Abstract
Objective:
To investigate the long-term effect of cryopreservation on hepatocyte function, as well as attempt to improve cell viability and function through the utilization of the hypothermic preservation solution, HypoThermosol (HTS), as the carrier solution.
Summary Background Data:
Advances in the field of bioartificial liver support have led to an increasing demand for successful, efficient means of cryopreservation of hepatocytes.
Methods:
Fresh rat hepatocytes were cryopreserved in suspension in culture media (Media-cryo group) or HTS (HTS-cryo group), both supplemented with 10% DMSO. Following storage up to 2 months in liquid nitrogen, cells were thawed and maintained in a double collagen gel culture for 14 days. Hepatocyte yield and viability were assessed up to 14 days postthaw. Serial measurements of albumin secretion, urea synthesis, deethylation of ethoxyresorufin (CYT P450 activity), and responsiveness to stimulation with interleukin-6 (IL-6) were performed.
Results:
Immediate postthaw viability was 60% in Media-cryo and 79% in HTS-cryo, in comparison with control (90%). Albumin secretion, urea synthesis and CYT P450 activity yielded 33%, 55%, and 59% in Media-cryo and 71%, 80%, and 88% in HTS-cryo, respectively, compared with control (100%). Assessment of cellular response to IL-6 following cryopreservation revealed a similar pattern of up-regulation in fibrinogen production and suppression of albumin secretion compared with nonfrozen controls.
Conclusions:
This study demonstrates that isolated rat hepatocytes cryopreserved using HTS showed high viability, long-term hepatospecific function, and response to cytokine challenge. These results may represent an important step forward to the utilization of cryopreserved isolated hepatocytes in bioartificial liver devices.
This study describes a new method for the cryopreservation of isolated hepatocytes in suspension. For the first time, high long-term cell survival is reported with an intact hepatospecific function.
Major steps have been taken recently in the development of treatments for hepatocellular disease including bioartificial liver (BAL) devices and hepatocyte transplantation. Reports on the utilization of BAL devices have detailed improvements in biochemical and neurologic parameters, as well as overall survival in several animal models.1,2 In addition to these reports, several systems have been evaluated for biocompatibility and safety in phase I clinical trials, and presently at least 1 BAL is involved in a large phase II/III clinical trial.3–5 In inherited metabolic diseases of the liver, hepatocyte transplantation offers another therapeutic option in attempt to restore liver function.6 Furthermore, transplantation of isolated hepatocytes may provide a vehicle for hepatocyte-directed ex vivo gene therapy.7–9 For both BAL and hepatocytes transplantation to fully reach their clinical potential, however, isolated hepatocytes need to be preserved for significant periods of time (months to years) so that they can be appropriately banked and distributed for on-demand utilization. In addition, cellular preservation would allow for extensive testing and validation of cell sources to assess the safety and efficacy of the bioproduct. Moreover, freshly isolated and cultured hepatocytes constitute suitable model systems for use in molecular biology, genetic studies, pharmacology, toxicology, cancer, and parasitology. Hepatocyte utilization in these areas would be enhanced if there were reliable, reproducible, and effective techniques for long-term storage of hepatocytes. Cryopreservation represents one tenable option for long-term hepatocyte preservation.
Various strategies have been described over the last 25 years for cryopreserving hepatocytes. Although progress has been reported for cultured hepatocytes,10,11 attempts to cryopreserved isolated primary hepatocytes have resulted in limited success. Reports detailing cell viability are demonstrated high viability, ranging from 30% to 90% immediately postthaw12–17 and reveal a continuous decline in cell number within a few hours.18 Additionally, many investigators have reported that despite high viability, few hepatocytes were able to attach to culture surfaces and survive for extended periods of time.13,19 Reports detailing assessment of cellular function (protein secretion, urea synthesis, CP450 activity) have also focused on short-term (hours, 1–2 days) analysis. A recent report using University of Wisconsin solution as a carrier solution or cryopreservation showed high postthaw viability (85%), but the viability dropped to 62.5% after 7 days.20 The use of postthaw processing, such as centrifugation in Percoll gradients, has resulted in selected assessment of viable cell function and, when unquantified, fails to provide an accurate evaluation of the efficacy of a given cryopreservation protocol. Overall, studies that carefully detail the survival and function of entire populations of cryopreserved isolated hepatocytes indicate low cell recovery and greatly impaired metabolic activity.
Recent reports that showed significant beneficial effects of an intracellular-like solution, HypoThermosol (HTS), during hypothermic storage at 4°C prompted us to evaluate this solution for the cryopreservation of primary hepatocytes.21,22 Based upon previous findings, we hypothesized the utilization of HTS would improve overall hepatocyte viability through a modulation of the cellular biochemical response to the cryopreservation process, as well as maintaining improved cellular function in comparison to nonpreserved cells. In the present study, we demonstrate that cryopreservation of isolated hepatocytes using HTS results in a yield and long-term survival that are significantly higher than reported in earlier studies. Further, concordant functional results bring the use of isolated cryopreserved hepatocytes in BAL devices within reach.
MATERIALS AND METHODS
Materials
Cell culture medium consisted of Dulbecco modified Eagle medium with 4.5 g/L glucose (DMEM; Life Technologies, Gaithersburg, MD) supplemented with 7 ng/mL glucagon (Lilly, Indianapolis, IN), 7.5 μg/mL hydrocortisone (Upjohn, Kalamazoo, MI), 0.5 U/mL insulin (Squibb, Princeton, NJ), 20 ng/mL epidermal growth factor (Becton Dickinson, Bedford, MA), 200 U/mL penicillin (J.R. Scientific, Woodland, CA), 200 μg/mL streptomycin (J.R. Scientific), and 10% fetal bovine serum (J.R. Scientific). Human recombinant IL-6 (ED50 = 200–800 pg/mL) was obtained from R&D Systems (Minneapolis, MN). Syto13 Green, ethidium bromide, ethoxyresorufin, and resorufin reference standard were from Molecular Probes (Eugene, OR). Chromatographically purified rat fibrinogen was obtained from Sigma Chemical (St. Louis, MO). Chromatographically purified rat albumin, goat antiserum to rat fibrinogen, peroxidase-conjugated rabbit antibody to goat IgG, and peroxidase-conjugated sheep IgG to rat albumin were obtained from Cappel (Aurora, OH).
Hepatocyte Isolation
All animal procedure were performed in accordance with National Research Council guidelines and approved by the subcommittee on Research Animal Care at the Massachusetts General Hospital. Hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Boston, MA) weighing 150 to 200 g, according to the 2-step in situ collagenase digestion method as modified by Dunn et al.23 Routinely, 200 to 300 million cells were isolated from an 8-g liver, with viability ranging from 85% to 94%, as determined by Trypan Blue exclusion.
Cryopreservation, Thawing and Culture
Following isolation, hepatocyte were incubated in preconditioned media (37°C, 10% CO2) for 20 minutes at a cell concentration of ∼1 million cells/mL at 37°C under an atmosphere of 90% air and 10% CO2. At the end of this incubation, cells were centrifuged at 50 × g and resuspended in culture media (control), ice-cold HTS (BioLife Solutions, Binghamton, NY) with 10% DMSO (HTS-cryo; trade name: CryoStor CS10) or ice-cold culture media with 10% DMSO (Media-cryo), at a cell concentration of 2 × 106 cells/mL. Cell suspensions (1.0 mL) were transferred to cryogenic vials (Nalge Company, Rochester, NY) and incubated on ice for 10 minutes, then placed into a controlled rate cooler (Kryo 10; Planer, Middlesex, UK) and frozen under the following protocol. From 4°C, samples were cooled at −1°C/min to −6°C and held for 10 minutes to allow for sample equilibration. Extracellular ice formation was then initiated by application of cold forceps to the exterior of the cryovial. After another 10-minute hold, samples were cooled at −1°C/min to −80°C, then transferred to liquid nitrogen for storage (24 hours to 2 months). Following storage, cells were rapidly thawed in a 37°C water bath until all visible ice disappeared while being gently agitated to achieve uniform thawing resulting in a calculated warming rate of ∼155°C/min. Samples were then diluted 1:10 in preconditioned culture media and incubated for 10 minutes. The suspension was centrifuged for 5 minutes at 50 × g at room temperature. The supernatant was decanted and the cell pellet was resuspended in warm culture media. Cells were then seeded in Petri dishes and placed into culture (37°C, 10% CO2). Fresh hepatocytes seeded right after isolation were used as nonfrozen controls.
Hepatocyte Culture
For long-term survival and function experiments, hepatocytes were cultured in a double collagen-gel configuration. One milliliter of a mixture of 1 part of concentrated (10 ×) DMEM and 9 parts of collagen (1.12 mg/mL) was distributed evenly over a 60 mm tissue culture dish (Falcon, Lincoln Park, NJ). Type I collagen was prepared from rat-tail tendon by a modified procedure of Elsdale and Bard.24 The collagen solution was allowed to gel at 37°C for 60 minutes before cell seeding. Two milliliters of cell suspension (1 million hepatocytes) in culture media were seeded on the dishes and incubated in a humidified atmosphere of 90% air/10% CO2 at 37°C for 24 hours. The medium was then aspirated and a second layer of collagen-DMEM solution (1 mL) was added. After 60 minutes incubation at 37°C to allow for gel solidification, 2 mL of fresh culture medium was added and replaced daily thereafter. Culture aspirates were collected daily and stored at −80°C for further analysis. Cultures were maintained for up to 14 days, after which the cells were harvested and stored at 4°C for analysis.
Yield and Viability
Hepatocyte yield and viability was determined immediately postthaw by Trypan Blue exclusion. For this analysis, 50 μL of cell suspension was added to 50 μL of Trypan Blue. The numbers of dead (blue) and live (white) cells were scored under light microscopy in a Bürker chamber.
Cell Attachment
Cells were seeded at low density (0.25 million cells) in a collagen-coated P35 Petri dish and cultured at 37°C. Cell attachment was evaluated 20–24 hours after seeding on both nonfrozen control and cryopreserved hepatocytes. The culture medium was aspirated off to remove floating dead cells and replaced by 0.5 mL of Syto13 and ethidium bromide in culture media (1:100 and 1:200, respectively). Fluorescent microscopy pictures were taken at 200 × magnification through a matrix to standardize the measurement. Cells which were positively fluoresced green (Syto 13), were negative for ethidium bromide counterstaining, and were spread out on the collagen-coated surface were counted as live cells and comprised the attached, viable population. The calculated attachment rate of cryopreserved hepatocytes represents the percentage attached cryopreserved cells relative to nonfrozen control (set at 100%), on a batch-to-batch basis. The absolute attachment rate of each sample was not determined by this method.
Lactate Dehydrogenase (LDH) Assay
At the termination of experiments, hepatocytes were liberated from the collagen gel by incubation with 0.2% collagenase in Krebs buffer at 37°C for 30 minutes. The cells were then pelleted, washed in phosphate-buffered saline, and resuspended in 2 mL of 0.1% Triton X-100 in culture media. Total intracellular LDH-content was measured with a commercially available assay kit (Roche, Mannheim, Germany) and quantified by comparison with LDH standards (Lin-Trol; Sigma Diagnostics, St. Louis, MO). The absorbance was measured in a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA).
Protein and Urea Assays
Collected medium samples were analyzed for rat albumin and fibrinogen concentration by enzyme-linked immunosorbent assay, in triplicate, as previously described.23 Urea concentration was determined via its specific reaction with diacetyl monoxime with a commercially available assay kit (Sigma Chemical, catalog no. 535) scaled for use in a 96-well plate. The absorbance was measured in a Thermomax microplate reader (Molecular Devices).
Ethoxyresorufin Deethylation Assay
This assay was based on the time-dependent formation of resorufin from ethoxy resorufin due to activity of the specific isoenzyme P450IA1 as described by Behnia et al.25 The formation of resorufin was monitored by a fluorometer at various time intervals (5, 15, 25, and 35 minutes.) in the samples (100 μL) collected from the Petri dishes. The rate of formation of resorufin was calculated from the slope of the early linear portion of the curve (fluorescence intensity versus time) for each dish and further converted to concentration (nmol/min) using a resorufin curve standard curve.
Cytokine Exposure
Starting on either the second or the seventh day of culture, cells were fed daily with culture medium supplemented with 2.5 ng/mL IL-6 for a total of 4 days. The cultures were returned to cytokine-free medium hereafter.
Morphology
Phase-contrast microscopy pictures were taken of hepatocytes in the double gel matrix after 14 days in culture, at a magnification of 200 × with a Nikon Diaphot inverted microscope and cellular morphology was assessed qualitatively in comparison to controls.
Statistical Analysis of Data
Quantitative results were expressed as mean ± SD of at least 2 replicate dishes for each condition with a minimum of 3 experimental repeats, with each experimental repeat using cells from a different hepatocytes isolation procedure. Data were normalized to noncryopreserved experimental controls at each time interval in the same experiment. Statistical significance was calculated using a 2-tailed Student t test for paired data and ANOVA as applicable. The threshold for statistical significance was considered P < 0.05.
RESULTS
Survival
Yield, Postthaw Viability and Attachment
Total cell numbers recovered after cryopreservation and washing steps were compared between the 2 cryopreservation groups and control. A small but significant loss of cells was seen in the media-cryo group (but not in the HTS-cryo group) when compared with control (P = 0.03). Immediate postthaw viability, as determined by Trypan Blue exclusion, revealed considerable differences between groups, with an absolute drop in viability of 30% in Media-cryo (60% ±7) and 11% in HTS-cryo (79% ±4) in comparison with nonfrozen controls (90% ±12) (Table 1). Assessment of cellular attachment revealed, as with viability, a significant difference between the media-cryo and HTS-cryo samples (55% versus 83% of control, respectively; P < 0.005) (Fig. 1).
TABLE 1. Postthaw Assessment of Hepatocytes Yield, Viability, and Attachment Following Cryopreservation
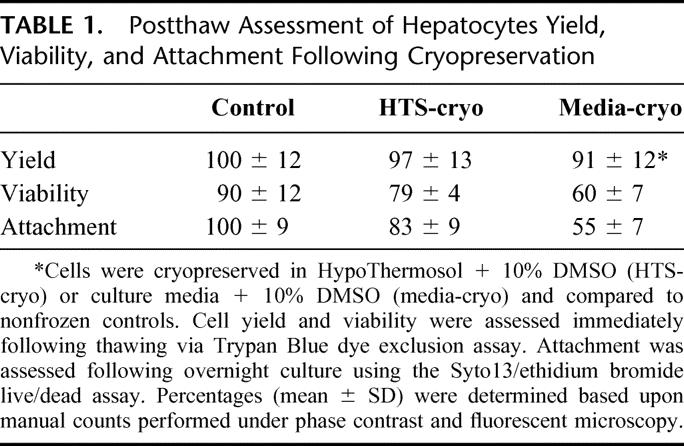
FIGURE 1. Fluorescent micrographs of hepatocytes after overnight culture following cryopreservation. Cells were cryopreserved in (B) HTS + 10% DMSO (HTS-cryo) or (C) culture media + 10% DMSO (Media-cryo), cultured overnight on collagen coated tissue culture ware and compared with (A) nonfrozen controls. Samples were stained with Syto13/ethidium bromide to distinguish living populations (green cells) and dead populations (red cells).
Long-term Survival
Hepatocyte survival was evaluated on days 1, 3, 7, and 14 by measurement of intracellular LDH content, which reflects relative cell number in comparison to controls at each respective time point. This assay was validated in our hands by comparing day 1 LDH content data with next day attachment rates for each of the 3 conditions and revealed comparable results between the 2 methods (Fig. 2). Evaluation of long-term survival revealed a small decline in cell number over the 14-day period in both the media-cryo and HTS-cryo populations in comparison to controls (Fig. 2). Despite the observed decline statistical analysis revealed no significant difference with in the respective populations between days 1 and 14 (P ≥ 0.05).
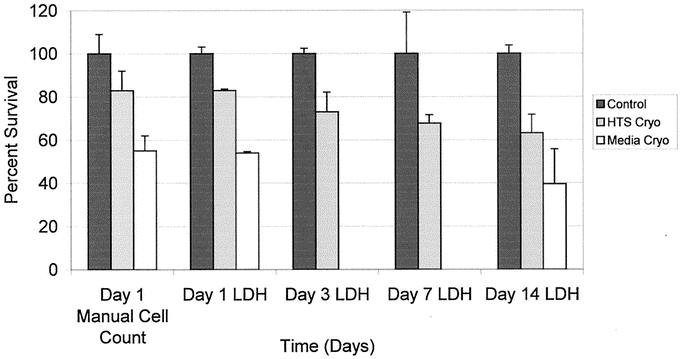
FIGURE 2. Hepatocyte viability over 14 days’ culture following cryopreservation. Long-term cell viability was assessed by determination of total intracellular LDH levels in experimental samples in comparison to controls.
Morphology
Morphologic assessment by phase-contrast microscopy at day 14 revealed a typical monolayer configuration of polygonal cells in both control and HTS-cryo samples (Fig. 3). In contrast, media-cryo samples demonstrated culture morphology characterized by a high density of disintegrating hepatocytes with irregular cell shape.
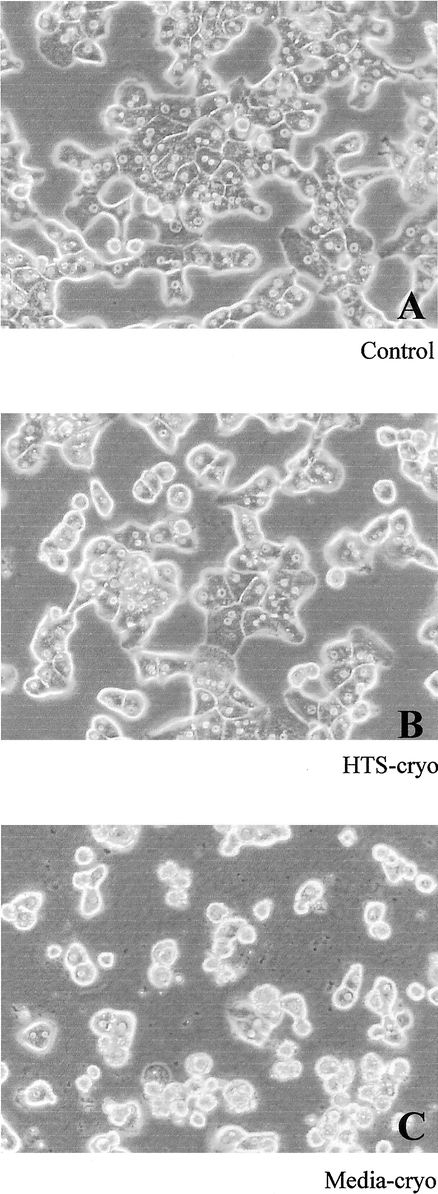
FIGURE 3. Phase contrast micrographs of hepatocytes following 14 days of culture. Cells were cultured in a collagen gel 3-dimensional configuration for 14 days. Cells cryopreserved in (B) HTS + 10% DMSO exhibited morphology similar to (A) controls (intact, hexagonal cells), whereas (C) media + 10% DMSO–cryopreserved cells were morphologically different, characterized by cellular disintegration and irregular cell shape.
Storage
To determine the influence of duration of storage in liquid nitrogen, HTS-cryo samples from the same experiments were analyzed after 1-day and 2-month storage in liquid nitrogen. One-day and 2-month cryopreserved samples showed no differences in yield (1.1 ± 0.13 and 1.15 ± 0.15 million cells per sample, respectively) and postthaw viability (80 ± 2.9 and 78 ± 1.8%, respectively) (n = 12). Albumin excretion was compared in n = 4 only, but showed identical results (data not shown).
Cellular Function
Albumin Excretion and Urea Synthesis
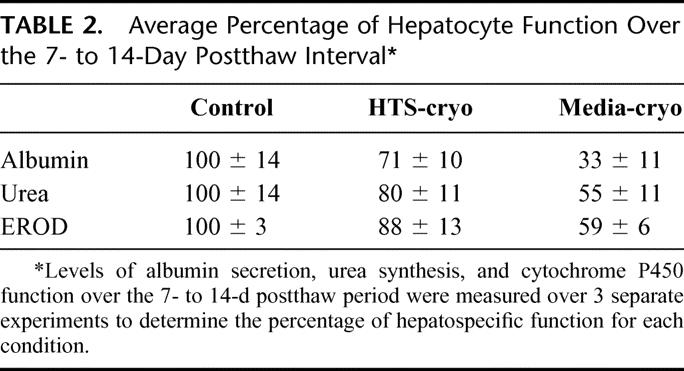
Samples of culture media were collected on a daily basis over a 14-day period and analyzed for albumin and urea content. Following assessment, albumin and urea levels were normalized to viable cell number of the hepatocyte population before cryopreservation. Both albumin excretion and urea synthesis typically stabilized following the first week of culture, after which a plateau phase was observed in all 3 groups (Fig. 4). Comparison between groups was performed by averaging albumin and urea levels in the plateau phase (days 8 to 14) (Table 2). Evaluation of albumin revealed a reduction in secretion in cryopreserved samples in comparison to controls (P < 0.01), yet when comparing the samples, the HTS-cryo hepatocytes yielded improved function over that of the media-cryo population (71% versus 33%, respectively, P < 0.005). Urea synthesis in both the cryopreserved samples was reduced from that of control levels as well (80% and 55% versus 100%, P < 0.01). As with albumin secretion, urea synthesis in the HTS-cryo samples was significantly greater than that of the media frozen samples (80% versus 55%, P = 0.003).
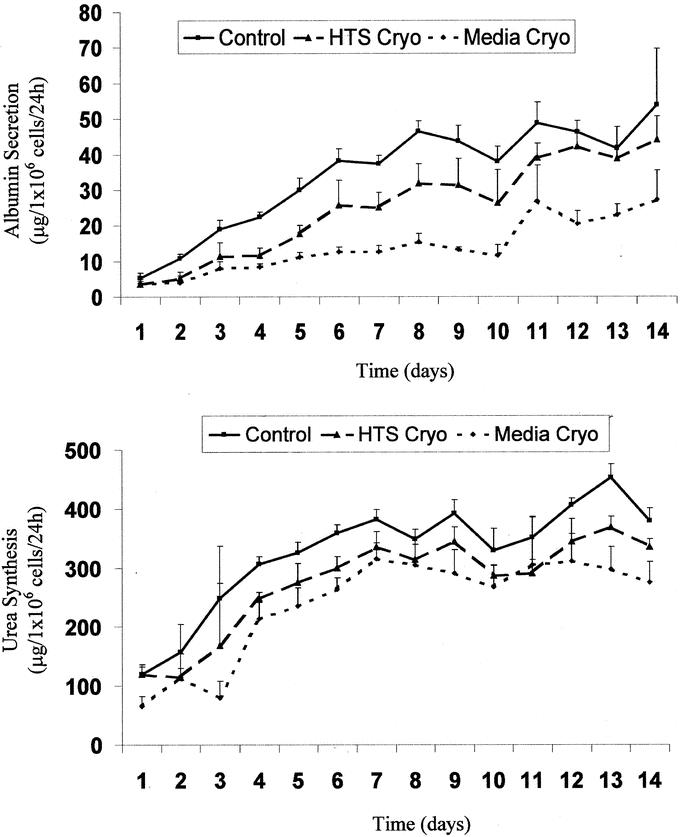
FIGURE 4. Levels of albumin secretion and urea synthesis of hepatocytes following cryopreservation. Figures represent typical graphical data obtained for (A) albumin secretion and (B) urea synthesis from experimental analysis. Analysis was repeated for 3 separate experiments and is reported in the text and Table 2.
TABLE 2. Average Percentage of Hepatocyte Function Over the 7- to 14-Day Postthaw Interval
Cytochrome P450 Activity
Cytochrome P450IA1 isoenzyme activity was assessed with the EROD assay and showed an initial delayed recovery in detoxification function of cryopreserved cells when compared with control (Day 3: HTS-cryo = 55%; Media-cryo = 46%). Following extended culture (14 days) cytochrome P450 function improved in both the HTS-cryo and media-cryo populations to 88% and 59% of controls, respectively.
IL-6 Response
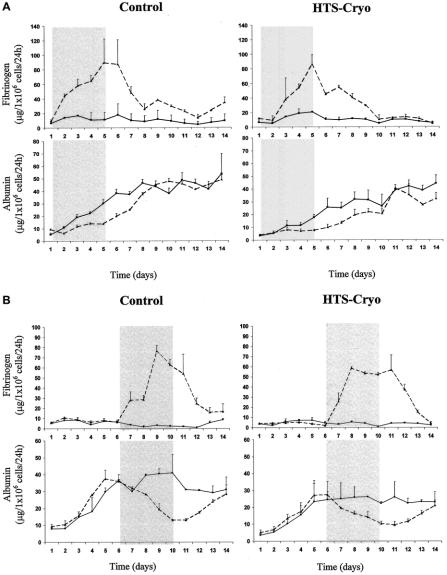
Responsiveness of cryopreserved hepatocytes to cytokine stimulation was evaluated in comparison with controls by measuring rates of fibrinogen and albumin production before, during, and after exposure to IL-6 and compared with identical nonstimulated cultures. Hepatocyte responsiveness to IL-6 was evaluated both after several days postthaw as well as immediately following thawing (Fig. 5). In all cases, groups showed a similar response during and following stimulation. IL-6 exposure resulted in a strong up-regulation of fibrinogen production, with a concurrent decrease in albumin secretion, which was maintained during the exposure period. After removal of IL-6, secretion rates of both albumin and fibrinogen in the HTS-cryo and control groups returned to typical base line levels (Fig. 5).
FIGURE 5. a, b, Hepatocyte responsiveness to cytokine stimulation following cryopreservation. Cells were exposed to IL-6 (2.5 ng/mL) at either 2 to 5 days (a) or 7 to 10 days (b) postthaw, and fibrinogen and albumin production was monitored as a determinant of hepatocyte responsiveness. Straight lines represent cultures not exposed to IL-6, dotted lines represent cultures exposed to 2.5 ng/mL human IL-6 at days 2 to 5 or 7 to 10 (shaded box). Data revealed that cells cryopreserved in HTS + 10% DMSO responded in a similar manner to controls in both an increase in fibrinogen production and a decrease in albumin production in response to IL-6 stimulation.
DISCUSSION
In this study, we evaluated the cryopreservation of isolated primary rat hepatocytes in suspension using the hypothermic preservation solution, HTS as the carrier solution. Critical to the interpretation of the data in this study is that survival and function of the cryopreserved samples were normalized to matched controls and reported as a percentage of control values. Additionally, unlike most prior studies, which focused on immediate or short-term (few days) function, we extended the frame of evaluation to 14 days’ postthaw assessment of survival and function. Our results demonstrate that hepatocytes cryopreserved in HTS yielded enhanced long-term function and survival in comparison to traditional media-based cryopreservation solution.
For assessment of long-term function, cryopreserved hepatocytes were kept for 14 days in a collagen gel sandwich configuration as described by Dunn et al.26 Isolated adult rat hepatocytes cultured in this sandwich system have been shown to maintain normal morphology and retention of differentiated hepatospecific function for up to 6 weeks. Rates of albumin and urea secretion of cryopreserved hepatocytes, like nonfrozen hepatocytes, reached a plateau of stable function after 8 days of culture. The daily increase of function in the first week is in accordance with earlier work on hepatocytes in a sandwich configuration.23,26 Overall functional levels of samples cryopreserved in HTS with 10% DMSO were comparable to control (12%-29% reduction), whereas media with 10% DMSO cryopreserved samples showed considerable losses (ie, 41–67%) compared with control.
To discern whether this decreased function is either due to cell death and thus less cells or less function per cell, yield and viability parameters were carefully studied. The differential in preservation efficacy between the HTS and media solutions was apparent after a careful assessment of postthaw viability, which revealed a decline in viable cell number compared with controls. Hepatocytes cryopreserved in HTS + DMSO yielded a 32% (overall 19%) improvement in cell viability over the media + DMSO samples and represented only an 11% drop in viability in comparison to controls. Samples were plated and cultured overnight to assess attachment and viability following 24 hours of culture. This assessment was performed due to recent reports of the involvement of apoptotic and necrotic cell death following cryopreservation leading to delayed onset cell death in several differing cell systems.21,22 Assessment of cellular survival at 24 hours and up to 14 days revealed that, consistent with previous reports, there was continued cell death, resulting in a decline in attachment rate and long-term survival in the media and HTS samples. On a per-cell basis, functional levels are comparable between the 3 groups. This indicates that the differences in overall function can be explained by cell death instead of less function per cell.
To date, reports on cryopreservation of isolated hepatocytes typically showed reasonable to excellent immediate postthaw viability (up to 98% of control) but were disappointing with regard to overall attachment and long-term function.27,28 Postthaw selection of viable cells by centrifugation over Percoll,13,19,29 as well as comparing cellular function on a per-cell basis,27,30 interferes with interpreting overall cell loss and residual function, which is crucial when considering use of cryopreserved cells in a BAL. Survival and long-term functional data in the present study are purposely expressed per batch, as a percentage of control, and clearly indicate that we were not only able to recover viable hepatocytes after thawing but also these hepatocytes functioned properly in a stable long-term culture configuration. Albumin secretion, urea synthesis, and EROD activity as important parameters of hepatospecific function were measured to evaluate a broad spectrum of hepatospecific functions. We consider an overall loss of function of 12% to 29% as in HTS-frozen cells as an important improvement of the cryopreservation process of isolated hepatocytes. If using up to 30% more cells can compensate this postcryopreservation cell loss, cryopreservation of hepatocytes will become a feasible option for use in BALs.
Cryopreservation of hepatocytes in the intracellular-type dextran-based preservation medium (ie, HTS) resulted in an enhancement in postthaw cell yield, viability, long-term survival, and hepatospecific function. Previous reports detailing the use of HTS as the carrier solution during cryopreservation have shown similar improvements in viability and survival in several different cell types.21,22 In these reports, it was shown that this improvement of cryopreservation outcome was due to modulation of the physiological and biochemical response to the cryopreservation process. This modulation has been shown to directly reduce the levels of apoptosis and necrosis following cryopreservation, thereby reducing the level of delayed-onset cell death and improving survival. Although we have not assessed the mechanism of cell death in this study, it is plausible that HTS imparted its beneficial effect through stabilization of cells against apoptotic cell death. This issue is currently under investigation.
In addition to basal hepatospecific function, responsiveness of hepatocytes to cytokine stimulation is essential for utilization in clinical applications; therefore, we tested cryopreserved hepatocyte responsiveness to elevated levels of IL-6. IL-6 is the major regulator cytokine of the acute-phase response to injury in hepatocytes,31 and therefore hepatocyte responsiveness is critical for cell utilization in BAL assist devices as the patients’ plasma perfusing the bioreactor will contain high levels of IL-6.32 Our data demonstrate that the response of fresh and cryopreserved hepatocytes is similar when stimulated with IL-6 both early in postthaw culture, as well as following culture stabilization. This observation contributes to our assertion that hepatocytes cryopreserved in HTS + DMSO are viable and functional following the cryopreservation process. One observation of note was that the recovery of albumin secretion following removal of cytokine stimulation was on the order of 5–6 days. We believe that this lag may reflect residual cytokine retention in the collagen gel matrix, which was gradually removed over time through washout by cytokine-free media over a period of days. Collagen-sandwiched hepatocytes have ∼2 mL of liquid retention in the collagen gel matrix, and given the 2-mL medium daily changes, one can predict that cytokine levels decrease by half daily. We believe that recovery times in a bioreactor will be shorter due to more rapid diluent turnover.
This study represents the first report on assessment of long-term hepatocellular function and acute-phase challenge response after cryopreservation and demonstrated that the improvement in preservation efficacy was not strictly limited to cell viability but translated into the maintenance of viable and functioning cells, as well. Through our study we not only demonstrated that HTS reduced the level of cell death but we further demonstrated that cryopreservation in HTS yielded improved maintenance of cellular and physiological function. These results may represent an important step forward to the utilization of cryopreserved isolated hepatocytes in regenerative medical applications such as in BAL devices and hepatocyte transplantation.
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Institutes of Health (DK 46270) and Shriners Hospitals for Children. Dr. Sosef was also supported by a Fulbright-grant, a Michael van Vloten Research Fellowship (The Netherlands), and a Travel Award from the Netherlands Organization for Scientific Research (NWO).
Footnotes
Address correspondence to: Mehmet Toner, PhD, Center for Engineering in Medicine and Surgical Services, Shriners Hospital for Children, 51 Blossom Street, Boston, MA 02114. E-mail: mtoner@sbi.org.
REFERENCES
- 1.Khalili TM, Navarro A, Ting P, et al. Bioartificial liver treatment prolongs survival and lowers intracranial pressure in pigs with fulminant hepatic failure. Artif Organs. 2001;25:566–570. [DOI] [PubMed] [Google Scholar]
- 2.Sosef MN, Abrahamse LS, van De Kerkhove MP, et al. Assessment of the AMC-bioartificial liver in the anhepatic pig. Transplantation. 2002;73:204–209. [DOI] [PubMed] [Google Scholar]
- 3.Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. [DOI] [PubMed] [Google Scholar]
- 4.van de Kerkhove MP, Di Florio E, Scuderi V, et al. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002;25:950–959. [DOI] [PubMed] [Google Scholar]
- 5.Stevens A, Busuttil RW, Hari S, et al. An interim analysis of a phase II/III prospective, randomized, multicenter, controlled trial of the HepatAssist bioartificial support system for the treatment of fulminant hepatic failure. Hepatology. 2001;34:299A. [Google Scholar]
- 6.Kim BH, Han YS, Dong SH, et al. Temporary amelioration of bilirubin conjugation defect in Gunn rats by transplanting conditionally immortalized hepatocytes. J Gastroenterol Hepatol. 2002;17:690–696. [DOI] [PubMed] [Google Scholar]
- 7.Cheung ST, Tsui TY, Wang WL, et al. Liver as an ideal target for gene therapy: expression of CTLA4Ig by retroviral gene transfer. J Gastroenterol Hepatol. 2002;17:1008–1014. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Malhi H, Gagandeep S, et al. Liver repopulation with hepatocyte transplantation: new avenues for gene and cell therapy. J Gene Med. 1999;1:386–392. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Dennis JA, Healy PJ, et al. Hepatocyte gene therapy in a large animal: a neonatal bovine model of citrullinemia. Proc Natl Acad Sci U S A. 1999;96:3981–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borel RI, Toner M, Sheeha SJ, et al. Long-term functional recovery of hepatocytes after cryopreservation in a three-dimensional culture configuration. Cell Transplant. 1992;1:281–292. [DOI] [PubMed] [Google Scholar]
- 11.Guyomard C, Rialland L, Fremond B, et al. Influence of alginate gel entrapment and cryopreservation on survival and xenobiotic metabolism capacity of rat hepatocytes. Toxicol Appl Pharmacol. 1996;141:349–356. [DOI] [PubMed] [Google Scholar]
- 12.Chesne C, Guillouzo A. Cryopreservation of isolated rat hepatocytes: a critical evaluation of freezing and thawing conditions. Cryobiology. 1988;25:323–330. [DOI] [PubMed] [Google Scholar]
- 13.Chesne C, Guyomard C, Fautrel A, et al. Viability and function in primary culture of adult hepatocytes from various animal species and human beings after cryopreservation. Hepatology. 1993;18:406–414. [DOI] [PubMed] [Google Scholar]
- 14.Coundouris JA, Grant MH, Simpson JG, et al. Drug metabolism and viability studies in cryopreserved rat hepatocytes. Cryobiology. 1990;27:288–300. [DOI] [PubMed] [Google Scholar]
- 15.Innes GK, Fuller BJ, Hobbs KE. Functional testing of hepatocytes following their recovery from cryopreservation. Cryobiology. 1988;25:23–30. [DOI] [PubMed] [Google Scholar]
- 16.Loretz LJ, Li AP, Flye MW, et al. Optimization of cryopreservation procedures for rat and human hepatocytes. Xenobiotica. 1989;19:489–498. [DOI] [PubMed] [Google Scholar]
- 17.Morsiani E, Pazzi P, Moscioni AD, et al. In vitro morphological and functional characterization of isolated porcine hepatocytes for extracorporeal liver support: bile acid uptake and conjugation. J Surg Res. 1998;79:54–60. [DOI] [PubMed] [Google Scholar]
- 18.De Loecker R, Fuller BJ, Gruwez J, et al. The effects of cryopreservation on membrane integrity, membrane transport, and protein synthesis in rat hepatocytes. Cryobiology. 1990;27:143–152. [DOI] [PubMed] [Google Scholar]
- 19.Fautrel A, Joly B, Guyomard C, et al. Long-term maintenance of drug-metabolizing enzyme activities in rat hepatocytes after cryopreservation. Toxicol Appl Pharmacol. 1997;147:110–114. [DOI] [PubMed] [Google Scholar]
- 20.Arikura J, Kobayashi N, Okitsu T, et al. UW solution: a promising tool for cryopreservation of primarily isolated rat hepatocytes. J Hepatobil Pancreat Surg. 2002;9:742–749. [DOI] [PubMed] [Google Scholar]
- 21.Baust JM, Vogel MJ, Van Buskirk R, et al. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant. 2001;10:561–571. [PubMed] [Google Scholar]
- 22.Baust JM, Van B, Baust JG. Cell viability improves following inhibition of cryopreservation- induced apoptosis. In Vitro Cell Dev Biol Anim. 2000;36:262–270. [DOI] [PubMed] [Google Scholar]
- 23.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. [DOI] [PubMed] [Google Scholar]
- 24.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behnia K, Bhatia S, Jastromb N, et al. Xenobiotic metabolism by cultured primary porcine hepatocytes. Tissue Eng. 2000;6:467–479. [DOI] [PubMed] [Google Scholar]
- 26.Dunn JC, Yarmush ML, Koebe HG, et al. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Ding Y, Zhang H. Cryopreservation of suckling pig hepatocytes. Ann Clin Lab Sci. 2001;31:391–398. [PubMed] [Google Scholar]
- 28.Wu L, Sun J, Qin G, et al. Cryopreserved porcine hepatocytes in a liver biodialysis system. Transplant Proc. 2001;33:1950–1951. [DOI] [PubMed] [Google Scholar]
- 29.Pang JM, Zaleski J, Kauffman FC. Toxicity of allyl alcohol in primary cultures of freshly isolated and cryopreserved hepatocytes maintained on hydrated collagen gels. Toxicol Appl Pharmacol. 1997;142:87–94. [DOI] [PubMed] [Google Scholar]
- 30.Naik S, Santangini HA, Trenkler DM, et al. Functional recovery of porcine hepatocytes after hypothermic or cryogenic preservation for liver support systems. Cell Transplant. 1997;6:447–454. [DOI] [PubMed] [Google Scholar]
- 31.Castell JV, Andus T, Kunz D, et al. Interleukin-6: the major regulator of acute-phase protein synthesis in man and rat. Ann N Y Acad Sci. 1989;557:87–99. [PubMed] [Google Scholar]
- 32.Hughes RD, Nicolaou N, Langley PG, et al. Plasma cytokine levels and coagulation and complement activation during use of the extracorporeal liver assist device in acute liver failure. Artif Organs. 1998;22:854–858. [DOI] [PubMed] [Google Scholar]