Abstract
The anchoring of microtubules (MTs) to subcellular structures is critical for cell shape, polarity, and motility. In mammalian cells, the centrosome is a prominent MT anchoring structure. A number of proteins, including ninein, p150Glued, and EB1, have been implicated in centrosomal MT anchoring, but the process is far from understood. Here we show that CAP350 and FOP (FGFR1 oncogene partner) form a centrosomal complex required for MT anchoring. We show that the C-terminal domain of CAP350 interacts directly with FOP and that both proteins localize to the centrosome throughout the cell cycle. FOP also binds to EB1 and is required for localizing EB1 to the centrosome. Depletion of either CAP350, FOP, or EB1 by siRNA causes loss of MT anchoring and profound disorganization of the MT network. These results have implications for the mechanisms underlying MT anchoring at the centrosome and they attribute a key MT anchoring function to two novel centrosomal proteins, CAP350 and FOP.
INTRODUCTION
In most animal cells, the centrosome plays an important role in the organization of MT networks (Rieder et al., 2001; Bornens, 2002; Nigg, 2004; Ou and Rattner, 2004; Doxsey et al., 2005). A single centrosome is composed of two centrioles that are surrounded by amorphous pericentriolar material (PCM). These two centrioles (sometimes referred to as mother and daughter) differ in structure, function and age (state of maturity). In particular, the fully mature centriole is characterized by the presence of appendages at its distal end. MTs are nucleated from so-called γ-tubulin ring complexes (γ-TuRCs; Moritz et al., 2004). These ring-shaped multiprotein complexes are present within PCM associated with both mother and daughter centrioles and, indeed, both centrioles are competent to nucleate MTs (Piel et al., 2000). Subsequent to nucleation, MTs are released (Keating et al., 1997; Abal et al., 2002). So, in order to remain associated with centrosomes, they need to be captured by centrosomal MT anchoring activities (Dammermann et al., 2003). Anchoring mechanisms remain incompletely understood, but the available evidence suggests that appendages of the mature centriole play a prominent role in MT anchoring (Piel et al., 2000). Moreover, transport of released MTs to appendages was shown to require dynein/dynactin activity (Piel et al., 2000; Clark and Meyer, 1999; Quintyne et al., 1999).
Several proteins have been shown to localize to centriole appendages. These include ninein (Mogensen et al., 2000), odf2/cenexin (Lange and Gull, 1995; Nakagawa et al., 2001; Ishikawa et al., 2005), centriolin (Gromley et al., 2003), ε-tubulin (Chang et al., 2003), Cep170 (Guarguaglini et al., 2005), and CEP110 (Ou et al., 2002). An involvement in MT anchoring has been clearly demonstrated for ninein (Mogensen et al., 2000; Dammermann and Merdes, 2002; Abal et al., 2002; Delgehyr et al., 2005). However, not all proteins implicated in MT anchoring are concentrated at appendages. In particular, evidence for a role in MT anchoring has been reported for PCM-1 (Dammermann and Merdes, 2002), BBS4 (Kim et al., 2004), and CEP135 (Ohta et al., 2002).
The MT plus-end-associated protein EB1 (Askham et al., 2002; Louie et al., 2004) and the dynein/dynactin complex (Quintyne et al., 1999; Quintyne and Schroer, 2002) have also been linked to MT anchoring. EB1 has attracted considerable interest because of its ability to interact with the tumor suppressor protein APC (adenomatous polyposis coli; Su et al., 1995). EB1 prominently localizes to growing MT plus ends and plays a role in the regulation of MT dynamics (Berrueta et al., 1998; Tirnauer et al., 1999; Mimori-Kiyosue et al., 2000; Nakamura et al., 2001). In addition, it associates with centrosomes, consistent with a function in centrosomal MT anchoring (Berrueta et al., 1998; Morrison et al., 1998; Askham et al., 2002; Louie et al., 2004). Specifically, the retention of MTs at the centrosome was shown to depend on an interaction between EB1 and the dynactin subunit p150Glued (Askham et al., 2002).
Here we have studied CAP350 and FOP, two centrosome components identified in a recent proteomics study (Andersen et al., 2003). We demonstrate that these two proteins form a complex and that both are required for MT anchoring to the centrosome. We further show that FOP binds to EB1 and is responsible for the centrosome association of this MT plus-end-tracking protein. CAP350 and FOP thus add to a growing list of centrosomal proteins required for MT anchoring to mammalian centrosomes, implying that MT anchoring is a more complicated process than hitherto surmised.
MATERIALS AND METHODS
Plasmid Constructions
The 3′ region of a CAP350 cDNA clone (KIAA0480) was obtained from Deutsches Ressourcenzentrum für Genomforschung (Berlin), and the overlapping KIAA2443 cDNA was kindly provided by Dr. S. Sugano (Institute of Medical Science, University of Tokyo, Japan). The missing 5′ region was amplified by RT-PCR from HeLa S3 total RNA, using sequence information from GenBank (Accession No. AF287356). cDNAs were ligated to yield full-length CAP350 cDNA, confirmed by DNA sequencing, and subcloned into pCRII-TOPO (Invitrogen, Carlsbad, CA). The FOP cDNA has previously been described (Andersen et al., 2003). N-terminally myc-tagged CAP350 and C-terminally flag-tagged FOP were constructed in mammalian pCMV expression vectors by standard procedures. The EB1-GFP plasmid was a kind gift from Dr. Y. Mimori-Kiyosue (KAN Research Institute, Kyoto, Japan).
For yeast two-hybrid screening, fragments of CAP350, FOP, and EB1 were amplified by PCR and subcloned into pACT2 (CLONTECH Laboratories, Palo Alto, CA) or the two-hybrid bait vector pFBT9 (a version of pGBT9; CLON-TECH Laboratories; modified to encode kanamycin resistance; kindly provided by Dr. F. Barr). Transformed yeast samples were plated on synthetic media either lacking leucine and tryptophan (-LW) or lacking leucine, tryptophan, histidine, and adenine, with 2% (wt/vol) glucose as the carbon source (QDO). All results were confirmed by streaking several independent colonies on both selective (QDO) and nonselective (-LW) plates.
Antibody Production
To generate a goat polyclonal antibody against CAP350, a fragment spanning residues 2115-2643 was fused to glutathione-S-transferase (GST), purified from Escherichia coli, and used as immunogen (500 μg protein for first injection, followed by 350 μg for first boost and 250 μg for second boost). Rabbit polyclonal anti-FOP antibodies were raised against full-length protein expressed in E. coli (Charles River Laboratories, St. Alban les Elbeuf, France).
Cell Culture, RNA Interference, and Immunofluorescence Microscopy
Human cervical carcinoma (HeLa S3), osteosarcoma (U2OS), epithelial adenocarcinoma (A549), and embryonal kidney cells (HEK293) as well as African green monkey kidney cells (COS7), were all grown at 37°C and 5% CO2 in DMEM (Invitrogen-BRL), supplemented with 10% fetal calf serum and penicillin-streptomycin (100 IU/ml and 100 mg/ml, respectively).
RNA interference was performed on HeLa S3, A549 cells, or U2OS cells. Transfections with duplex RNA were carried out using oligofectamine (Invitrogen) for 72 or 96 h, respectively. The following siRNA duplex oligonucleotides were used: CAP350: 5′ ATGAACGATATCAGTGCTATA 3′ (oligo 1) and 5′ CAGGTAGTAGTCATCTTATAA 3′ (oligo 2; QIAGEN, Hilden, Germany); FOP: 5′AAGTGATCAGGCGCTGTCAAC 3′ (oligo 1; Dharmacon Research, Boulder, CO) and 5′ CTCGAAGGTCGAGAGAATTTA 3′ (oligo 2; QIAGEN).
The siRNA duplexes used to deplete pericentrin (Dammermann and Merdes, 2002) and EB1 (Louie et al., 2004) were designed as described previously, and the duplex GL2 (Elbashir et al., 2001) was used for control.
Immunofluorescence (IF) microscopy was performed as described previously (Meraldi et al., 2002). Cells were grown on coverslips and fixed for 10 min in cold methanol. Primary antibodies were goat anti-CAP350 serum (1:2000), rabbit anti-FOP (R144) serum (1:1000), affinity-purified rabbit anti-pericentrin B antibody (kind gift of Dr. M. Takahashi, used as described previously; Takahashi et al., 2002), mouse anti-EB1 (1:300, BD Transduction Laboratories, Lexington, KY; Cat. 610535), mouse anti-α-tubulin-FITC (1:1000, DM1A, Sigma, St. Louis, MO) and mouse anti-γ-tubulin antibody (1:1000, GTU-88, Sigma). Secondary reagents were Alexa-Fluor-555-conjugated goat anti-mouse, Alexa-Fluor-488-conjugated goat anti-rabbit, Cy3-, Cy5-, and Cy2-conjugated donkey anti-mouse, anti-rabbit, and anti-goat IgG antibodies (1:1000, Molecular Probes, Eugene, OR). DNA was stained with 4,6-diamidino-2-phenylindole (DAPI, 2 μg/ml). IF microscopy was performed using a Zeiss Axioplan II microscope with 40× and 63× oil immersion objectives, respectively. Photographs were taken using a Micromax 1300 × 1030 pixel CCD camera (Princeton Instruments, Princeton, NJ) with Metavue software (Universal Imaging, West Chester, PA). Images were processed with Adobe Photoshop software (Adobe Systems, San Jose, CA).
Cell Extracts and Immunoprecipitation Experiments
For immunoprecipitation experiments, HEK293T cells were washed once with phosphate-buffered saline and lysed in 20 mM Tris-HCl, pH 7.4, 100 mM KCl, 0.1% NP40, 1 mM EDTA, 10% glycerol, and 50 mM NaF plus protease inhibitors (Roche, Grenzach-Wyhlen, Germany) for 30 min on ice. Lysates were clarified at 16,000 × g at 4°C for 8 min and preabsorbed on protein A (Bio-Rad Laboratories, Richmond, CA) or protein-G beads (Amersham, Piscataway, NJ) for 30 min at 4°C. Equal amounts of precleared lysates were incubated with 20 μl beads bearing 5 μg of goat anti-CAP350, rabbit anti-FOP, and rabbit IgG, or sheep IgG for control, at 4°C for 2 h. Immunoprecipitates were washed three times in wash buffer (20 mM Tris-HCl, pH 7.4, 250 mM KCl, 0.1% NP40, 1 mM EDTA, 10% glycerol, and 50 mM NaF) and resuspended in gel sample buffer.
For immunoprecipitation experiments performed with overexpressed FOP-FLAG and EB1-GFP, HEK293T cells were collected after transfection for 36 h. Cells were immunoprecipitated with anti-FLAG M2 beads (Sigma), and bound proteins were eluted with 0.8 mg of FLAG peptide/ml in a total volume of 30 μl (Zhou et al., 1998).
MT Regrowth Assays
HeLa S3 cells were transfected with different siRNA duplexes and MT regrowth assays were performed as described previously (Fry et al., 1998). In brief, MTs were depolymerized on ice for 30 min and regrowth was induced by incubation in prewarmed medium (37°C). For better visualization of MTs by IF, the cells were preextracted for 30 s with MT-stabilizing extraction buffer (20 mM Tris·Cl, pH 7.0, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100) before methanol fixation (Yan et al., 2003).
RESULTS
CAP350 Is a Cell Cycle-regulated Centrosome Protein
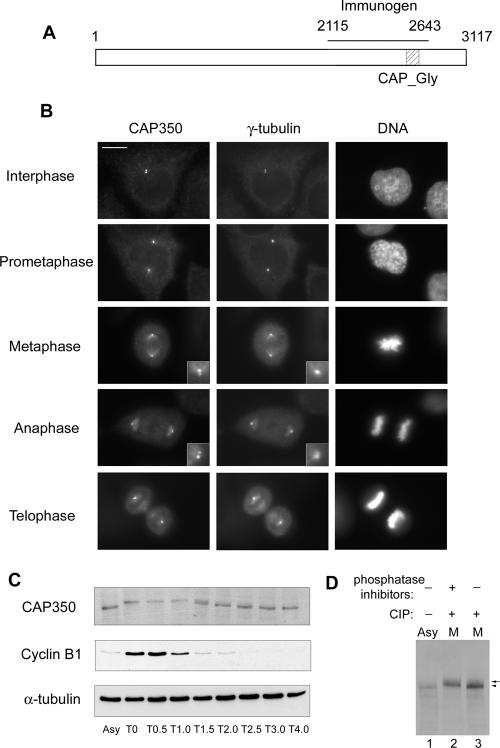
CAP350 was predicted to be a centrosomal protein by a protein correlation profiling (phencyclidine) algorithm used in a mass spectrometry-based proteomic study of the human centrosome (Andersen et al., 2003). In support of a MT-related function, CAP350 carries a distinctive CAP_Gly motif (Figure 1A), a domain of ∼70 amino acids with conserved glycine and hydrophobic residues (Riehemann and Sorg, 1993). Similar motifs have previously been described in several MT binding proteins, notably CLIP-170 (Diamantopoulos et al., 1999), CLIP-115 (Hoogenraad et al., 2000), and p150Glued (Vaughan et al., 2002). To study endogenous CAP350 in human cells, a goat polyclonal antibody was raised against a recombinant protein spanning residues 2115-2643. As shown by Western blotting, this antibody specifically recognized a protein of the expected molecular weight (350 kDa) in whole HeLa S3 cell lysates (Figure 1C; for the complete gel see Figure 2A). To examine the expression of CAP350 during the cell cycle, lysates were prepared from synchronized HeLa cells (Sillje et al., 1999) and analyzed by Western blotting. CAP350 protein was detectable at relatively constant levels during all stages of the cell cycle but displayed a retarded electrophoretic mobility during M phase, suggesting that it might be phosphorylated (Figure 1C). This possibility was confirmed by in vitro dephosphorylation, demonstrating that CAP350 is a substrate for mitosis-specific phosphorylation (Figure 1D). IF staining of asynchronously growing HeLa or U2OS cells with anti-CAP350 antibody revealed two closely spaced dots colocalizing with γ-tubulin, indicating that CAP350 associates with centrosomes throughout the cell cycle (Figure 1B and unpublished data). This centrosome association was also seen in nocodazole-treated cells, indicating that it was not dependent on MTs (unpublished data). During M phase, when γ-tubulin staining of poles was rather diffuse, two CAP350-positive dots could still be seen at each spindle pole, suggesting an association with centrioles (Figure 1B, insets). Additionally, association of CAP350 with MTs could be observed in mitotic but not interphase cells, and again, some colocalization with γ-tubulin on spindle microtubules was apparent (Figure 1B). Taken together, these results identify CAP350 as a core component of the human centrosome, confirming the prediction of the proteomics study (Andersen et al., 2003). Specifically, our data demonstrate that endogenous Cap350 localizes specifically to centrosomes and, to a lesser extent, to spindle MTs (Figure 1B). In contrast to a recent study detecting CAP350 at centrosomes but emphasizing a role of this protein in the regulation of nuclear hormone receptors (Patel et al., 2005), we could not detect significant amounts of endogenous CAP350 within the nucleus.
Figure 1.
CAP350 localizes to the centrosome and is phosphorylated in M phase. (A) Schematic representation of CAP350, indicating the CAP_Gly motif and the domain (residues 2115-2643 aa) used for antibody production. (B) IF staining in U2OS cells of endogenous CAP350 (left) and γ-tubulin (middle). DNA (right) was stained with DAPI. Insets shows enlargement of the centriolar staining of CAP350 and γ-tubulin at the poles. Bar, 10 μm. (C) CAP350 is modified in M phase. HeLa S3 were released from a nocodazole block, and samples were taken for Western blot analysis at the time points indicated (T0-T4.0 in hours). For comparison, asynchronously growing cells (Asy) were analyzed in parallel. (D) CAP350 is phosphorylated during mitosis. CAP350 was immunoprecipitated from asynchronously growing (Asy) or nocodazole-arrested (M) HeLa cells and treated with (+) or without (-) calf intestinal phosphatase (CIP) in the presence (+) or absence (-) of phosphatase inhibitors. Samples were separated by 6% SDS-PAGE and probed by Western blotting.
Figure 2.
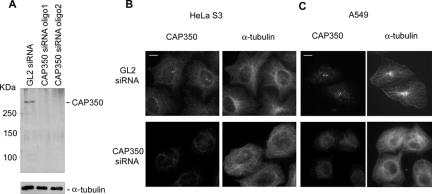
Depletion of CAP350 causes disorganization of the MT network. (A) Western blotting shows effective siRNA-mediated depletion of CAP350 in HeLa S3 cells. Cells were transfected with GL2 or CAP350-specific oligonucleotide duplexes for 72 h. Equal amounts of protein were separated by SDS-PAGE and probed by Western blotting with anti-CAP350 antibody (top) and anti-α-tubulin antibody for loading control (bottom). (B and C) IF staining of CAP-350 depleted (bottom) and control (top) HeLa S3 (B) and A549 cells (C) with antibodies to α-tubulin. Note that CAP350-depleted cells lack radial MT arrays focused on the centrosome. Bar, 10 μm.
Depletion of CAP350 Affects MT Anchoring
To explore the function of CAP350 in living cells, siRNA-mediated protein depletion experiments were performed. HeLa S3 or A549 cells were transfected for 72 h with either control (GL2; Elbashir et al., 2001) or one of two distinct CAP350-targeting siRNA oligonucleotide duplexes (oligo-1, oligo-2). As shown by Western blotting of HeLa total cell lysates, both CAP350 siRNA duplexes caused a strong reduction in CAP350 protein levels (Figure 2A). Depletion of the CAP350 protein could also be confirmed by IF microscopy with anti-CAP350 antibody (Figure 2, B and C). When compared with the astral MT arrays seen in cells treated with control GL2, cells depleted of CAP350 showed unfocused, disorganized MT networks (Figure 2, B and C).
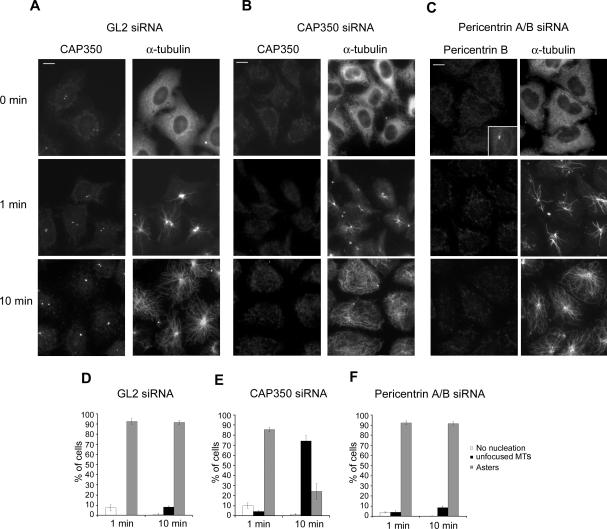
To explore the mechanism underlying the phenotype observed in CAP350-depleted cells, we next carried out MT regrowth assays, using both the GL2 duplex and pericentrin-A/B-specific siRNA for control (Figure 3). After depolymerization of MTs, the regrowth of MT asters was monitored at various time points between 1 and 10 min. IF microscopy (Figure 3, A-C) and quantitative analyses (Figure 3, D-F) of CAP350-depleted cells revealed that initial MT nucleation at the centrosome occurred (near-)normally, with at most a minor delay in aster formation, but at later time points ∼75% of the CAP350 depleted cells showed disorganized and unfocused MTs, indicating that MTs could be nucleated but not retained at the centrosome. Under comparable conditions, the depletion of pericentrin did not affect either the nucleation or the anchoring of MTs. Taken together, these results indicate that CAP350 is not required for MT nucleation but necessary for MT anchoring at the centrosome.
Figure 3.
Depletion of CAP350 affects MT anchoring. HeLa S3 cells were transfected with GL2 (A), CAP350 -2 (B), and pericentrin A/B siRNA (C) oligonucleotide duplexes. They were then subjected to MT regrowth assays and fixed at the time points indicated. CAP350 and pericentrin were visualized with appropriate antibodies (inset in C shows positive staining for pericentrin in GL2-treated control cells) and MTs were stained with FITC-labeled anti-α-tubulin antibody. Bar, 10 μm. (D-F) Transfected cells were classified according to their failure to nucleate MTs (white bars), or nucleate MTs and then form asters (gray bars) or nonfocused MT networks (black bars). Histograms show results from three independent experiments, counting 300 cells each, and error bars indicate SDs.
A Centrosomal Protein, FOP (FGFR1 oncogene partner), Interacts with CAP350
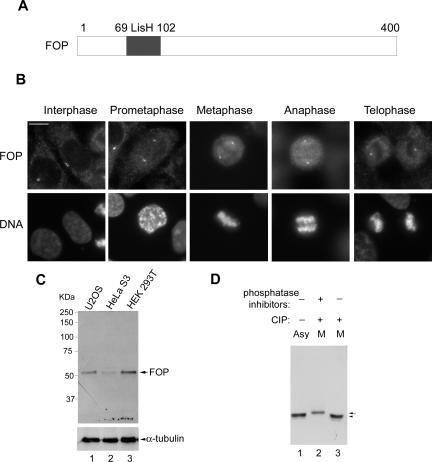
To better understand the role of CAP350 in MT anchoring, we searched for interacting proteins. Specifically, we carried out yeast two-hybrid screens with different fragments of CAP350 as bait and a testis cDNA library as prey. By using a C-terminal CAP350 fragment (residues 2159-3117) as bait, we identified 15 independent clones all expressing full-length FOP. This protein was originally identified as a fusion partner with fibroblast growth factor receptor 1 (FGFR1) in oncoproteins giving rise to stem cell myeloproliferative disorders (Popovici et al., 1999; Guasch et al., 2001; Vizmanos et al., 2004). Subsequently, FOP was shown to be a centrosomal protein (Andersen et al., 2003; Delaval et al., 2005b). Interestingly, FOP contains a LisH motif (Figure 4A), which in other proteins has been implicated in MT dynamics (Emes and Ponting, 2001; Figure 4A).
Figure 4.
FOP is a centrosomal protein and phosphorylated in mitosis. (A) Schematic representation of FOP, indicating the LisH domain (residues 69-102). (B) Subcellular localization of FOP at various cell cycle stages. U2OS were labeled with anti-FOP antibody (top) and DAPI to visualize DNA (bottom). Bar, 10 μm. (C) Western blotting of total HEK293T, U2OS, and HeLa S3 cell extracts with affinity purified anti-FOP antibody. Cell extracts, 15 μg, were used for each lane. (D) FOP was immunoprecipitated from asynchronously growing (Asy) or nocodazole-arrested (M) HeLa cells and treated with (+) or without (-) calf intestinal phosphatase (CIP) in the presence (+) or absence (-) of phosphatase inhibitors. Samples were separated by 12% SDS-PAGE and probed by Western blotting.
A rabbit antibody (R144) raised against full-length FOP labeled the centrosome throughout the cell cycle and this staining could readily be abolished by antigen competition (Figure 4B and unpublished data). FOP colocalized with γ-tubulin in interphase cells and, similar to CAP350 (Figure 1B), two bright FOP-positive spots were observed at each spindle pole of mitotic cells (Figure 4B). Moreover, a small portion of FOP could be seen to associate with spindle MTs (Figure 4B). These IF results indicate that FOP colocalizes with CAP350 throughout the cell cycle. By Western blotting, the anti-FOP antibody specifically recognized a ∼50 kDa protein in whole cell extracts prepared from HEK293T, U2OS, or HeLa S3. Attesting to the specificity of this reaction, FOP immunoreactivity disappeared after siRNA-mediated depletion of FOP (see Figure 7). Similar to CAP350, FOP also displayed a retarded mobility in M phase samples, due to phosphorylation (Figure 4 D).
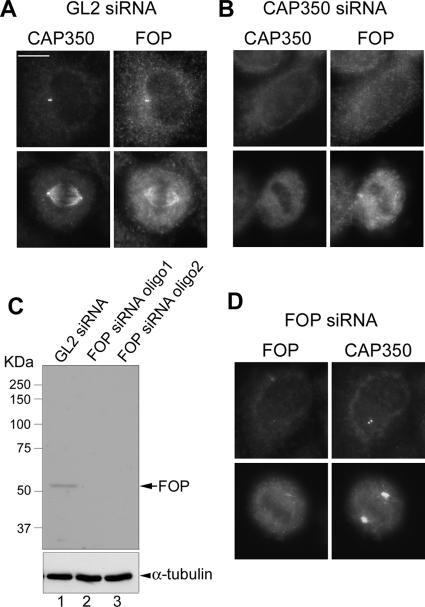
Figure 7.
Effect of CAP350 depletion on FOP localization and vice versa. (A, B, and D) FOP targeting to centrosome requires CAP350, whereas spindle association of CAP350 requires FOP. HeLa S3 cells were treated for 72 h with the GL2 control duplex (A), a CAP350-specific duplex (B), or a FOP-specific duplex (D) and then interphase and mitotic cells were analyzed by IF microscopy, using the antibodies indicated. Bar, 10 μm. (C) Western blotting shows effective depletion of FOP by 72-h treatment with two different siRNA oligonucleotide duplexes. Equal amounts of protein were separated by SDS-PAGE and probed by Western blotting with anti-FOP antibody (top) and anti-α-tubulin antibody as a loading control (bottom).
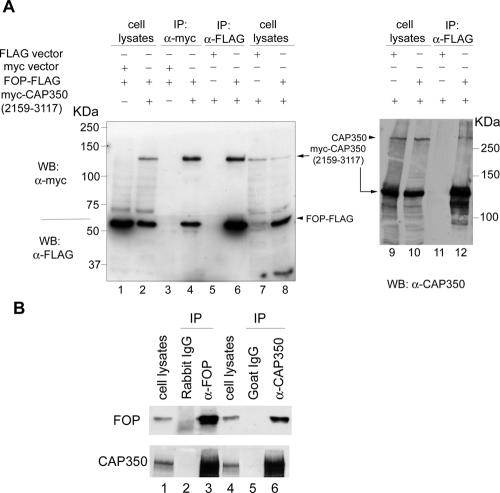
To confirm the yeast two-hybrid results and determine whether CAP350 and FOP interact in human cells, coimmunoprecipitation experiments were carried out. First, experiments were performed with lysates prepared from 293T cell co-overexpressing FOP-FLAG and the myc-tagged C-terminal CAP350 fragment (residue 2159-3117). As shown in Figure 5A, FLAG-tagged FOP was specifically coprecipitated with the myc-tagged CAP350 fragment (compare lanes 4 and 3) and, in a reciprocal experiment, the C-terminal CAP350 fragment was specifically coprecipitated with FLAG-tagged FOP (compare lanes 6 and 5). Furthermore, endogenous CAP350 could readily be detected in the FLAG-tagged FOP immunoprecipitate (lane 12), but not in the control sample (lane 11). To demonstrate an interaction between endogenous proteins, coimmunoprecipitation experiments were then carried out with anti-CAP350 and anti-FOP antibodies. As shown in Figure 5B, CAP350 could readily and specifically be coprecipitated with FOP (compare lanes 3 and 2), and vice versa (compare lanes 6 and 5). Taken together, these data clearly demonstrate that FOP interacts with CAP350 in vivo and that this interaction requires the C-terminal part of CAP350.
Figure 5.
CAP350 interacts with FOP in vivo. (A) FOP interacts with a C-terminal fragment of CAP350. Immunoprecipitation experiments were performed on lysates from 293T cells after cotransfection with the indicated plasmids. Antibodies used were anti-myc (lanes 3 and 4) and anti-FLAG (lanes 5 and 6), coupled to beads. Immunoprecipitated proteins were then analyzed by Western blotting, using the indicated antibodies, and whole cell lysates were analyzed in parallel (lanes 1 and 2). In a separate experiment, anti-FLAG immunoprecipitates were also separated by 6% SDS-PAGE and probed with anti-CAP350 antibody to detect endogenous CAP350 (lanes 9-12). (B) Endogenous FOP interacts with endogenous CAP350. HEK293T cell extracts were immunoprecipitated with control rabbit IgG (lane 2) or goat IgG (lane 5) and anti-FOP (lane 3) or anti-CAP350 antibody (lanes 6). Immunoprecipitates were subject to Western blotting with anti-FOP (top) or anti-CAP350 (bottom) antibodies; whole cell lysates are shown in lanes 2 and 4.
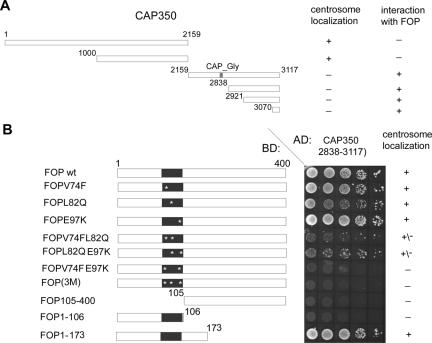
To more precisely map the interaction domains between CAP350 and FOP, yeast two-hybrid experiments were performed (Figure 6). These studies showed that the C-terminal 47 amino acids of CAP350 were sufficient for the interaction with FOP (Figure 6A) and the N-terminal 175 residues of FOP, including the LisH domain, were required for binding to CAP350 (Figure 6B). The LisH motif was not sufficient for binding of FOP to CAP350, as indicated by the inability of FOP1-106 to bind. However, the LisH motif was clearly required in that point mutations within this motif severely reduced or abolished binding (Figure 6B). Interestingly, these studies also revealed a strict correlation between the ability of FOP mutants to interact with CAP350 and their ability to localize to the centrosome (Figure 6B), suggesting that the centrosome association of FOP is mediated by CAP350.
Figure 6.
Mapping the interaction domain between CAP350 and FOP. (A) Summary of FOP interactions, as determined by yeast two-hybrid analyses with different C-terminal constructs of CAP350, and of centrosome localizations, as determined by IF microscopy. (B) Yeast two-hybrid analyses of interactions between the indicated C-terminal CAP350 fragment and FOP constructs. The wild-type and mutant FOP constructs analyzed are indicated schematically on the left and their ability to localize to the centrosome on the right. The abbreviation FOP(3M) stands for FOPV74FL82QE97K. The central panel shows the results of growing transformed yeast cells on selective medium (QDO), to test for interactions with the CAP350 bait. BD, binding domain; AD, activation domain.
Requirements for CAP350 and FOP Localization
To further explore the possibility that CAP350 and FOP might determine each other's localization, the two proteins were depleted by siRNA, using two different oligonucleotide duplexes (oligo1 and oligo2) for targeting each protein (Figure 7). Depletion efficiency was demonstrated by both Western blotting (Figures 2 and 7C, respectively) and IF microscopy (Figures 2 and 7, B and D). Depletion of CAP350 virtually abolished the association of FOP with centrosomes in both interphase and M phase cells (Figure 7B), confirming and extending the data shown in Figure 6. In contrast, depletion of FOP did not detectably influence the centrosome association of CAP350 in interphase cells (Figure 7D). Interestingly, however, it abolished CAP350 association with spindle MTs in M phase cells and instead caused the accumulation of CAP350 at spindle poles in (compare Figure 7D with Figure 1B). These results demonstrate that CAP350 is required for recruiting FOP to the centrosome and that FOP is necessary for CAP350 association with spindle MTs.
By deletion analysis, we found that the central part of CAP350 (residues 1000-2159) was required for centrosome association, but the CAP_Gly motif was not necessary (unpublished data). Exactly how CAP350 is targeted to the centrosome remains to be established.
FOP Interacts with the + TIP Protein EB1
When FLAG-tagged FOP was expressed in cells, it colocalized with γ-tubulin at centrosomes as expected, at least when expressed at relatively low levels (Figure 8A, top). Remarkably, however, at higher expression levels, FOP showed a pronounced tendency to associate with MT plus ends, as demonstrated by double-labeling with the plus-end marker (+TIP) protein, EB1 (Figure 8A, bottom). Considering that two different +TIP proteins, EB1 and p150Glued, have previously been implicated in MT anchoring (Quintyne et al., 1999; Askham et al., 2002; Louie et al., 2004), we asked whether FOP could interact with either EB1 or p150Glued. Yeast two-hybrid experiments revealed no interaction between FOP and p150Glued (unpublished data), but binding could readily be seen with EB1 (Figure 8C). Moreover, the ability of different FOP mutants to localize to MT plus ends was correlated with their ability to interact with EB1 (Figure 8C). An interaction between FOP and EB1 could also be demonstrated by coimmunoprecipitation from 293T cell lysates coexpressing GFP-tagged EB1 and FLAG-tagged FOP (Figure 8B). However, we have so far been unable to detect endogenous EB1 in anti-FOP immunoprecipitates (unpublished data), suggesting that the interaction is transient and/or that only a small amount of EB1 interacts with FOP at the centrosome.
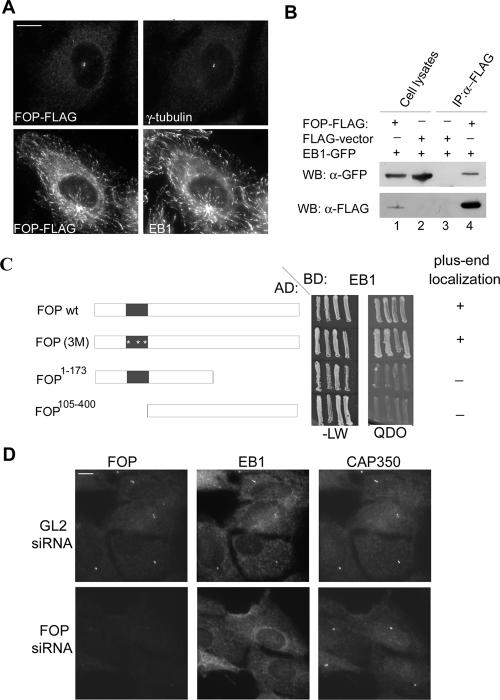
Figure 8.
FOP interacts with EB1 and is required for its centrosome localization. (A) U2OS cells were transfected with FLAG-tagged FOP and costained with anti-FLAG and either anti-γ-tubulin or anti-EB1 antibodies. Bar, 10 μm. (B) FOP interacts with EB1 in vivo. HEK293T cells were transfected for 36 h with GFP-tagged EB1 and FLAG-tagged FOP (lanes 1 and 4) or empty FLAG vector (lanes 2 and 3). After lysis, immunoprecipitations were performed with anti-FLAG antibodies coupled to beads and proteins were subject to SDS-PAGE and transferred to membranes. Western blots were probed with anti-FLAG (bottom) or anti-GFP (top) antibodies. (C) Yeast two-hybrid analysis of the interaction between EB1 and various FOP constructs. The wild-type and mutant FOP constructs analyzed are indicated schematically on the left and their ability to localize to MT plus ends, as determined by transient transfection, on the right. The abbreviation FOP(3M) stands for FOPV74FL82QE97K. The central panel shows the results of growing transformed yeast cells on selective medium (QDO), to test for interactions with the CAP350 bait, and on nonselective medium (-LW) to control for viability. BD, binding domain; AD, activation domain. (D) U2OS cells were transfected with a FOP-specific siRNA duplex for 96 h, treated for 4 h with 6 μg/ml nocodazole, and then subjected to IF microscopy, using anti-FOP (left), anti-EB1 (middle), and anti-CAP350 (right) antibodies. Bar, 10 μm.
Prompted by the above findings, we next asked whether depletion of endogenous FOP would impair the localization of EB1 at either MT plus ends or centrosomes. To allow the visualization of EB1 at the centrosome, MTs were depolymerized by nocodazole treatment (Fry et al., 1998) and CAP350 was used as a centrosome marker. Depletion of FOP virtually abolished centrosome localization of EB1 (Figure 8D) but did not detectably influence its MT plus-end localization (unpublished data). Moreover, overexpression of a FOP-binding C-terminal fragment of CAP350 (2159-3117) in COS7 cells caused the displacement of both endogenous FOP and EB1 from centrosomes (unpublished data). These data indicate that a CAP350-FOP complex is required for the accumulation of EB1 at the centrosome.
CAP350, FOP, and EB1 Are All Required for MT Anchoring
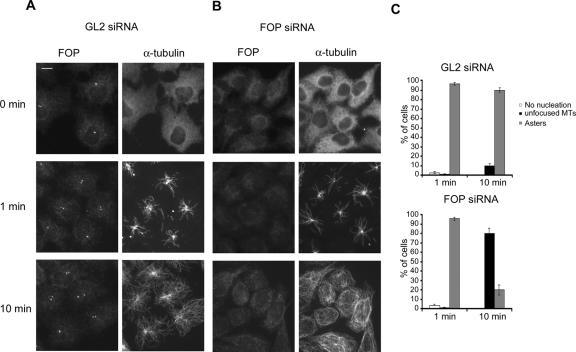
Having established that CAP350, FOP, and EB1 interact, we sought evidence to demonstrate that all three proteins are required for MT anchoring at the centrosome. When either FOP or EB1 were depleted by siRNA, the depleted cells showed disorganized MT arrays (Figure 9, A and B), very similar to the results observed upon depletion of CAP350 (Figure 2B). Moreover, when FOP was displaced from the centrosome by overexpression of a C-terminal CAP350 fragment (2159-3117), the MT network of COS7 cells also became disorganized (Figure 9C). To determine whether the disorganization caused by FOP depletion was the result of impaired MT anchoring, MT regrowth assays were performed in siRNA-treated cells (Figure 10). To assess MT nucleation and MT anchoring, respectively, samples were analyzed at different time points after induction of regrowth. After depletion of FOP, MTs were nucleated as efficiently as in the GL2-treated controls (Figure 10, compare 1-min time points), but with increasing time ∼80% of the FOP-depleted cells lacked focused radial MT arrays, indicating that MTs could not be retained at the centrosome (Figure 10, compare 10-min time points). In the case of EB1-depleted cells, MTs were also nucleated, although nucleation was delayed (unpublished data), presumably because polymerized MTs were less stable in the absence of EB1 (Louie et al., 2004). Similar to the FOP depleted cells, most of the EB1-depleted cells subsequently failed to develop focused MT arrays after 10-15 min of regrowth (unpublished data; see Figure 9B). These data demonstrate that MTs could be nucleated in the absence of either FOP or EB1, but they could not subsequently be retained at the centrosome.
Figure 9.
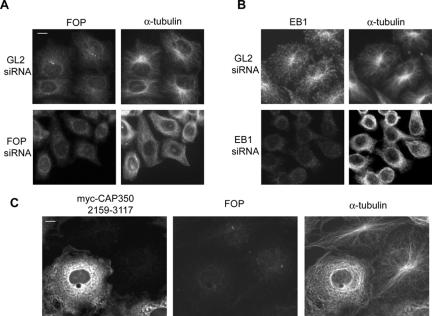
Depletion of FOP or EB1 causes disorganization of the MT network. (A and B) HeLa S3 cells were transfected for 72 h with the indicated siRNA duplexes and then subjected to IF staining with the indicated antibodies. Note that both FOP- and EB1-depleted cells lack radial MT arrays focused on the centrosome. Bar, 10 μm. (C) COS7 cells were transfected for 48 h with the myc-tagged C-terminal CAP350 fragment, a domain known to bind FOP (Figure 6), and then stained with anti-myc (left), anti-FOP (middle), and anti-α-tubulin antibodies. Note the transfected cells, but not neighboring controls, lack radial MT arrays. Bar, 10 μm.
Figure 10.
Depletion of FOP affects MT anchoring. HeLa S3 cells were transfected with GL2 (A) or a FOP-specific siRNA oligonucleotide duplex (B). They were then subjected to MT regrowth assays and fixed at the time points indicated. FOP was visualized with anti-FOP antibodies and MTs were stained with FITC-labeled anti-α-tubulin antibody. Bar, 10 μm. (C) Transfected cells were classified according to their failure to nucleate MTs (white bars), or nucleate MTs and then form asters (gray bars) or nonfocused MT networks (black bars). Histograms show results from three independent experiments, counting 300 cells each, and error bars indicate SDs.
Taken together, our data indicate that CAP350 provides a docking site for FOP at the centrosome. In turn, FOP then contributes to recruiting EB1, and all three proteins cooperate in MT anchoring at the centrosome.
DISCUSSION
The centrosome is best known for its role in MT nucleation, but recent studies provide increasing evidence that this organelle is also a major site of MT anchoring in many animal cells (Bornens, 2002; Ou and Rattner, 2004). MT anchoring at the centrosome results in radial MT arrays, and although MT anchoring is dependent on MT nucleation, it clearly represents a distinct aspect of centrosome function. A number of proteins have previously been implicated in MT anchoring (Mogensen et al., 2000; Askham et al., 2002; Dammermann and Merdes, 2002; Ohta et al., 2002; Kim et al., 2004; Delgehyr et al., 2005), but our understanding of the structures and mechanisms required for this important process remains limited. Here we characterize CAP350 and FOP, two novel proteins recently shown or predicted to localize to the centrosome (Andersen et al., 2003). We demonstrate that both proteins localize to the centrosome throughout the cell cycle. Moreover, we show that CAP350 and FOP interact directly with each other, and that both are required for MT anchoring. Finally, we identify EB1 as an interaction partner of FOP and confirm a role for this +TIP protein in MT anchoring (Askham et al., 2002; Louie et al., 2004).
Database searches readily identify CAP350 and FOP homologues in vertebrates but we have been unable to find obvious homologues in invertebrates or yeast. Interestingly, proteins showing partial sequence similarity to FOP have also been identified in the plant Arabidopsis (Traas et al., 1995; Delaval et al., 2005a). Although higher plants lack centrosomes, the products of these FOP-related plant genes (termed tonneau-1a and -1b) also function in MT organization (Delaval et al., 2005a). Both CAP350 and FOP carry structural motifs that have also been observed in other proteins implicated in cytoskeletal networks.
CAP350 harbors a CAP_Gly motif, a domain of ∼70 residues (Riehemann and Sorg, 1993). Similar motifs have been shown to be essential for MT association of CLIP-170 (Diamantopoulos et al., 1999), CLIP-115 (Hoogenraad et al., 2000), and p150Glued (Vaughan et al., 2002), but the precise role of CAP_Gly motifs is not well understood. Our study shows that the CAP_Gly motif of CAP350 was not required for either binding to FOP or centrosome localization of CAP350. Instead, mapping studies identified the last 47 C-terminal amino acids of CAP350 as being both necessary and sufficient for FOP binding. The structure of this fragment is unknown, but its functional importance is confirmed by a very high degree of sequence conservation in the C-termini of vertebrate CAP350 homologues.
FOP has originally been identified as a fusion partner for FGF-receptor 1 in chromosomal translocations giving rise to myeloproliferative disorders (Popovici et al., 1999; Guasch et al., 2001; Vizmanos et al., 2004). Although it is well established that constitutive activation of tyrosine kinase activity is critical for oncogenesis in leukemia patients carrying chimeric kinase fusion proteins, the role of FOP has not been thoroughly investigated. A priori, it is possible that FOP merely contributes an activating dimerization domain to the FGF-receptor kinase. Alternatively, it is attractive to speculate that targeting of the FOP-FGF-receptor fusion protein to the centrosome contributes to oncogenesis (Delaval et al., 2005b). The most striking feature of FOP is the presence of a LisH motif within the N-terminal half of the protein. Named after the lissencephaly gene product Lis1 (Emes and Ponting, 2001), LisH motifs have been implicated in both MT dynamics (Traas et al., 1995; Sapir et al., 1999) and protein dimerization (Cahana et al., 2001). In the case of FOP, we have shown that the LisH motif is required but not sufficient for complex formation with CAP350. In contrast, it was not required for FOP binding to EB1.
The MT plus-end tracking protein EB1 is well known to interact with the tumor suppressor APC (adenomatous polyposis coli; Su et al., 1995) and also with the dynactin component p150Glued (Berrueta et al., 1999; Askham et al., 2002; Ligon et al., 2003; Hayashi et al., 2005). Most of the studies on EB1 have emphasized its roles in MT stabilization at MT plus ends (Mimori-Kiyosue et al., 2000, 2005; Tirnauer and Bierer, 2000; Nakamura et al., 2001). However, EB1 has also been detected at the centrosome (Berrueta et al., 1998; Morrison et al., 1998), where it has been implicated in MT anchoring (Askham et al., 2002; Louie et al., 2004). Interestingly, this latter function requires the ability of EB1 to interact with p150Glued (Askham et al., 2002). Here, we have discovered that EB1 also interacts with FOP. Although the interface between EB1 and p150Glued has recently been resolved by crystallography (Hayashi et al., 2005), we have presently no information on how EB1 interacts with FOP. Importantly, however, FOP is clearly required for the accumulation of EB1 at the centrosome.
Our data demonstrate that all three proteins studied here, CAP350, FOP, and EB1, are required for MT anchoring. Whether they play a direct role in anchoring itself or instead contribute to the organization of MT minus ends requires further study. Our results also point to a hierarchy in the centrosome association of the three proteins. In the absence of CAP350, FOP could not localize to centrosomes. Moreover, overexpression of a CAP350 fragment spanning the FOP interaction domain resulted in the displacement of FOP from centrosomes. On the other hand, FOP was not required for the centrosome association of CAP350, but its absence impaired the ability of CAP350 to bind to spindle MTs during mitosis. CAP350 and FOP constitute genuine centrosomal proteins, but the properties of EB1 suggest that it acts as a more distal component in MT anchoring.
How exactly the three proteins characterized here, CAP350, FOP, and EB1, cooperate mechanistically in MT anchoring remains to be determined. One attractive possibility is that a CAP350-FOP complex provides centrosomal docking sites for EB1, which then cooperates with the p150Glued-dynactin complex to capture newly nucleated MTs after their release from γ-TuRCs (Askham et al., 2002). In the future, it will be important to investigate how the CAP350-FOP complex interacts with other centrosome components implicated in MT anchoring, notably ninein (Mogensen et al., 2000; Abal et al., 2002; Dammermann and Merdes, 2002; Delgehyr et al., 2005) and the BBS4/PCM-1 complex (Dammermann and Merdes, 2002; Kim et al., 2004). Finally, we note that MT anchoring to the interphase centrosome is expected to differ from MT anchoring to the mitotic spindle pole (Khodjakov et al., 2000; Bornens, 2002). In this context it may be relevant that both CAP350 and FOP were found to be subject to mitotic phosphorylation. It will be interesting, therefore, to explore the cell cycle regulation of these proteins.
Acknowledgments
We thank Drs. S. Sugano (Institute of Medical Science, University of Tokyo, Japan), Francis Barr (Max-Planck Institute of Biochemistry, Martinsried, Germany), M. Takahashi (Kobe University, Japan) and Y. Mimori-Kiyosue (Kan Research Institute, Kyoto, Japan) for antibodies, cDNAs, and plasmids. We also thank Elena Nigg for excellent technical assistance and Martina Casenghi, Sébastien Lavoie, Eunice Chan, and Susanne Bahe for many helpful discussions. This work was supported by the Max-Planck-Society and the Fonds der Chemischen Industrie.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0810) on November 28, 2005.
Abbreviations used: MT, microtubule; IF, immunofluorescence; siRNA, small interfering RNA; +TIP, plus end-tracking protein.
References
- Abal, M., Piel, M., Bouckson-Castaing, V., Mogensen, M., Sibarita, J. B., and Bornens, M. (2002). Microtubule release from the centrosome in migrating cells. J. Cell Biol. 159, 731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, J. S., Wilkinson, C. J., Mayor, T., Mortensen, P., Nigg, E. A., and Mann, M. (2003). Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570-574. [DOI] [PubMed] [Google Scholar]
- Askham, J. M., Vaughan, K. T., Goodson, H. V., and Morrison, E. E. (2002). Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol. Biol. Cell 13, 3627-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta, L., Kraeft, S. K., Tirnauer, J. S., Schuyler, S. C., Chen, L. B., Hill, D. E., Pellman, D., and Bierer, B. E. (1998). The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA 95, 10596-10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta, L., Tirnauer, J. S., Schuyler, S. C., Pellman, D., and Bierer, B. E. (1999). The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 9, 425-428. [DOI] [PubMed] [Google Scholar]
- Bornens, M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25-34. [DOI] [PubMed] [Google Scholar]
- Cahana, A. et al. (2001). Targeted mutagenesis of Lis1 disrupts cortical development and LIS1 homodimerization. Proc. Natl. Acad. Sci. USA 98, 6429-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, P., Giddings, T. H., Jr., Winey, M., and Stearns, T. (2003). Epsilon-tubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 5, 71-76. [DOI] [PubMed] [Google Scholar]
- Clark, I. B., and Meyer, D. I. (1999). Overexpression of normal and mutant Arp1alpha (centractin) differentially affects microtubule organization during mitosis and interphase. J. Cell Sci. 112, 3507-3518. [DOI] [PubMed] [Google Scholar]
- Dammermann, A., Desai, A., and Oegema, K. (2003). The minus end in sight. Curr. Biol. 13, R614-R624. [DOI] [PubMed] [Google Scholar]
- Dammermann, A., and Merdes, A. (2002). Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159, 255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaval, B., Lelievre, H., and Birnbaum, D. (2005a). Myeloproliferative disorders: the centrosome connection. Leukemia 19, 1739-1744. [DOI] [PubMed] [Google Scholar]
- Delaval, B., Letard, S., Lelievre, H., Chevrier, V., Daviet, L., Dubreuil, P., and Birnbaum, D. (2005b). Oncogenic tyrosine kinase of malignant hemopathy targets the centrosome. Cancer Res. 65, 7231-7240. [DOI] [PubMed] [Google Scholar]
- Delgehyr, N., Sillibourne, J., and Bornens, M. (2005). Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565-1575. [DOI] [PubMed] [Google Scholar]
- Diamantopoulos, G. S., Perez, F., Goodson, H. V., Batelier, G., Melki, R., Kreis, T. E., and Rickard, J. E. (1999). Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J. Cell Biol. 144, 99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey, S., McCollum, D., and Theurkauf, W. (2005). Centrosomes in cellular regulation. Annu. Rev. Cell Dev. Biol. 21, 411-434. [DOI] [PubMed] [Google Scholar]
- Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- Emes, R. D., and Ponting, C. P. (2001). A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 10, 2813-2820. [DOI] [PubMed] [Google Scholar]
- Fry, A. M., Mayor, T., Meraldi, P., Stierhof, Y. D., Tanaka, K., and Nigg, E. A. (1998). C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2105. J. Cell Biol. 141, 1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley, A., Jurczyk, A., Sillibourne, J., Halilovic, E., Mogensen, M., Groisman, I., Blomberg, M., and Doxsey, S. (2003). A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarguaglini, G., Duncan, P. I., Stierhof, Y. D., Holmstrom, T., Duensing, S., and Nigg, E. A. (2005). The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol. Biol. Cell 16, 1095-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch, G., Ollendorff, V., Borg, J. P., Birnbaum, D., and Pebusque, M. J. (2001). 8p12 stem cell myeloproliferative disorder: the FOP-fibroblast growth factor receptor 1 fusion protein of the t(6;8) translocation induces cell survival mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt/mTOR pathways. Mol. Cell. Biol. 21, 8129-8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, I., Wilde, A., Mal, T. K., and Ikura, M. (2005). Structural basis for the activation of microtubule assembly by the EB1 and p150(Glued) complex. Mol. Cell 19, 449-460. [DOI] [PubMed] [Google Scholar]
- Hoogenraad, C. C., Akhmanova, A., Grosveld, F., De Zeeuw, C. I., and Galjart, N. (2000). Functional analysis of CLIP-115 and its binding to microtubules. J. Cell Sci. 113, 2285-2297. [DOI] [PubMed] [Google Scholar]
- Ishikawa, H., Kubo, A., Tsukita, S., and Tsukita, S. (2005). Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7, 517-524. [DOI] [PubMed] [Google Scholar]
- Keating, T. J., Peloquin, J. G., Rodionov, V. I., Momcilovic, D., and Borisy, G. G. (1997). Microtubule release from the centrosome. Proc. Natl. Acad. Sci. USA 94, 5078-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., Cole, R. W., Oakley, B. R., and Rieder, C. L. (2000). Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 10, 59-67. [DOI] [PubMed] [Google Scholar]
- Kim, J. C. et al. (2004). The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 36, 462-470. [DOI] [PubMed] [Google Scholar]
- Lange, B. M., and Gull, K. (1995). A molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol. 130, 919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon, L. A., Shelly, S. S., Tokito, M., and Holzbaur, E. L. (2003). The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization. Mol. Biol. Cell 14, 1405-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie, R. K., Bahmanyar, S., Siemers, K. A., Votin, V., Chang, P., Stearns, T., Nelson, W. J., and Barth, A. I. (2004). Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J. Cell Sci. 117, 1117-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., Honda, R., and Nigg, E. A. (2002). Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 21, 483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y. et al. (2005). CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168, 141-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., Shiina, N., and Tsukita, S. (2000). The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 10, 865-868. [DOI] [PubMed] [Google Scholar]
- Mogensen, M. M., Malik, A., Piel, M., Bouckson-Castaing, V., and Bornens, M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013-3023. [DOI] [PubMed] [Google Scholar]
- Moritz, M., Rice, L.M.A., and Agard, D. A. (2004). Microtubule nucleation. In: Centrosomes in Development and Disease, ed. E. A. Nigg, Weinheim: Wiley-VCH, 27-41.
- Morrison, E. E., Wardleworth, B. N., Askham, J. M., Markham, A. F., and Meredith, D. M. (1998). EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene 17, 3471-3477. [DOI] [PubMed] [Google Scholar]
- Nakagawa, Y., Yamane, Y., Okanoue, T., Tsukita, S., and Tsukita, S. (2001). Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol. Biol. Cell 12, 1687-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, M., Zhou, X. Z., and Lu, K. P. (2001). Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr. Biol. 11, 1062-1067. [DOI] [PubMed] [Google Scholar]
- Nigg, E. A. (2004). Centrosomes in Development and Disease, Weinheim: Wiley-VCH.
- Ohta, T., Essner, R., Ryu, J. H., Palazzo, R. E., Uetake, Y., and Kuriyama, R. (2002). Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J. Cell Biol. 156, 87-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, Y., and Rattner, J. B. (2004). The centrosome in higher organisms: structure, composition, and duplication. Int. Rev. Cytol. 238, 119-182. [DOI] [PubMed] [Google Scholar]
- Ou, Y. Y., Mack, G. J., Zhang, M., and Rattner, J. B. (2002). CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J. Cell Sci. 115, 1825-1835. [DOI] [PubMed] [Google Scholar]
- Patel, H., Truant, R., Rachubinski, R. A., and Capone, J. P. (2005). Activity and subcellular compartmentalization of peroxisome proliferator-activated receptor alpha are altered by the centrosome-associated protein CAP350. J. Cell Sci. 118, 175-186. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici, C., Zhang, B., Gregoire, M. J., Jonveaux, P., Lafage-Pochitaloff, M., Birnbaum, D., and Pebusque, M. J. (1999). The t(6;8)(q27;p11) translocation in a stem cell myeloproliferative disorder fuses a novel gene, FOP, to fibroblast growth factor receptor 1. Blood 93, 1381-1389. [PubMed] [Google Scholar]
- Quintyne, N. J., Gill, S. R., Eckley, D. M., Crego, C. L., Compton, D. A., and Schroer, T. A. (1999). Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N. J., and Schroer, T. A. (2002). Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159, 245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C. L., Faruki, S., and Khodjakov, A. (2001). The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11, 413-419. [DOI] [PubMed] [Google Scholar]
- Riehemann, K., and Sorg, C. (1993). Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem. Sci. 18, 82-83. [DOI] [PubMed] [Google Scholar]
- Sapir, T., Cahana, A., Seger, R., Nekhai, S., and Reiner, O. (1999). LIS1 is a microtubule-associated phosphoprotein. Eur. J. Biochem. 265, 181-188. [DOI] [PubMed] [Google Scholar]
- Sillje, H. H., Takahashi, K., Tanaka, K., Van Houwe, G., and Nigg, E. A. (1999). Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 18, 5691-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. K., Burrell, M., Hill, D. E., Gyuris, J., Brent, R., Wiltshire, R., Trent, J., Vogelstein, B., and Kinzler, K. W. (1995). APC binds to the novel protein EB1. Cancer Res. 55, 2972-2977. [PubMed] [Google Scholar]
- Takahashi, M., Yamagiwa, A., Nishimura, T., Mukai, H., and Ono, Y. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell 13, 3235-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., and Bierer, B. E. (2000). EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J. Cell Biol. 149, 761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., O'Toole, E., Berrueta, L., Bierer, B. E., and Pellman, D. (1999). Yeast Bim1p promotes the G1-specific dynamics of microtubules. J. Cell Biol. 145, 993-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas, J., Bellini, C., Nacry, P., Kronenberger, J., Bouchez, D., and Caboche, M. (1995). Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature 375, 676-677. [Google Scholar]
- Vaughan, P. S., Miura, P., Henderson, M., Byrne, B., and Vaughan, K. T. (2002). A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J. Cell Biol. 158, 305-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizmanos, J. L., Hernandez, R., Vidal, M. J., Larrayoz, M. J., Odero, M. D., Marin, J., Ardanaz, M. T., Calasanz, M. J., and Cross, N. C. (2004). Clinical variability of patients with the t(6;8)(q27;p12) and FGFR1OP-FGFR1 fusion: two further cases. Hematol. J. 5, 534-537. [DOI] [PubMed] [Google Scholar]
- Yan, X., Li, F., Liang, Y., Shen, Y., Zhao, X., Huang, Q., and Zhu, X. (2003). Human Nudel and NudE as regulators of cytoplasmic dynein in poleward protein transport along the mitotic spindle. Mol. Cell. Biol. 23, 1239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q., Chen, D., Pierstorff, E., and Luo, K. (1998). Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 17, 3681-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]