Abstract
Reversible phosphorylation of the SR family of splicing factors plays an important role in pre-mRNA processing in the nucleus. Interestingly, the SRPK family of kinases specific for SR proteins is localized in the cytoplasm, which is critical for nuclear import of SR proteins in a phosphorylation-dependent manner. Here, we report molecular dissection of the mechanism involved in partitioning SRPKs in the cytoplasm. Common among all SRPKs, the bipartite kinase catalytic core is separated by a unique spacer sequence. The spacers in mammalian SRPK1 and SRPK2 share little sequence homology, but they function interchangeably in restricting the kinases in the cytoplasm. Removal of the spacer in SRPK1 had little effect on the kinase activity, but it caused a quantitative translocation of the kinase to the nucleus and consequently induced aggregation of splicing factors in the nucleus. Rather than carrying a nuclear export signal as suggested previously, we found multiple redundant signals in the spacer that act together to anchor the kinase in the cytoplasm. Interestingly, a cell cycle signal induced nuclear translocation of the kinase at the G2/M boundary. These findings suggest that SRPKs may play an important role in linking signaling to RNA metabolism in higher eukaryotic cells.
INTRODUCTION
Pre-mRNA splicing in mammalian cells requires many auxiliary factors, an important class of which is the SR family of splicing factors. SR proteins are characterized by one or two RNA recognition motifs (RRMs) at the N terminus and an arginine-serine-rich (RS) domain enriched in serine and arginine repeats at the C terminus (reviewed by Fu, 1995; Manley and Tacke, 1996; Graveley, 2000). RRMs are important for sequence-specific binding to commit pre-mRNA to the splicing pathway (Fu, 1993), whereas the RS domain in SR proteins is thought to promote protein-protein interactions during spliceosome assembly (Wu and Maniatis, 1993). Recently, the RS domain in SR proteins was found to directly bind RNA in assembled spliceosomes (Shen et al., 2004; Shen and Green, 2004).
SR proteins are regulated by reversible phosphorylation. Phosphorylated SR proteins seem to be required for initiating spliceosome assembly at the earliest detectable stage (Mermoud et al., 1994; Kohtz et al., 1994; Cao et al., 1997). Once the spliceosome is fully assembled, dephosphorylation seems to be essential for splicing to take place in the spliceosome (Mermoud et al., 1992; Cao et al., 1997). Interestingly, a later study indicates that a given SR protein may not be obligated to go through a complete phosphorylation-dephosphorylation cycle in each splicing reaction, implying that a dephosphorylated SR protein is able to substitute for dephosphorylation-resistant SR proteins to fulfill the late requirement in splicing (Xiao and Manley, 1998). Consistent with these biochemical observations, ASF/SF2 mutants containing negatively charged aspartic acids or glutamic acids in place of serines in the RS domain are fully functional in complementing the essential function of ASF/SF2 in cell viability (Lin et al., 2005).
Reversible phosphorylation of SR proteins has also been shown to play a critical role in coupling splicing with both upstream and downstream events during gene expression. It has been well established that splicing takes place cotranscriptionally in the nucleus; thus, splicing factors have to be recruited to nascent transcripts. This recruitment process seems to require proper phosphorylation of SR proteins (Misteli et al., 1997, 1998). A series of in vitro and in vivo experiments show that phosphorylation is important for SR proteins to interact with the C-terminal domain of polymerase II (Pol II) to coordinate transcription with splicing (Misteli and Spector, 1999; Hirose and Manley, 2000). In linking splicing to RNA export, SR proteins have been found to function as adaptors for nuclear export of spliced mRNA (Huang and Steitz, 2001; Huang et al., 2003, 2004; Lai and Tarn, 2004). The adaptor function seems to be confined within a subset of SR proteins capable of shuttling between the nucleus and the cytoplasm. Interestingly, several shuttling SR proteins can directly interact with the nuclear export factor TAP via sequences in the N-terminal RRMs, but the interaction requires dephosphorylation of the C-terminal RS domain. Although prevention of a single SR protein from shuttling is not sufficient to impair mRNA export in vivo, it remains to be determined whether shuttling SR proteins as a class are important for mRNA export (Lin et al., 2005). Once exported to the cytoplasm, SR proteins are reimported to the nucleus by interacting with the import receptor hMtr10/Transportin-SR in a phosphorylation-dependent manner (Yun and Fu, 2000; Lai et al., 2000, 2001; Gilbert et al., 2001; Yun et al., 2003). It has been proposed that phosphorylation of shuttling SR proteins in the cytoplasm may act as a switch between mRNA unloading and SR protein reimport (Gilbert and Guthrie, 2004).
Mammalian cells express several kinases key to phosphorylation regulation of SR proteins and RNA metabolism in general. The first SR protein-specific kinase SRPK1 was cloned during the characterization of an activity in regulating SR protein redistribution during the cell cycle (Gui et al., 1994). We now know that SRPK1 belongs to a family of kinases that are evolutionarily conserved from budding yeast to humans (Takeuchi and Yanagida, 1993; Wang et al., 1998; Kuroyanagi et al., 1998; Siebel et al., 1999; Koizumi et al., 1999; Tang et al., 1998, 2000). All SRPK family members share highly conserved kinase domains, which are separated by a unique spacer sequence in individual family members, indicating that specific SRPKs may be uniquely regulated by the spacer sequence. The second family of SR protein kinases is called Clk/Sty (for cyclin-like kinase or serine/threonine/tyrosine kinase), which has three family members in mammalian cells. Clk/Sty or Clk-1 was initially cloned as a cyclin-like kinase by degenerate PCR (Ben-David et al., 1991; Howell et al., 1991). Its connection to SR proteins was made when Clk/Sty was shown to interact with SR proteins in a two-hybrid screen (Colwill et al., 1996). The best evidence for its involvement in splicing regulation came from studies in Drosophila where a Clk/Sty orthologue could phosphorylate endogenous SR proteins and mutations in the kinase altered specific alternative splicing events in the sex determination pathway (Du et al., 1998).
Here, we report the mechanism for the regulation of SRPKs by a unique spacer sequence in each kinase. The cytoplasmic localization of SRPK1 is controlled by multiple elements in the spacer, deletion of which had little effect on kinase activity, but caused a quantitative translocation of the kinase to the nucleus. Contrary to an early work indicating the presence of active nuclear export signal (NES) in the spacer of the SRPK family member in fission yeast, we found that cellular partitioning of SRPK1 is likely achieved by a spacer-mediated anchoring mechanism in mammalian cells. The importance of partitioning the kinase during interphase is demonstrated by induced aggregation of splicing factors when an excessive amount of a spacer-deleted kinase is present in the nucleus. Interestingly, the kinase translocates to the nucleus in response to a cell cycling signal before the initiation of the M phase, indicating that the kinase may play a role in cell cycle progression. Together, these findings raise the possibility that the SRPK family of kinases may function as an important class of signal molecules for splicing regulation in higher eukaryotic cells.
MATERIALS AND METHODS
Plasmids
Myc- or FLAG-tagged SRPKs were cloned into the pUHD vector (Gossen and Bujard, 1992) or pcDNA3 (Invitrogen, Carlsbad, CA). SRPK1-K109M contains one point mutation at position 109 from Lys to Met, which impairs the ATP binding site in the kinase (Wang et al., 1998). The nuclear localization signal (NLS) from the SV40 large T antigen was engineered into the N terminus of the kinase coding sequence by using an extended PCR primer. Spacer deletion series were constructed using proper restriction sites in the spacer in combination with PCR. Details in plasmid construction were available upon request. All mutant constructs were confirmed by sequencing.
Kinetic Analysis
The phosphorylation of glutathione S-transferase (GST)-ASF/SF2 was carried out in a buffer containing 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 7.0, 10 mM Mg2+, 1 mg/ml bovine serum albumin (BSA), and [γ-32P]ATP (600-1000 cpm pmol-1) as described previously (Aubol et al., 2003). Typically, enzyme and substrate were preincubated for 3 min before reaction initiation with 0.2 mM ATP in a total volume of 20 μl. Upon reaction quenching with 10 μl of SDS-PAGE loading buffer after designated time periods, phosphorylated GST-ASF/SF2 was separated from unreacted [32P]ATP and enzyme in a 12% SDS-PAGE gel. The appropriate bands were cut out of the dried gel and counted in liquid scintillant. The amount of phosphorylated GST-ASF/SF2 was calculated using the specific activity of the [32P]ATP. As described previously (Aubol et al., 2003), steady-state kinetics was used to derive the Km, and single turnover experiments over a range of kinase concentrations were performed to calculate the Kd for both wild-type (wt) and spacer-deleted SRPK1.
Cell Culture and Synchronization
HeLa and HeLa tTA cells were cultured in DME supplemented with 10% fetal bovine serum. For immunofluorescence staining, cells were first seeded on coverslips coated with 0.1% polylysine. Transfection was carried out using Lipofectamine 2000 reagent (Invitrogen). Cells were synchronized to S phase by adding 2.5 mM thymidine to the growth medium for 24 h as described previously (Gui et al., 1994). G2/M phase cells were prepared by releasing S-phase cells after the thymidine block to the culture media for 7-8 h. Transcription and translation were blocked by using 50 μg/ml actinomycin D and 100 μg/ml cycloheximide for 3 h, respectively. NES-mediated nuclear export was blocked with 10 ng/ml leptomycin B (LMB) for 1 h.
Immunofluorescence Microscopy
Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at room temperature followed by permeabilization for 10 min in 0.1% Triton X-100/phosphate-buffered saline. We used 0.1% BSA in PBS to block nonspecific binding during antibody staining. Endogenous SRPK1 was detected using monoclonal anti-SRPK1 (catalog no. 611072; BD Transduction Laboratories, Lexington, KY), and SC35 was stained with either polyclonal anti-SC35 (Himmelspach et al., 1995) or monoclonal anti-SC35 (Fu and Maniatis, 1990). Transfected wild-type and mutant SRPK1 were localized by using polyclonal rabbit anti-myc (catalog no. 2272; Cell Signaling Technology, Beverly, MA). Transfected wild-type and spacer-deleted SRPK2 were localized by using monoclonal anti-FLAG (M2 from Sigma-Aldrich, St. Louis, MO). Fluorescent-conjugated secondary antibodies were Alexa 594-conjugated donkey anti-mouse IgG and Alexa 488-conjugated goat anti-rabbit IgG (catalog nos. A-21203 and A-11070, Molecular Probes, Eugene, OR). Coverslips were mounted in a mounting solution containing 4,6-diamidino-2-phenylindole (DAPI) (Vectashield; Vector Laboratories, Burlingame, CA) and analyzed under a Zeiss Axiophot microscope and images acquired with a Hamamatsu ORCA-ER digital camera with Improvision OpenLab 3.1.5 software. Deconvolution images were taken in the image core of the University of California San Diego Cancer Center with a DeltaVision restoration microscope system (Applied Precision, Issaquah, WA) using a Photometrics Sony CoolSNAP HQ charged-coupled device camera system (10 MHz; 12 bit; 1392 × 1040) attached to an inverted, wide-field fluorescent microscope (Nikon TE-200).
Heterokaryon Assay
HeLa tTA cells were transfected with various SRPK constructs. Transfected cells were trypsinized 2 h later and mixed with mouse embryonic fibroblasts at the ratio of 2:3. After overnight coculturing, cycloheximide was added to block protein synthesis at 10 μg/ml for 1 h and then increased to 100 μg/ml for 30 min before cell fusion. Cell fusion was induced with 50% polyethylene glycol (PEG)3350 (wt/vol) in PBS for 2 min at 37°C. PEG was washed away with PBS (5 times), and treated cells were incubated in the growth medium containing 100 μg/ml cycloheximide for additional 2 h. Actin was labeled with Alexa 594-phaloidin to identify fused cells.
RESULTS
Regulation of SRPKs by Unique Spacer Sequences
SR protein-specific kinases were previously shown to localize in the cytoplasm in transfected cells (Wang et al., 1998; Yeakley et al., 1999). To determine the localization of endogenous kinase, we stained HeLa cells with a specific monoclonal antibody against SRPK1 (see Materials and Methods). The endogenous kinase was mainly localized in the cytoplasm, consistent with our previous observations. In addition, a fraction of the kinase was visible in the nucleus under a standard inverted microscope (Figure 1, A-C). This result was further confirmed by confocal microscopic analysis of center sections of the nucleus (Figure 1, D-F). No signal was detected if primary antibodies were omitted (Figure 1, G-I). Colocalization of SRPK1 with endogenous SC35 revealed that nuclear SRPK1 seems generally associated with SC35 in nuclear speckles under a standard inverted microscope (Figure 1C, note that staining with the polyclonal anti-SC35 peptide antibodies used here is more diffused than the pattern detected with the monoclonal, phosphoepitope-specific, anti-SC35 antibody, see images in Figure 3). However, both the kinase and the SR protein clearly have a large population diffusely distributed in the nucleus as seen under a confocal microscope (Figure 1F).
Figure 1.
Localization of SRPK1 in interphase HeLa cells. Endogenous SRPK1 and SC35 were localized using a mouse monoclonal anti-SRPK1 and rabbit polyclonal anti-SC35 antibodies (A and B). SRPK1 is mainly present in the cytoplasm with a fraction clearly detectable in the nucleus. Some colocalization of SRPK1 with SC35 in nuclear speckles is evident under a standard inverted fluorescence microscope (C). Confocal microscopy confirmed the localization of SRPK1 in both the cytoplasm and the nucleus (D). SRPK1 and SC35 are diffused in the nucleus at a single focal level (E and F). No staining was detected in the absence of primary antibodies (G, red channels; H, green channels). Nuclei were stained with DAPI (I).
Figure 3.
The spacer of SRPK1 lacks detectable NES. Both Myc-tagged full-length SRPK1 (A) and inactive SRPK1 containing a mutation in the ATP binding site (K109M) (D) were mainly localized in the cytoplasm of transfected HeLa cells. Removal of the spacer (K1-ΔS) resulted in exclusive localization of the kinase in the nucleus (G), colocalizing with phosphorylated SC35 in nuclear speckles 8 h posttransfection (H and I). Prolonged expression of the spacer-deleted kinase (24 h posttransfection, J) induced aggregation of splicing factors in the nucleus (K and L). Nuclear entrance seems to be saturable as indicated by a level of cytoplasmic kinase (J). Inactivation of the kinase activity in spacer-deleted SRPK1 (K-ΔS-K109M) prevented nuclear translocation (M). SC35 was detected with a monoclonal anti-SC35 antibody (B, E, H, K, and N). Potential colocalization was determined by merged images (C, F, I, L, and O).
The substrates for SR kinases are the SR family of splicing factors. Previous studies demonstrate that both hypo- and hyperphosphorylation of SR proteins are detrimental to splicing (Cao et al., 1997; Prasad et al., 1999). Therefore, kinases for SR proteins must be regulated, which, in SRPKs, seems to be achieved by partitioning the kinase in the cytoplasm, analogous to many protein kinases involved in signaling (Cyert, 2001). Although SRPKs are serine-specific kinases, they resemble tyrosine kinases by having a unique spacer that separates the small and large catalytic lobes. Spacers in tyrosine kinases are frequently serving as docking sites for signaling molecules and thus are important regulatory modules in those kinases (Hunter, 2000). Deletion of the spacer in both SRPK1 and SRPK2 resulted in translocation of the kinases to the nucleus (Figure 2, A-H). Similar nuclear translocation was also reported for the SRPK family members in fission and budding yeast (Takeuchi and Yanagida, 1993; Siebel et al., 1999). Interestingly, the spacers in SRPK1 and SRPK2 seem to function as an autonomous unit because insertion of the spacer of SRPK2 into SRPK1 restored the cytoplasmic localization of the kinase (Figure 2, I and J). These findings, coupled with previous studies of SRPK orthologues in low eukaryotic cells, demonstrate that the spacers are regulatory domains critical for the cellular distribution of the SRPK family of kinases.
Figure 2.

Cellular partitioning of SRPK1 and SRPK2 by spacer sequences. Myc-tagged full-length SRPK1 (A), spacer-deleted SRPK1 (C), FLAG-tagged full-length SRPK2(E), and spacer-deleted SRPK2 (G) were localized in transfected HeLa cells. Both full-length kinases were localized in the cytoplasm, whereas deletion of the spacers resulted in translocation of the kinases to the nucleus. To test the interchangeability of spacers, the spacer from SRPK2 was used to replace the spacer sequence in SRPK1 and the hybrid kinase was localized in the cytoplasm. Nuclei were stained with DAPI (right).
Kinase Activity Required for Nuclear Targeting
Both wt and mutant SRPK1 lacking the kinase activity (because of a mutation in the ATP binding site) were localized in the cytoplasm (Figure 3, A-C and D-F). When the spacer was deleted, the kinase translocated to the nucleus where it colocalized with splicing factors in nuclear speckles (Figure 3, G-I). Accumulation of the spacer-deleted SRPK1 in the nucleus then induced aggregation of splicing factors (Figure 3, J-L), which is similar to the cellular response to inhibition of splicing by injected oligos or by the transcription inhibitor actinomycin D (O'Keefe et al., 1994). These observations are consistent with the finding that expression of the spacerdeleted Sky1p in budding yeast resulted in a lethal phenotype, together indicating that cellular partitioning of SRPKs is essential for cell physiology (Siebel et al., 1999).
To determine whether the kinase activity is critical for induced aggregation of splicing factors in the nucleus, we tested the spacer-deleted SRPK1 in which the ATP binding site was mutated. Surprisingly, the mutation efficiently blocked the accumulation of the spacer-deleted kinase in the nucleus (Figure 3, M-O). Thus, the kinase activity seems to be essential for nuclear entrance of the spacer-deleted SRPK1.
Impact of the Spacer Sequence on Kinase Activity
The requirement for an enzymatically active kinase to enter the nucleus is consistent with our previous biochemical studies that the spacer-deleted SRPKs are active in phosphorylating SR proteins in vitro (Siebel et al., 1999; Nolen et al., 2001). It has been unclear, however, whether deletion of the spacer further activates the kinase (as recombinant full-length SRPKs are already active) or modulates other biochemical aspects of the kinase because many kinases translocate to the nucleus upon activation (Cyert, 2001). We therefore set out to conduct detailed kinetic characterization of wt and the spacer-deleted SRPK1 using ASF/SF2 as a model substrate.
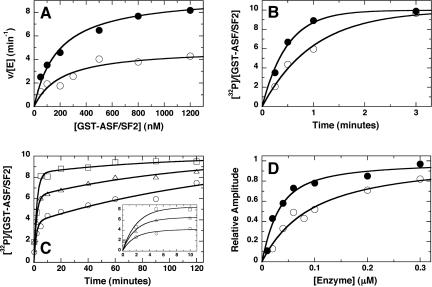
In steady-state kinetic assays (Figure 4A), the spacer increased the turnover number (k) by a factor of 2 (5 versus 10 min-1), but it had no impactcaton the K for ASF/SF2 (Km of ∼200 nM). To ensure that the observed changes in kcat were not because of inactive enzyme in our purification, single turnover experiments were performed under conditions of excess enzyme concentration. Under these constraints, the observed rate constant for substrate phosphorylation should be independent of the total enzyme concentration and thus report directly on the overall phosphorylation rate in the active site. As shown in Figure 4B, the single exponential rate constants for ASF/SF2 phosphorylation using excess SRPK1 and SRPK1ΔS (i.e., [E] ≫ Kd) differed by approximately threefold, indicating that the spacer enhanced substrate turnover by a small but real amount.
Figure 4.
Effects of the SRPK1 spacer on the phosphorylation kinetics of ASF/SF2. Steady-state kinetics (A). Initial velocities were measured as a function of varying GST-ASF/SF2 using SRPK1 (•) and K1-ΔS (○). For SRPK1, kcat and Km are 9.5 ± 0.5 min-1 and 190 ± 40 nM, respectively, and for K1-ΔS, kcat and Km are 4.9 ± 0.4 min-1 210 ± 60 nM, respectively. and Single-turnover kinetics (B). GST-ASF/SF2 (200 nM) was preequilibrated with 1 μM SRPK1 (•) and K1-ΔS (○) and allowed to react with [32P]ATP for varying periods of time. Data were fit to a single exponential function to obtain rate constants of 2.5 and 0.9 min-1 for SRPK1 and K1-ΔS. Enzyme-dependent single turnover kinetics (C). GST-ASF/SF2 (10 nM), preequilibrated with 50 nM (○), 150 nM (▵), and 360 nM (□) K1-ΔS, was mixed with [γ-32P]ATP for varying periods of time, and the number of sites phosphorylated was determined and expressed as a ratio of32P-incorporated and the total GST-ASF/SF2 concentration. The amplitudes of the first kinetic phase are 3.7, 6.2, and 8 at 50, 150, and 360 nM K1-ΔS, respectively. Determination ofKd values for SRPK1 and K1-ΔS (D). The amplitudes of the first phases in single turnover experiments were normalized to the reaction endpoints and plotted against the total concentrations of SRPK1 (•) and K1-ΔS(○). A quadratic function was used to obtain K values of 20 ± 6 and 80 ± 14 nM for SRPK1 and K1-ΔS, respectively.d
Whether the spacer altered the interaction between the kinase and ASF/SF2 was unclear from steady-state kinetic analyses because Km is an apparent dissociation constant and does not commonly measure the real affinity of the substrate and enzyme. Because of these limitations, we measured directly the Kd for ASF/SF2 based on single turnover experiments. As shown in Figure 4C, the single exponential transient for ASF/SF2 phosphorylation was clearly biphasic at lower enzyme concentrations because of the dissociation of the kinase-substrate complex near or below the Kd value. The initial phase reflects the phosphorylation of ASF/SF2 in the enzyme-substrate complex, whereas the slower phase reflects the phosphorylation of ASF/SF2 upon recombination of the free enzyme and substrate. As a result, the amplitude of the first phase monitors the concentration of enzyme-bound substrate at any given amount of total enzyme. Measuring the relative amplitude of the initial phase as a function of total enzyme concentration provided Kd values of 20 and 80 nM for wt and spacer-deleted SRPK1 (Figure 4D), respectively. Based on these data, we conclude that the spacer does not hinder ASF/SF2 phosphorylation but rather enhances the stability of the complex by approximately four-fold. These observations suggest that the translocation of the spacer-deleted SRPK1 does not seem to be the result of large changes in binding or catalytic properties imposed by the removal of the spacer sequence.
Signals in the Spacer and Potential Action Mechanism
Because the effect of spacer removal from SRPK1 on kinase activity does not explain the quantitative shift of the kinase to the nucleus, we next focused on potential signals in the spacer sequence that may dictate the cellular distribution of SRPK1. A previous study showed that a motif in the spacer of the fission yeast kinase Dsk1 could act as a NES in an LMB-sensitive manner (Fukuda et al., 1997). However, the motif does not seem to be conserved among SRPK family members. To pursue the possibility that the cytoplasmic localization of SRPK1 may be directed by a divergent NES, we treated the cell with LMB. Although nuclear export of c-Abl was effectively blocked by LMB as documented previously (Taagepera et al., 1998), we observed no effect of a similar treatment on SRPK1 localization (Figure 5A). To substantiate this observation, we determined the potential NES signal in SRPK1 by examining its ability to shuttle between the nucleus and the cytoplasm. For this purpose, a classic NLS from the SV40 large T antigen was fused to the kinase, and the nucleus-targeted kinase was tested in a heterokaryon assay. The NLS was sufficient to shift the kinase to the nucleus, indicating that the NLS acted in a dominant manner over potential NES. However, the nuclear-targeted kinase was unable to shuttle (Figure 5B, a-c). Similarly, we fused the spacer from SRPK1 to an NLS-green fluorescent protein (GFP) reporter and found that the fusion protein was also unable to shuttle (Figure 5B, d-f). These data indicate that the spacer in SRPK1 does not carry a detectable NES.
Figure 5.
(A) Detection of potential NES by using LMB. LMB treatment had not effect on SRPK1 cellular distribution (A, a and b), but it induced nuclear accumulation of GFP-c-Abl (A, d and e). Nuclei were stained with DAPI (A, c and f). (B) Heterokaryon assay. HeLa cells were transfected with Myc and NLS-tagged full-length SRPK1 (B, a), GFP-NLS-fused SRPK1 spacer (B, d), spacer-deleted active K1-ΔS (B, g), and inactive K1-ΔS-K109M (B, j). Cell fusion with coculture mouse cells (showing more punctuated DAPI staining in the nucleus in b, e, h, and k) indicates that full-length SRPK1 (a and c) and the spacer alone (d and f) were unable to shuttle, whereas both active (g and i) and inactive (j and l) spacer-deleted kinases had the capacity to shuttle between the nucleus and the cytoplasm. Cell fusion was monitored by staining actin with Alexa 594-phaloidin (c, f, i, and l).
Interestingly, in contrast to the full-length kinase, the spacer-deleted kinase was able to shuttle (Figure 5B, g-i). The shuttling capacity was detectable regardless of the presence of an extra NLS. Because nuclear accumulation of the spacer-deleted kinase requires the catalytic activity, we asked whether the ability to export was also dependent on the catalytic activity of the kinase. We fused an NLS to the spacer-deleted kinase containing the point mutation in the ATP binding site and found that this mutant kinase was able to shuttle in the heterokaryon assay (Figure 5B, j-l). Thus, although entrance of the kinase to the nucleus requires an active enzyme, nuclear export is independent of the kinase activity. Based on these observations, we speculate that the kinase may be transported in and out of the nucleus via its interaction with substrate SR proteins but use distinct mechanisms for import and export (see Discussion).
Dissection of the Localization Signal in the Spacer
The dramatic effect of spacer deletion on the localization of SRPK1 does not seem to be explained by a large change in enzymatic kinetics or the presence of a measurable NES. We therefore considered the possibility that the spacer may carry a cytoplasmic anchoring signal(s), which may enable the tight regulation of the kinase in the cell. To pursue this possibility and pinpoint potential regulatory motifs in the spacer, we conducted deletion analysis to characterize the potential cytoplasmic anchoring signal in SRPK1. Systematic deletion from the N or C terminus of the spacer indicated that none of the smaller deletions had a major effect on shifting the kinase to the nucleus (our unpublished data), which again argued against the existence of a single functional NES in the regulation of the cellular distribution of the kinase.
Inspection of the impact on kinase distribution by a large number of spacer deletion mutants suggests that the spacer may be roughly divided into three segments. Any combination of two segments (deletion of any single segment) was sufficient to retain the kinase in the cytoplasm, whereas any single segment (deletion of any two segments) had a partial effect, resulting in both cytoplasmic and nuclear localization of the kinase in transfected cells (Figure 6). These observations suggest that a simple conformational change may not be sufficient to explain the dramatic effect of spacer deletion on nuclear translocation of the kinase (because at least some single deletions tested were expected to cause a major conformational change in the spacer). Instead, the spacer may contain multiple redundant elements involved in interaction with cytoplasmic proteins, which together promote the localisation of the kinase in the cytoplasm. Interestingly, whereas the SRPK family of kinases is evolutionarily conserved from yeast to human, the spacer sequences in them are highly divergent (Siebel et al., 1999), suggesting that spacer sequences in SRPKs may have coevolved with multiple interaction partners in different species and that SRPK family members may be uniquely regulated via their spacer sequences.
Figure 6.
Dissection of signals in the SRPK1 spacer. (A) The structure of SRPK1 is diagrammed on the top with indicated domain structure and amino acids at boundary positions. The spacer can be divided into three regions (a-c) based on the presence of convenient restriction sites for subcloning. The deletion series are illustrated below the kinase structure. (B) Localization of SRPK1 mutants. HeLa cells were fixed at 8 h after transfection, and expressed SRPK1 mutants were detected with rabbit anti-myc antibodies. Nuclei were stained with DAPI. Constructs a, b, and c showed partial translocation to the nucleus, whereas ab, bc, and ac exhibited largely cytoplasmic localization as seen with wt full-length SRPK1.
Regulation of SRPK1 by Signal-induced Nuclear Translocation
Our kinetic studies showed that the spacer in SRPK1 is able to modulate the interaction of the kinase with SR protein substrates. The spacer sequence may also interact with specific cellular proteins to anchor the kinase to the cytoplasm. These observations suggest that the kinase may be subject to regulation by signaling via its spacer sequence. Little is known about how pre-mRNA splicing and RNA transport might be regulated by a specific signal, although several pilot studies have indicated that changes in localization of splicing factors and regulators could be induced by stress-related signaling. For example, inhibition of transcription by actinomycin D or osmatic stress had been shown to induce nuclear export of heterogeneous nuclear ribonucleoprotein A1 (Pinol-Roma and Dreyfuss, 1992) and the localization of the RNA binding proteins in stress granules in the cytoplasm (van der Houven van Oordt et al., 2000; Allemand et al., 2005). However, none of these treatment conditions were found to alter the localization of SRPK1 in HeLa cells (our unpublished data). It was reported earlier that Dsk1 entered to the nucleus in the M phase during the cell cycle in fission yeast, indicating that a cell cycle signal may trigger the translocation of the kinase to the nucleus to facilitate cell cycle progression at the M phase (Takeuchi and Yanagida, 1993). To determine whether SRPKs in mammalian cells were able to respond to cell cycle signaling, we examined SRPK1 localization in synchronized HeLa cells (Figure 7). On release of thymidine-blocked HeLa cells at different time points, we observed that a significant fraction of SRPK1 became accumulated in the nucleus before the beginning of the M phase (compare SRPK1 localization in cells released from the thymidine block by 7 and 7.5 h in Figure 7, A-D).
Figure 7.
Translocation of SRPK1 to the nucleus at the G2/M boundary. HeLa cells were arrested with thymidine block. Localization of the endogenous SRPK1 was carried out upon releasing the cells from the cell cycle arrest for 7 (A) and 7.5 h (C). Nuclei were stained by DAPI (B, D, G, and K). Nuclear staining of SRPK1 became predominant at the end of the G2 phase after cells were released from the thymidine block for 7.7 h (E and H). Nuclear envelope was monitored by anti-Lamin A/C (F, H, J, and L). Cells at the end of the G2 phase were analyzed under an inverted microscope (E-H) or a deconvolution microscope (I-L).
Because nuclear envelop breaks down during the cell cycle in mammalian cells (which does not happen in fission yeast), the localization experiment during the G2 to M transition requires careful monitoring of the integrity of the nuclear envelop to determine whether SRPK1 entered the nucleus before nuclear envelop breakdown in response to cell cycle signaling. We accomplished this by monitoring the structure of nuclear lamins. As shown in Figure 7, E-H, we detected nuclear translocation of SRPK1 at the late G2 phase (7.5-7.7 h after releasing from the thymidine block)when nuclear envelope was still intact. To make sure that the kinase was not merely piled up on top of the nuclear envelop, we conducted deconvolution microscopy, and the data clearly showed the presence of the kinase within the nucleus (Figure 7, I-L). These data demonstrate that SRPK1 is able to respond to cell cycle signaling to enter the nucleus during the cell cycle. It will be interesting to determine in the future how SRPK1 and other SRPK family members may respond to specific signaling in interphase cells.
DISCUSSION
Requirement for Cytoplasmic Localization of SRPKs
RNA metabolism in mammalian cells is a highly orchestrated process, which is regulated by reversible phosphorylation. In our present study, we demonstrate that the SRPK family of kinases is subject to tight regulation in mammalian cells by cellular partitioning. Bulk of the kinase population is restricted in the cytoplasm, and this localization pattern may reflect the functional requirement of SRPKs in several key pathways of RNA metabolism. First, cytoplasmic SRPKs play an essential role for nuclear import of newly synthesized SR proteins because most of them are imported by hMtr10 in a phosphorylation-dependent manner (Lai et al., 2000; Yun et al., 2000, 2003). Second, a fraction of SRPKs is also detected in the nucleus where they may join other SR protein kinases such as the Clk/Sty family members to regulate the function of SR proteins in many different steps from splicing to export. Because both hypo- and hyperphosphorylation of SR proteins are harmful to splicing, the kinase activity has to be regulated. Here, we show that the presence of a large amount of active SRPK1 in the nucleus after deleting the regulatory spacer caused aggregation of splicing factors, which likely leads to general inhibition of gene expression. One of the key aspects affected may be at the transition from splicing to export because dephosphorylation of shuttling SR proteins is required for interaction with the key export factor TAP (Huang et al., 2003, 2004; Lai and Tarn, 2004). It is thus conceivable that inhibition of SR protein dephosphorylation in the presence of an excessive amount of an SR protein kinase would be detrimental to the orderly transition from splicing to export. Finally, localization of SRPKs in the cytoplasm may also play a key role in releasing shuttling SR proteins from exported mRNA and for proper recycling of shuttling SR proteins back to the nucleus (Gilbert and Guthrie, 2004). Together, these works clearly demonstrate a strategic role for the localization of SRPKs in the cytoplasm. We now provide key evidence that such cellular partitioning is mainly achieved by the action of the unique spacer sequences within the kinases in mammalian cells.
Potential Import Mechanism for SRPKs
By immunocytochemistry, bulk of the endogenous SRPK1 is found in the cytoplasm with a fraction of the kinase in the nucleus, indicating that SRPKs are able to enter the nucleus. Nuclear import of SRPK1 is accelerated when the “anchoring signal” in the spacer is removed. Interestingly, nuclear accumulation of the spacer-deleted SRPK1 depends on its kinase activity, which suggests a potential mechanism for how the kinase might be imported. Proteins may be imported by diffusion (if the size is small enough), by receptor-mediated nuclear transport, or by a so-called piggy-back mechanism. The diffusion mechanism clearly does not apply to SRPK1 as the kinase is a large protein of 655 amino acids, migrating as an ∼90-kDa protein in SDS-PAGE (Gui et al., 1994). We considered the possibility for receptor-mediated import of SRPK1. Our current results show that the kinase core without the spacer is sufficient to enter the nucleus, but its import depends on the kinase activity. This observation indicates that the kinase core, but not the spacer, may carry an NLS. If this is the case, such a putative NLS has to be fully exposed in the active kinase and become tightly masked in the inactive kinase containing a point mutation in the ATP binding site. We considered this scenario less likely because the mutation in the ATP binding site is not expected to cause a major conformation change in the kinase. Therefore, the piggy-back mechanism becomes a viable choice and is most consistent with the observations we have made so far.
SRPK1 is known to bind tightly to SR protein substrates via a mitogen-activated protein kinase kinase insert in the kinase (Ngo et al., 2005). In addition, RS domain phosphorylation is catalyzed by SRPK1 in a processive manner, which seems to be mediated by a docking motif upstream the RS domain (Aubol et al., 2003; Ngo et al., 2005). As a result, the substrate remains docked on the kinase without being released during or even after phosphorylation. Because only phosphorylated RS domain in typical SR proteins is able to interact with hMtr10 (Lai et al., 2000; Yun et al., 2003), the interaction may result in the formation of a tripartite complex, which is competent for nuclear import. By this mechanism, the kinase may be piggy-backed to the nucleus in a manner that is dependent on the phosphorylation state of its substrates. The spacer may carry a dominant signal to prevent efficient nuclear translocation of SRPK1, thus keeping a balanced distribution of the kinase in the cell. When the spacer is removed, increased binding and/or decreased anchoring may result in efficient import of the kinase to the nucleus.
In contrast to nuclear import, nuclear export of the spacer-deleted kinase may be because of the association of the kinase with shuttling SR proteins. Shuttling SR proteins are dephosphorylated after splicing and remain associated with spliced mRNA during mRNA export (Huang et al., 2003, 2004; Lai and Tarn, 2004; Lin et al., 2005). SRPK1 may interact with a fraction of dephosphorylated shuttling SR proteins during the transition from splicing to export. Because export of shuttling SR proteins actually requires them in a hypophosphorylation state (Lin et al., 2005), the associated SRPK1 may be exported as part of the ribonucleoprotein complex to the cytoplasm.
Signals in the Spacer for Cytoplasmic Localization of SRPK1
It is clear that the spacer in the kinase plays a key regulatory role. Our kinetic analysis indicates that removal of the spacer modestly increased the stability of the kinase-substrate complex, which may contribute to nuclear import of the spacer-deleted kinase via a potential piggy-back mechanism. This model is consistent with the observation that overexpression of ASF/SF2 increased nuclear localization of the full-length kinase in transfected cells (Ngo et al., 2005). However, the ASF/SF2 induced shift was not as quantitative as we detected with the spacer-deleted kinase. Thus, although a modest enhancement of the kinase-substrate stability may have helped nuclear translocation of the kinase upon the removal of the spacer, it is unlikely to count for the quantitative shift observed. Furthermore, it is unlikely that SR protein synthesis and/or shuttling are significantly elevated at the end of the G2-phase to massively induce the nuclear translocation of the endogenous kinase.
A previous study in fission yeast indicated an active NES in the spacer of kinase Dsk1 (Fukuda et al., 1997). By a number of criteria, we could not detect any NES activity in the spacer of SRPK1. We therefore considered some sort of anchoring mechanism for the localization of SRPK1 in the cytoplasm of mammalian cells. Consistent with this possibility, we found multiple elements in the spacer that seem to act in a combinatory manner to cause the localization of the kinase in the cytoplasm. Strikingly, although the spacers of SRPK1 and SRPK2 share little homologue in their sequences, they seem to function interchangeably in the regulation of kinase cellular partitioning. Interestingly, we also found that the full-length SRPK1 did not efficiently export out of the nucleus in comparison with the spacer-deleted SRPK1. One explanation might be that the spacer may also exert some anchoring effect in the nucleus by engaging in interactions with some nonshuttling nucleus proteins (Nikolakaki et al., 2001). Future studies are required to identify specific spacer binding proteins in order to formally test these potential mechanisms.
Function and Regulation of SRPK1 beyond Splicing?
The finding that SRPKs can translocate to the nucleus in response to a cell cycle signal indicates that the kinase system has the potential to be regulated by signaling in interphase cells. How SRPK1 may respond to signals remains unknown and represents an interesting subject for future studies. Although SRPKs seem to be constitutive kinases, a recent study indicates that SRPK1 may be further activated by CK2 (Mylonis and Giannakouros, 2003). However, it remains to be determined whether CK2 has any effect on nuclear translocation of the kinase.
The functional aspects of nuclear translocation of SRPK1 in the end of the G2 phase are also interesting, which may or may not relate to phosphorylation regulation of pre-mRNA processing. In keeping with this possibility, SRPK1 has been shown to phosphorylate substrates that are not related to pre-mRNA splicing. For example, SRPK1 has been found to phosphorylate a RS repeat region in the lamin B receptor, and SRPK1-mediated phosphorylation seems to play a role in the attachment of the lamin B receptor to chromatin (Takano et al., 2004). This function may require the translocation SRPK1 to the nucleus before initiation of the M phase.
Acknowledgments
We are grateful to S. Stevenin for anti-SC35 antibodies and to X.-D. Xu for critical comments on the manuscript. This work was supported by National Institutes of Health Grant GM-52872 (to X.-D. Fu), National Institutes of Heath Grant GM-67969 and National Science Foundation Grant MCB-111068 (to J.A.A.), and National Institutes of Health Training Grant GM-07752 (to J. H.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-10-0963) on November 30, 2005.
References
- Allemand, E., Guil, S., Myers, M., Moscat, J., Caceres, J. F., and Krainer, A. R. (2005). Regulation of heterogenous nuclear ribonucleoprotein A1 transport by phosphorylation in cells stressed by osmotic shock. Proc. Natl. Acad. Sci. USA 102, 3605-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubol, B. E., Chakrabarti, S., Ngo, J., Shaffer, J., Nolen, B., Fu, X. D., Ghosh, G., and Adams, J. A. (2003). Processive phosphorylation of alternative splicing factor/splicing factor 2. Proc. Natl. Acad. Sci. USA 100, 12601-12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David, Y., Letwin, K., Tannock, L., Bernstein, A., and Pawson, T. (1991). A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 10, 317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, W., Jamison, S. F., and Garcia-Blanco, M. A. (1997). Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro. RNA 3, 1456-1467. [PMC free article] [PubMed] [Google Scholar]
- Colwill, K., Pawson, T., Andrews, B., Prasad, J., Manley, J. L., Bell, J. C., and Duncan, P. I. (1996). The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 15, 265-275. [PMC free article] [PubMed] [Google Scholar]
- Cyert, M. S. (2001). Regulation of nuclear localization during signaling. J. Biol. Chem. 276, 20805-20808. [DOI] [PubMed] [Google Scholar]
- Du, C., McGuffin, M. E., Dauwalder, B., Rabinow, L., and Mattox, W. (1998). Protein phosphorylation plays an essential role in the regulation of alternative splicing and sex determination in Drosophila. Mol. Cell 2, 741-750. [DOI] [PubMed] [Google Scholar]
- Fu, X. D. (1993). Specific commitment of different pre-mRNAs to splicing by single SR proteins. Nature 365, 82-85. [DOI] [PubMed] [Google Scholar]
- Fu, X. D. (1995). The superfamily of arginine/serine-rich splicing factors. RNA 1, 663-680. [PMC free article] [PubMed] [Google Scholar]
- Fu, X. D., and Maniatis, T. (1990). Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343, 437-441. [DOI] [PubMed] [Google Scholar]
- Fukuda, M., Asano, S., Nakamura, T., Adachi, M., Yoshida, M., Yanagida, M., and Nishida, E. (1997). CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308-311. [DOI] [PubMed] [Google Scholar]
- Gilbert, W., and Guthrie, C. (2004). The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell 13, 201-212. [DOI] [PubMed] [Google Scholar]
- Gilbert, W., Siebel, C. W., and Guthrie, C. (2001). Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA 7, 302-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen, M., and Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley, B. R. (2000). Sorting out the complexity of SR protein functions. RNA 6, 1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, J. F., Lane, W. S., and Fu, X. D. (1994). A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature 369, 678-682. [DOI] [PubMed] [Google Scholar]
- Himmelspach, M., Cavaloc, Y., Chebli, K., Stevenin, J., and Gattoni, R. (1995). Titration of serine/arginine (SR) splicing factors during adenoviral infection modulates E1A pre-mRNA alternative splicing. RNA 1, 794-806. [PMC free article] [PubMed] [Google Scholar]
- Hirose, Y., and Manley, J. L. (2000). RNA polymerase II and the integration of nuclear events. Genes Dev. 14, 1415-1429. [PubMed] [Google Scholar]
- Howell, B. W., Afar, D. E., Lew, J., Douville, E. M., Icely, P. L., Gray, D. A., and Bell, J. C. (1991). STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol. Cell. Biol. 11, 568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Gattoni, R., Stevenin, J., and Steitz, J. A. (2003). SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell 11, 837-843. [DOI] [PubMed] [Google Scholar]
- Huang, Y., and Steitz, J. A. (2001). Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell 7, 899-905. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Yario, T. A., and Steitz, J. A. (2004). A molecular link between SR protein dephosphorylation and mRNA export. Proc. Natl. Acad. Sci. USA 101, 9666-9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T. (2000). Signaling-2000 and beyond. Cell 100, 113-127. [DOI] [PubMed] [Google Scholar]
- Kohtz, J. D., Jamison, S. F., Will, C. L., Zuo, P., Luhrmann, R., Garcia-Blanco, M. A., and Manley, J. L. (1994). Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368, 119-124. [DOI] [PubMed] [Google Scholar]
- Koizumi, J., Okamoto, Y., Onogi, H., Mayeda, A., Krainer, A. R., and Hagiwara, M. (1999). The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs). J. Biol. Chem. 274, 11125-11131. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi, N., Onogi, H., Wakabayashi, T., and Hagiwara, M. (1998). Novel SR-protein-specific kinase, SRPK2, disassembles nuclear speckles. Biochem. Biophys. Res. Commun. 242, 357-364. [DOI] [PubMed] [Google Scholar]
- Lai, M. C., Lin, R. I., Huang, S. Y., Tsai, C. W., and Tarn, W. Y. (2000). A human importin-beta family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J. Biol. Chem. 275, 7950-7957. [DOI] [PubMed] [Google Scholar]
- Lai, M. C., Lin, R. I., and Tarn, W. Y. (2001). Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc. Natl. Acad. Sci. USA 98, 10154-10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. C., and Tarn, W. Y. (2004). Hypophosphorylated ASF/SF2 binds TAP and is present in messenger ribonucleoproteins. J. Biol. Chem. 279, 31745-31749. [DOI] [PubMed] [Google Scholar]
- Lin, S., Xiao, R., Sun, P., Xu, X., and Fu, X.-D. (2005). Dephosphorylation dependent sorting of SR splicing factors during mRNP maturation. Mole Cell 20, 413-425. [DOI] [PubMed] [Google Scholar]
- Manley, J. L., and Tacke, R. (1996). SR proteins and splicing control. Genes Dev. 10, 1569-1579. [DOI] [PubMed] [Google Scholar]
- Mermoud, J. E., Cohen, P., and Lamond, A. I. (1992). Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res. 20, 5263-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermoud, J. E., Cohen, P. T., and Lamond, A. I. (1994). Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 13, 5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli, T., Caceres, J. F., Clement, J. Q., Krainer, A. R., Wilkinson, M. F., and Spector, D. L. (1998). Serine phosphorylation of SR proteins is required for their recruitment to sites of transcription in vivo. J. Cell Biol. 143, 297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli, T., Caceres, J. F., and Spector, D. L. (1997). The dynamics of a pre-mRNA splicing factor in living cells. Nature 387, 523-527. [DOI] [PubMed] [Google Scholar]
- Misteli, T., and Spector, D. L. (1999). RNA polymerase II targets pre-mRNA splicing factors to transcription sites in vivo. Mol. Cell 3, 697-705. [DOI] [PubMed] [Google Scholar]
- Mylonis, I., and Giannakouros, T. (2003). Protein kinase CK2 phosphorylates and activates the SR protein-specific kinase 1. Biochem. Biophys. Res. Commun. 301, 650-656. [DOI] [PubMed] [Google Scholar]
- Ngo, J., Chakrabarti, S., Ding, J. H., Velazquez-Dones1, A., Nolen, B., Adams, J. A., Fu, X.-D., and Ghosh1, G. (2005). Interplay between SRPK and Clk/Sty kinases in phosphorylation of the splicing factor ASF/SF2 is regulated by a docking motif in ASF/SF2. Mol. Cell 20, 77-89. [DOI] [PubMed] [Google Scholar]
- Nikolakaki, E., Kohen, R., Hartmann, A. M., Stamm, S., Georgatsou, E., and Giannakouros, T. (2001). Cloning and characterization of an alternatively spliced form of SR protein kinase 1 that interacts specifically with scaffold attachment factor-B. J. Biol. Chem. 276, 40175-40182. [DOI] [PubMed] [Google Scholar]
- Nolen, B., Yun, C. Y., Wong, C. F., McCammon, J. A., Fu, X. D., and Ghosh, G. (2001). The structure of Sky1p reveals a novel mechanism for constitutive activity. Nat. Struct. Biol. 8, 176-183. [DOI] [PubMed] [Google Scholar]
- O'Keefe, R. T., Mayeda, A., Sadowski, C. L., Krainer, A. R., and Spector, D. L. (1994). Disruption of pre-mRNA splicing in vivo results in reorganization of splicing factors. J. Cell Biol. 124, 249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinol-Roma, S., and Dreyfuss, G. (1992). Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355, 730-732. [DOI] [PubMed] [Google Scholar]
- Prasad, J., Colwill, K., Pawson, T., and Manley, J. L. (1999). The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing. Mol. Cell. Biol. 19, 6991-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H., and Green, M. R. (2004). A pathway of sequential arginine-serinerich domain-splicing signal interactions during mammalian spliceosome assembly. Mol. Cell 16, 363-373. [DOI] [PubMed] [Google Scholar]
- Shen, H., Kan, J. L., and Green, M. R. (2004). Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol. Cell 13, 367-376. [DOI] [PubMed] [Google Scholar]
- Siebel, C. W., Feng, L., Guthrie, C., and Fu, X. D. (1999). Conservation in budding yeast of a kinase specific for SR splicing factors. Proc. Natl. Acad. Sci. USA 96, 5440-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taagepera, S., McDonald, D., Loeb, J. E., Whitaker, L. L., McElroy, A. K., Wang, J. Y., and Hope, T. J. (1998). Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl. Acad. Sci. USA 95, 7457-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, M., Koyama, Y., Ito, H., Hoshino, S., Onogi, H., Hagiwara, M., Furukawa, K., and Horigome, T. (2004). Regulation of binding of lamin B receptor to chromatin by SR protein kinase and cdc2 kinase in Xenopus egg extracts. J. Biol. Chem. 279, 13265-13271. [DOI] [PubMed] [Google Scholar]
- Takeuchi, M., and Yanagida, M. (1993). A mitotic role for a novel fission yeast protein kinase dsk1 with cell cycle stage dependent phosphorylation and localization. Mol. Biol. Cell 4, 247-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z., Kuo, T., Shen, J., and Lin, R. J. (2000). Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1. Mol. Cell. Biol. 20, 816-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z., Yanagida, M., and Lin, R. J. (1998). Fission yeast mitotic regulator Dsk1 is an SR protein-specific kinase. J. Biol. Chem. 273, 5963-5969. [DOI] [PubMed] [Google Scholar]
- van der Houven van Oordt, W., Diaz-Meco, M. T., Lozano, J., Krainer, A. R., Moscat, J., and Caceres, J. F. (2000). The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149, 307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. Y., Lin, W., Dyck, J. A., Yeakley, J. M., Songyang, Z., Cantley, L. C., and Fu, X. D. (1998). SRPK 2, a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J. Cell Biol. 140, 737-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. Y., and Maniatis, T. (1993). Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75, 1061-1070. [DOI] [PubMed] [Google Scholar]
- Xiao, S. H., and Manley, J. L. (1998). Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J. 17, 6359-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeakley, J. M., Tronchere, H., Olesen, J., Dyck, J. A., Wang, H. Y., and Fu, X. D. (1999). Phosphorylation regulates in vivo interaction and molecular targeting of serine/arginine-rich pre-mRNA splicing factors. J. Cell Biol. 145, 447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, C. Y., and Fu, X. D. (2000). Conserved SR protein kinase functions in nuclear import and its action is counteracted by arginine methylation in Saccharomyces cerevisiae. J. Cell Biol. 150, 707-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, C. Y., Velazquez-Dones, A. L., Lyman, S. K., and Fu, X. D. (2003). Phosphorylation-dependent and -independent nuclear import of RS domain-containing splicing factors and regulators. J. Biol. Chem. 278, 18050-18055. [DOI] [PubMed] [Google Scholar]