the loss of large myelinated fibers worsens the truncal ataxia and motor disorders seen in these mice.
DISCUSSION
Inactivation of one calnexin allele did not show any obvious consequences, because these mice showed no detectable differences from their wild-type littermates. However, inactivation of both calnexin alleles resulted in a clear phenotype, and all calnexin gene-deficient mice were immediately distinguishable from their calnexin heterozygous and wild-type littermates. Calnexin knockout mice were about one-third smaller than their littermates and showed severe body shaking and a very wobbly, unsteady gait.
A thorough SHIRPA analysis revealed that surviving calnexin gene knockout mice had normal reflexes and normal hearing and visual capacity but showed behavioral abnormalities such as negative geotaxis and retropulsion. Retropulsion can be caused by defects in the cholinergic pathway or receptors, but no obvious AChR abnormalities were found in calnexin gene-deficient mice. Tremor is also a feature of many mutants. With a pure tremor phenotype, abnormalities of myelin development, such as in pmp22 mutations (1), are often seen, but this too was not a feature of calnexin gene knockout, as the distribution of myelin was normal. Motor defects may also have an origin in the motor cortex, corticospinal tract, or lower motor neuron, as seen in wobbler and G93 SOD1 transgenic animals (2). However, electron microscopy of sciatic nerves of wild-type and calnexin gene knockout mice showed a profound reduction (>60%) in the numbers of large and medium myelinated nerve fibers in the calnexin gene knockouts, while the number of small myelinated fibers remained largely unaltered. With decreasing numbers of large myelinated fibers, the diameter of the sciatic nerve was reduced proportionally. The substantial loss of these motor nerve fibers is most likely the cause of the observed ataxia and tremor. In accordance with this hypothesis was the observation that calnexin gene-deficient mice showing severe symptoms suffered from a substantial loss of large myelinated fibers, whereas mice with mild phenotypes showed only marginal reductions in the number of large myelinated nerve fibers.
The molecular mechanism which causes the selective loss of the large myelinated fibers remains unsolved. Considering the chaperone function of calnexin, it is plausible to assume that one or more essential proteins (survival factors or their receptors) for these nerve fibers require interaction with calnexin during the folding process. Clearly, just as calnexin cannot compensate for the loss of calreticulin (calreticulin gene-deficient mice die in utero due to defective heart development), calreticulin is unable to compensate for the loss of calnexin as the animals develop specialized physiological functions that characterize postnatal viability.
Acknowledgments
A.D. was funded by a BBSRC/GlaxoWellcome industrial fellowship. M.M. was funded by the Max Cloetta Foundation, Fondazione per lo studio delle malattie neurodegenerative, A+D-Fond of the Swiss Academy of Medical Sciences, and National Center for Competence in Research (NCCR), “Neuronal Plasticity and Repair.” J.E.M. and S.V. are supported by the Motor Neuron Disease Association, the Birth Defects Foundation, and the Annie Lindsell Trust.
REFERENCES
- 1.Adlkofer, K., R. Martini, A. Aguzzi, J. Zielasek, K. V. Toyka, and U. Suter. 1995. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 11:274-280. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, J. M., M. B. Gardner, F. J. Wolfgram, G. W. Ellison, D. D. Porter, and W. W. Brandkamp. 1974. Studies on a murine form of spontaneous lower motor neuron degeneration—the wobbler (wa) mouse. Am. J. Pathol. 76:63-78. [PMC free article] [PubMed] [Google Scholar]
- 3.Gelman, M. S., W. Chang, D. Y. Thomas, J. J. Bergeron, and J. M. Prives. 1995. Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J. Biol. Chem. 270:15085-15092. [DOI] [PubMed] [Google Scholar]
- 4.Hammond, C., I. Braakman, and A. Helenius. 1994. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 91:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert, D. N., J. X. Zhang, W. Chen, B. Foellmer, and A. Helenius. 1997. The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin. J. Cell Biol. 139:613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helenius, A. 1994. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol. Biol. Cell 5:253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helenius, A., and M. Aebi. 2001. Intracellular functions of N-linked glycans. Science 291:2364-2369. [DOI] [PubMed] [Google Scholar]
- 8.Howell, D. N., P. E. Andreotti, J. R. Dawson, and P. Cresswell. 1985. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J. Immunol. 134:971-976. [PubMed] [Google Scholar]
- 9.Hurtley, S. M., and A. Helenius. 1989. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 5:277-307. [DOI] [PubMed] [Google Scholar]
- 10.Ihara, Y., M. F. Cohen-Doyle, Y. Saito, and D. B. Williams. 1999. Calnexin discriminates between protein conformational states and functions as a molecular chaperone in vitro. Mol. Cell 4:331-341. [DOI] [PubMed] [Google Scholar]
- 11.Jannatipour, M., and L. A. Rokeach. 1995. The Schizosaccharomyces pombe homologue of the chaperone calnexin is essential for viability. J. Biol. Chem. 270:4845-4853. [DOI] [PubMed] [Google Scholar]
- 12.Keller, S. H., J. Lindstrom, and P. Taylor. 1998. Inhibition of glucose trimming with castanospermine reduces calnexin association and promotes proteasome degradation of the alpha-subunit of the nicotinic acetylcholine receptor. J. Biol. Chem. 273:17064-17072. [DOI] [PubMed] [Google Scholar]
- 13.Lewandoski, M., K. M. Wassarman, and G. R. Martin. 1997. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 7:148-151. [DOI] [PubMed] [Google Scholar]
- 14.Margolese, L., G. L. Waneck, C. K. Suzuki, E. Degen, R. A. Flavell, and D. B. Williams. 1993. Identification of the region on the class I histocompatibility molecule that interacts with the molecular chaperone, p88 (calnexin, IP90). J. Biol. Chem. 268:17959-17966. [PubMed] [Google Scholar]
- 15.Mesaeli, N., K. Nakamura, E. Zvaritch, P. Dickie, E. Dziak, K. H. Krause, M. Opas, D. H. MacLennan, and M. Michalak. 1999. Calreticulin is essential for cardiac development. J. Cell Biol. 144:857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalak, M., R. E. Milner, K. Burns, and M. Opas. 1992. Calreticulin. Biochem. J. 285:681-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parlati, F., D. Dignard, J. J. Bergeron, and D. Y. Thomas. 1995. The calnexin homologue cnx1+ in Schizosaccharomyces pombe, is an essential gene which can be complemented by its soluble ER domain. EMBO J. 14:3064-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pipe, S. W., J. A. Morris, J. Shah, and R. J. Kaufman. 1998. Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J. Biol. Chem. 273:8537-8544. [DOI] [PubMed] [Google Scholar]
- 19.Prasad, S. A., J. W. Yewdell, A. Porgador, B. Sadasivan, P. Cresswell, and J. R. Bennink. 1998. Calnexin expression does not enhance the generation of MHC class I-peptide complexes. Eur. J. Immunol. 28:907-913. [DOI] [PubMed] [Google Scholar]
- 20.Rogers, D. C., E. M. Fisher, S. D. Brown, J. Peters, A. J. Hunter, and J. E. Martin. 1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome 8:711-713. [DOI] [PubMed] [Google Scholar]
- 21.Sadasivan, B. K., A. Cariappa, G. L. Waneck, and P. Cresswell. 1995. Assembly, peptide loading, and transport of MHC class I molecules in a calnexin-negative cell line. Cold Spring Harbor Symp. Quant. Biol. 60:267-275. [DOI] [PubMed] [Google Scholar]
- 22.Scott, J. E., and J. R. Dawson. 1995. MHC class I expression and transport in a calnexin-deficient cell line. J. Immunol. 155:143-148. [PubMed] [Google Scholar]
- 23.Trombetta, E. S., and A. Helenius. 1998. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 8:587-592. [DOI] [PubMed] [Google Scholar]
- 24.Witzemann, V., H. Schwarz, M. Koenen, C. Berberich, A. Villarroel, A. Wernig, H. R. Brenner, and B. Sakmann. 1996. Acetylcholine receptor epsilon-subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc. Natl. Acad. Sci. USA 93:13286-13291. [DOI] [PMC free article] [PubMed] [Google Scholar]