Abstract
Most human immunodeficiency virus (HIV) type 1 infections occur by the mucosal route. Thus, it is important to assess the immune responses to HIV in the vaginal, cervical, and rectal compartments. Here we quantitated the virus-specific CD8+ T-cell response and characterized the phenotype of lymphocytes in the genital tracts of naive macaques, macaques acutely or chronically infected with simian immunodeficiency virus SIVmac251, and macaques chronically infected with chimeric simian/human immunodeficiency virus SHIVKU2. Vaginal biopsy samples or samples obtained at the time of euthanasia were used in this analysis. The percentage of Gag-specific, tetramer-positive T cells was as high as 13 to 14% of the CD3+ CD8+ T-cell population in the vaginal and cervical laminae propriae of both SIVmac251 and SHIVKU2 chronically infected macaques. In most cases, the frequency of this response in the cervicovaginal compartment far exceeded the frequency in the blood or the draining iliac lymph node. Vaginal laminae propriae of naive macaques contained 55 to 65% CD3+ CD8+ cells and 28 to 34% CD3+ CD4+ cells, while the majority of intraepithelial cells were CD8+ T cells (75 to 85%). For the same cells, the surface expression of CD62L was low whereas that of αEβ7 was high. No difference in the expression of CD45RA on CD8+ T cells was observed in the chronic stage of SIVmac251 infection. Although no decrease in the percentage of CD4+ cells in the genital tract was observed within the first 12 days of infection, by 6 weeks from SIVmac251 infection and thereafter the percentage of CD4+ T cells was decreased in the laminae propriae of the vagina and cervix. Expression of CD45RA did not differ in naive and acutely SIVmac251 infected macaques. Information on the quality and quantity of local immune responses may help in the design of vaccine strategies aimed at containing viral replication at the site of viral encounter.
In most newly acquired human immunodeficiency virus type 1 (HIV-1) infections, especially in the developing world (70 to 80%), the virus is transmitted by the mucosal route. Recent studies, focused on genital HIV-specific immune responses in individuals who remain uninfected despite continuous exposure to HIV-1, have found high levels of specific immunoglobulin A in the mucosa (5, 21, 40). Whether such mucosal immunoglobulin A responses contribute directly to immune protection, however, is not clear (49), and it is possible that these apparently protected individuals may have local, virus-specific, protective CD8+ T cells. In support of this possibility, a recent study on a cohort of sex workers who are resistant to HIV-1 infection despite substantial exposure to the virus has demonstrated the presence of HIV-specific, gamma interferon-producing CD8+ T cells in the cervical mucosa (20). This cohort of apparently uninfected individuals had higher levels of HIV-1-specific CD8+ T cells in mucosal tissues than in blood, while HIV-1-infected individuals had higher HIV-1 responses in blood than in mucosal tissues (20). Neither cervical nor blood HIV-1-specific responses were found in low-risk control donors from the same area (20). These findings raise important questions about the role of mucosal immune responses in the vagina and cervix in protection from HIV-1 infection. However, many of these questions are difficult to address in humans.
The immunodeficiency induced with simian immunodeficiency virus SIVmac251 in macaques (10, 28, 32) closely resembles the disease induced in humans by HIV-1, thus providing a suitable model in which to study virus-specific systemic and mucosal immune responses. Virus-specific cytolytic activity in the vaginal mucosae of SIVmac251-infected macaques has previously been demonstrated in the CD8+ intraepithelial-lymphocyte (IEL) compartment (33). In addition, it has been demonstrated that intravaginal inoculation with SIVmac251 can sometimes induce occult systemic infection (37, 41). In these animals, persistent SIVmac-specific CD4+ T-cell proliferative responses in vaginal mucosae, vaginal antibodies, and cytotoxic T-lymphocyte (CTL) responses have been detected (41).
Because HIV-1 is mainly transmitted mucosally, there is a need to further assess CTL responses in the macaque model, not only in the blood but also at the mucosal sites. However, because of the limitation in the number of lymphocytes that can be obtained from vaginal and cervical tissues, most studies so far on CTLs from the genital tracts of female macaques have been performed following prior expansion of lymphocytes in vitro (33, 41). This approach allows for accurate assessment of CTL function but has limitations regarding the phenotypic characterization of T cells and the quantitation of virus-specific CD8+ T cells. Here, we have investigated the phenotype of T cells in the genital tract directly from biopsy specimens as well as from tissue collected postmortem from SIVmac251-infected macaques. We studied the expression of CD4, CD8, CD45RA, CD62, and CD103 in naive and SIVmac251-infected macaques. In addition, in a group of Mamu-A*01-positive macaques infected by SIVmac251 or chimeric simian/human immunodeficiency virus SHIVKU2, we also quantitated, by tetramer technology, the frequency of virus-specific CD8+ T-cell responses to the SIV Gag protein.
MATERIALS AND METHODS
Animals.
Specimens from a total of 19 nulliparous female macaques, aged 6 to 22, were used for this study. Vaginal biopsy specimens (diameter, 4 mm) from nine healthy macaques were collected, washed, and maintained in RPMI medium containing 10% fetal calf serum (FCS) plus penicillin-streptomycin prior to T-cell isolation from the lamina propria and intraepithelial compartments. All biopsy specimens were taken from the dorsal aspect of the vault of introitus of the vagina. Since it has been previously determined by others that the menstrual cycle does not affect the number and distribution of immune cells (36), samples were taken randomly (i.e., at different stages of the menstrual cycle).
Two female Mamu-A*01-positive macaques were sacrificed during primary infection following intrarectal exposure to undiluted SIVmac251/561 stock virus (45a) (animal 817) or intravenous exposure to a 1:3,000 dilution of the same viral stock (animal 819) (Table 1). From these macaques, blood was also drawn on days 4, 8, and 12 after infection. Animals were sacrificed at the times indicated in Table 1, and mucosal tissues from the vagina, cervix, draining iliac lymph nodes, and, in some animals, the gut were also collected postmortem and stored in RPMI medium containing 10% FCS plus penicillin-streptomycin until use.
TABLE 1.
Genetic, virological, and clinical features of the macaques
| Macaque | SIVmac251/SHIVKU2 status at time of tissue collection | Mamu-A*01 | Tissue collected | Absolute no. of CD3+ CD4+ cells/mm3 in blood at time of tissue collection | Absolute no. of CD3+ CD8+ cells/mm3 in blood at time of tissue collection | Viral load at time of tissue collection (RNA copies/ml of plasma)a |
|---|---|---|---|---|---|---|
| 815 | Naive | + | Vaginal biopsy | 2,491 | 1,212 | N/A |
| 816 | Naive | + | Vaginal biopsy | 1,841 | 882 | N/A |
| 814 | Naive | + | Vaginal biopsy | 1,441 | 901 | N/A |
| 818 | Naive | + | Vaginal biopsy | 1,269 | 483 | N/A |
| 582 | Naive | + | Vaginal biopsy | 917 | 416 | N/A |
| 536 | Naive | + | Vaginal biopsy | 1,668 | 2,398 | N/A |
| 727 | Naive | − | Vaginal biopsy | Not available | Not available | N/A |
| 582 | Naive | + | Vaginal biopsy | Not available | Not available | N/A |
| 654 | Naive | + | Vaginal biopsy | Not available | Not available | N/A |
| 819 | Primary infection, intravenous (12 days) | + | Vagina, cervix, and iliac lymph nodes | 474 | 198 | 1,158,220 |
| 817 | Primary infection, intrarectal (12 days) | + | Vagina, cervix, and iliac lymph nodes | 450 | 213 | 27,518 |
| 730 | Primary infection (6 wk) | Not known | Vaginal biopsy | 310 | 494 | 1,114,840 |
| 738 | Primary infection (6 wk) | Not known | Vaginal biopsy | 363 | 448 | 664,960 |
| 479 | Chronic SIVmac251 infection (69 wk) | − | Vagina | 141 | 1,354 | 141,800 |
| 408 | Chronic SIVmac251 infection (69 wk) | + | Blood, vagina, cervix, and iliac lymph nodes | 170 | 384 | 1,343,140 |
| 669 | Chronic SIVmac251 infection (70 wk) | + | Blood, vagina, cervix, and iliac lymph nodes | 572 | 283 | <2,000 |
| 455 | Chronic SHIVKU2 infection (156 wk) | + | Blood, vagina, and iliac lymph nodes | 956 | 737 | <2,000 |
| 454 | Chronic SHIVKU2 infection (156 wk) | + | Blood, vagina, and iliac lymph nodes | 780 | 888 | <2,000 |
| 409 | Chronic SHIVKU2 infection (129 wk) | + | Blood, vagina, and iliac lymph nodes | 1,234 | 1,747 | <2,000 |
N/A, not applicable.
Vaginal biopsy specimns were also obtained from two additional female macaques (animals 730 and 738) during primary infection at 6 weeks following exposure to SIVmac251/561 by the vaginal route (Table 1).
Among the chronically infected macaques, three females infected with SIVmac251 (animals 408, 479, and 669) and three females infected with SHIVKU2 were used. All six macaques were challenged by the intrarectal route, and vaginal, cervical, and iliac lymph node specimens were collected postmortem as described above (Table 1).
Isolation of tissue lymphocytes.
Mononuclear cells were isolated from the iliac lymph nodes, vagina, and cervix as well as from intestinal tissues. On the average, biopsy specimens obtained from the vagina yielded about 2 × 106 cells from the lamina propria. Postmortem specimens included the ectocervix and endocervix, and all vaginal samples consisted of half of the vagina, split in a cranial-caudal manner. Mononuclear cells from iliac lymph nodes were isolated by mechanical dissociation of the tissue and consecutive separation by Ficoll gradient centrifugation. Vaginal and cervical tissues were treated with 1 mM dithiothreitol (ICN Biomedicals Inc., Aurora, Ohio) for 30 min, followed by stirring in 1 mM EDTA in calcium- and magnesium-free Hanks balanced salt solution (Life Technologies, Baltimore, Md.), pH 7.2, at room temperature for 1 h (four to five times) to remove the epithelial layer. IELs were isolated from the collected supernatants through a 40% Percoll gradient. Microscopic examination of hematoxylin-and-eosin staining of sections of the remaining tissue revealed that all of the epithelium was removed and that the lamina propria was intact.
Lamina propria lymphocytes were separated following the removal of epithelium and IELs. The remaining tissue was cut into small pieces and incubated with 0.5 mg of collagenase IV (Sigma Chemical Co., St. Louis, Mo.)/ml in Iscove’s modified Dulbecco’s medium (Life Technologies, Palsley, Pa.) supplemented with 10% FCS and penicillin-streptomycin for 2 to 3 h at 37°C. Mononuclear cells were isolated from the supernatant containing dissociated cells by Percoll gradient centrifugation. Intestinal lymphocytes underwent a similar procedure but were incubated in Hanks balanced salt solution-EDTA three times, and the lamina propria was dissociated by using 400 U of collagenase D (Boehringer GmbH, Mannheim, Germany)/ml.
Flow cytometry.
A total of 5 × 104 cells were used for staining of each sample. Mononuclear cells were directly stained with phycoerythin (PE)-conjugated tetrameric complexes for Gag181 (24), Env622 (14), and Tat28 (1). Peridinin chlorophyll (PerCP)-conjugated anti-CD3ɛ@ (PharMingen, San Diego, Calif.) and fluorescein isothiocyanate (FITC)-conjugated anti-CD8α/β (Becton Dickinson, San Jose, Calif.) were used in conjunction with the tetrameric complexes. An antibody that recognizes CD8α/β was chosen to label CD8+ T cells, since it has been shown that in most monkeys most of the CD8+ cells express this marker and that in most, but not all, cases more than 95% of tetramer-positive cells for the SIVmac251 immunodominant p11C Gag epitope are of the CD8α/β+ type (24, 26).
In addition, cells were stained with anti-CD45RA (2H4)-HR1 (phycoerythin) (Coulter Corp., Miami, Fla.), anti-CD4-PE (Becton Dickinson), anti-CD62L-FITC, and anti-CD103-FITC (Coulter Immunotech, Marseille, France).
Briefly, 5 × 105 lymphocytes isolated by Ficoll diatrizoate or Percoll gradient centrifugation were incubated with 1 μg of tetrameric complexes and/or selected antibodies for 30 min at room temperature. After cells were washed twice in Dulbecco’s phosphate-buffered saline supplemented with 2% FCS and fixed in 1% paraformaldehyde (pH 7.4), samples were analyzed by flow cytometry using the FACScalibur (Becton Dickinson) instrument. Unstained cells as well as cells stained with appropriate isotype controls were used as negative controls.
RESULTS
Decrease in percentages of CD4+ T cells in the cervicovaginal laminae propriae of SIVmac251-infected macaques.
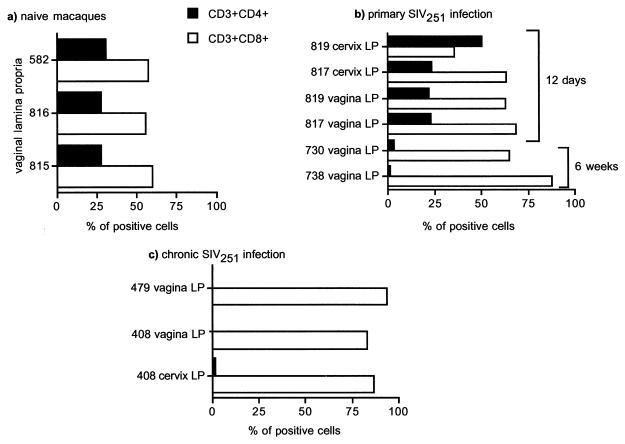
The frequency and phenotypic markers of CD4+ and CD8+ T cells in the genital tracts of healthy macaques are not fully defined. Therefore, the distribution and enumeration of CD4+ and CD8+ T cells in the vagina were initially determined for healthy, naive macaques. Following vaginal biopsies, the intraepithelial cells and lamina propria lymphocytes were isolated and each population was stained simultaneously with antibodies against the CD4, CD8, and CD3 antigens. Analysis of CD4- and CD8-expressing cells was performed by gating first on the whole lymphocyte population as determined by forward and side scatter and then on the CD3+ population. The percentages of CD4- and CD8-positive cells were assessed by quadrant quantification using the Cellquest software. For the three naive macaques studied (animals 582, 816, and 815), the vaginal lamina propria contained 55 to 65% CD3+ CD8+ cells and 28 to 34% CD3+ CD4+ cells (Fig. 1a). IELs were predominantly CD8+ (75 to 85%), and only a small fraction of these cells expressed the CD4 marker (data not shown).
FIG. 1.
Enumeration of T cells in vagina laminae propriae of naive and SIVmac251-infected macaques. Shown are percentages of CD3+ CD4+ and CD3+ CD8+ cells in vaginal laminae propriae of naïve macaques (a) and of SIVmac251-infected macaques during primary (b) or chronic (c) infection. Cells were gated through the CD3+ population (4 × 104 cells were acquired through this gate for each sample analyzed), and quadrant analysis for CD4-PE- and CD8-PerCP-positive cells was used to generate the percentages shown in the histograms. LP, lamina propria.
Since apparent depletion of CD4+ T cells in the gut-associated lymphoid tissue (GALT) occurs early following SIVmac251 infection of macaques (56, 60), we examined the percentages of CD4+ T cells in the urogenital tracts of all macaques studied. For the two acutely infected macaques (macaques 817 and 819), sacrificed at day 12 postinfection, the percentages of CD4+ and CD8+ T cells in the genital tract did not differ from those of naive macaques (compare Fig. 1a to Fig. 1b). In contrast, analysis of vaginal biopsy specimens obtained from macaques 730 and 738 at 6 weeks postinfection revealed a decrease in the percentage of CD4+ T cells in the lamina propria (Fig. 1b). Similarly, in the vaginal and cervical laminae propriae of macaques chronically infected with SIVmac251, such as animals 408 and 479 (see Table 1), CD4+ T cells represented only 0.13 and 0.24% of the total T-cell population, respectively (Fig. 1c). For the chronically SHIVKU2 infected macaques studied here, the percentage of CD4+ cells in the vaginal lamina propria was similar to that for naive macaques (data not shown), and there was no depletion of CD4+ cells in the blood (Table 1).
It is not clear whether the decrease in the percentage of CD4+ cells represents a local depletion of CD4+ cells or is due to a relative increase in CD8+ cells. Others have shown that, in the intestinal tissues, the decreased percentage of CD4+ cells may be due to both factors (39).
Phenotype of the cervicovaginal mucosal lymphocytes.
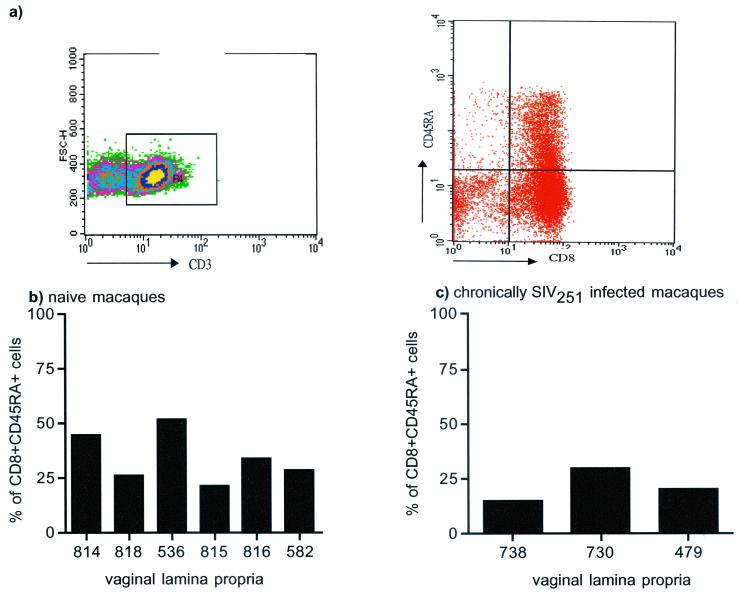
The CD8+ T-cell phenotype, which is important in cell-mediated responses to SIVmac251 (9, 33), was further characterized. Lamina propria CD8α/β+ T cells were assessed for expression of the CD45RA, CD62L, and CD103 markers. For the six healthy naive macaques studied here, 28 to 46% of vaginal lamina propria T cells expressed both CD45RA and CD8α/β (Fig. 2b), and the relative frequency of these cells in the three chronically SIVmac251 infected macaques studied here did not differ (Fig. 2c).
FIG. 2.
CD3+ CD8+ CD45RA+ frequency in the vaginal tissues of naive and chronically infected macaques. Shown are the percentages of CD3+ CD8+ cells of the vaginal lamina propria that express the CD45RA marker in naive (b) or chronically infected (c) macaques. Tricolor staining for CD3-FITC, CD8-PerCP, and CD45RA-HRD was used to generate these data. Cells were gated through the CD3+ population (4 × 104 cells were acquired through this gate for each sample analyzed) and then analyzed for expression of CD8 and CD45RA using quadrant analysis (a).
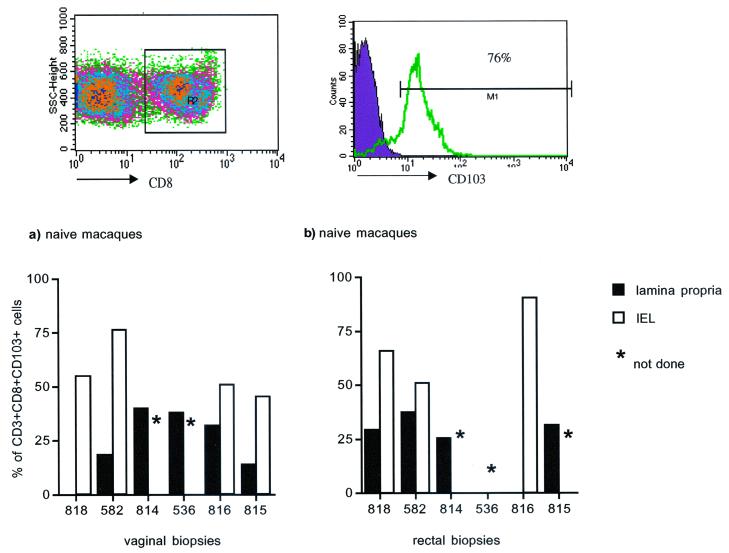
CD103 is a marker for αEβ7 integrin, a molecule acquired by recent thymic emigrants (43) that is believed to mediate T-cell adhesion to epithelial cells by binding to the E-cadherin molecule (7, 8) expressed on the epithelial cells of skin (11, 54), renal epithelial cells (13), and epithelial cells of mucosal surfaces, including the vagina and cervix (7, 16, 19). CD103 therefore represents a marker highly expressed on IELs. The expression of αEβ7 (CD103) on mucosal lymphocytes from the guts of humans and mice has been studied previously (12, 35), but less is known about the distribution of this marker in macaques. Long-term CD8+ T-cell lines, derived from the vaginal epithelia of macaques, have been shown to express the CD103 marker (42), but no direct estimation of the frequency of this marker in ex vivo CD8+ T cells has been carried out. In vaginal tissues of naive macaques, approximately half of CD8α/β+ IELs expressed αEβ7, whereas expression on CD8α/β+ lymphocytes from the vaginal lamina propria was lower (range, 14 to 31%) (Fig. 3a). This marker was also quantified on intestinal lymphocytes of naive macaques. αEβ7 was expressed on more than 60% of the population of intestinal CD8α/β+ IELs of the three healthy females studied, whereas in the intestinal lamina propria, this marker was expressed on 25 to 40% of the CD8+ cells (Fig. 3b).
FIG. 3.
CD8+ CD103+ T cells in the cervicovaginal and rectal mucosae of naive macaques. Bar graphs show CD103 (αEβ7) expression in intraepithelial and lamina propria CD8+ T lymphocytes of vaginal (a) and rectal (b) tissues of naive macaques. As shown in the top two panels, two-color staining was done for CD8 and CD103. Cells were gated on the CD8+ population (104 cells were acquired through this gate), and expression of CD103 on this population was determined by histogram analysis as shown.
In primary infection of macaque 817, which was infected intrarectally, the percentages of CD8+ αEβ7+ intestinal IELs were three- to sixfold lower than those for macaque 819, which was infected intravenously (data not shown) or those for naive macaques. Lamina propria CD8+ T-cell expression of this marker was similar in both animals and corresponded to the levels seen in healthy macaques. L-selectin (CD62L), which is highly expressed on lymph node lymphocytes and has low expression in mucosal lymphocytes, was also studied. Expression of CD62L on vaginal laminae propriae of healthy macaques was also low, as expected (42, 46), ranging from 0.22 to 1.73% of CD8+ lymphocytes (data not shown). The frequencies of the CD8+ T cells expressing αEβ7 as well as CD62L in both the genital and intestinal tracts are similar among mice, macaques, and humans.
High frequency of MHC class I-restricted responses in vaginal and cervical laminae propriae of SIVmac251- and SHIVKU2-infected macaques.
Virus-specific CTLs play a significant role in containing HIV and SIV infections (6, 17, 25, 38, 47, 51). By use of the tetramer technology (2), CTLs specific for the SIV Gag protein have been demonstrated in the blood, lymph nodes, and semen of SIV-infected macaques (27) as well as in the gut (14a, 58). Some of the macaques studied here were positive for the major histocompatibility complex (MHC) class I Mamu-A*01 molecule (Table 1). Therefore, a tetrameric complex conjugated with the Mamu-A*01-restricted, dominant p11C, C-M epitope was used to measure the frequency of the virus-specific CD8+ T-cell response (24). Quantification of the Mamu-A*01-positive CD8+ T-cell response was performed on purified lymphocytes from vaginal tissue and the draining iliac lymph node for two chronically SIVmac251 infected macaques and for three macaques infected with SHIVKU2 (18) (Table 1).
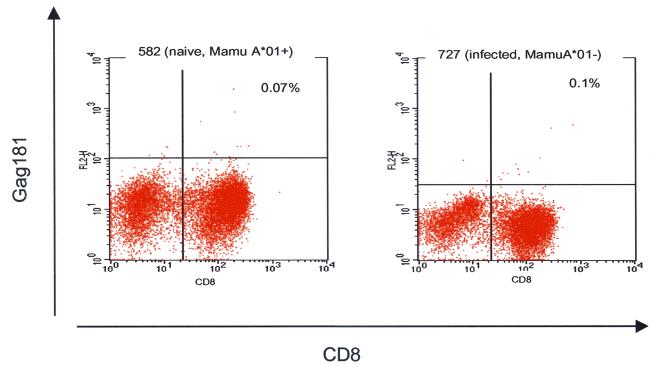
The specificity of the Gag181 tetramer binding was assessed in vaginal biopsy specimens obtained from Mamu-A*01-positive naive macaques and Mamu-A*01-negative infected macaques (Fig. 4). Tetrameric complexes with specificity for Gag antigens did not stain more than 0.12% of the total CD3+ CD8+ T-cell population in cervicovaginal mucosae, blood, or lymph nodes.
FIG. 4.
Staining of control tissues with the Gag181 tetramer. Vaginal biopsy specimens obtained from naive Mamu-A*0-positive macaques and from infected Mamu-A*01-negative macaques were used as negative controls for the Gag181 tetramer staining.
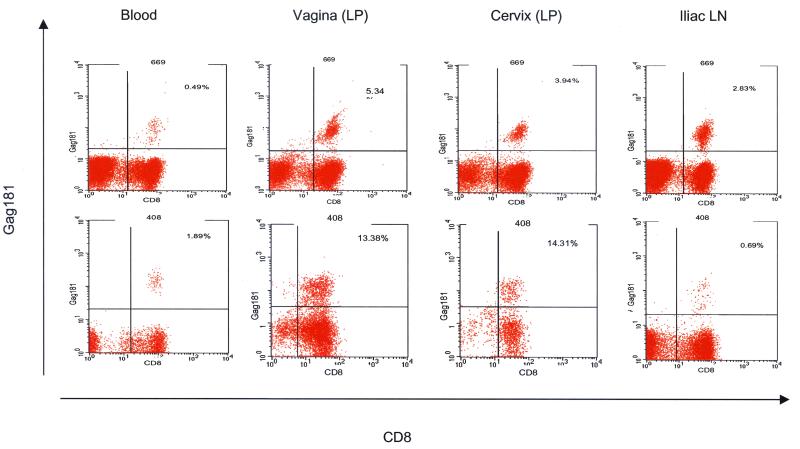
In the macaques chronically infected with SIVmac251 studied here (Table 1), the frequency of virus-specific CD8+ T cells was greater in the genital lamina propria than in the blood or iliac lymph nodes. Analysis of the cervicovaginal tissues, blood, and iliac lymph nodes from the two chronically SIVmac251 infected macaques, 408 and 668 (Table 1), revealed that 14% of the CD3+ CD8+ T cells in the lamina propria of the vagina of macaque 408 were specific for the immunodominant Gag181 peptide; the percentage for the same compartment of animal 669 was 5%. For both animals the frequency of this Gag-specific CD8+ T-cell response in the cervicovaginal compartment exceeded that found in the blood or the iliac lymph nodes (Fig. 5).
FIG. 5.
Frequency of Gag181 tetramer-specific CD8+ T cells in the systemic and genital compartments of chronically SIVmac251 infected macaques. The percentage in the upper right portion of each panel represents tetramer-positive CD3+ CD8+ T cells. Cells were gated through the CD3+ population, and 104 CD3+ CD8+ cells were acquired for analysis of each sample where possible. Staining was done on lymphocytes from the blood, vaginal and cervical laminae propriae (LP), and iliac lymph nodes (LN), as indicated.
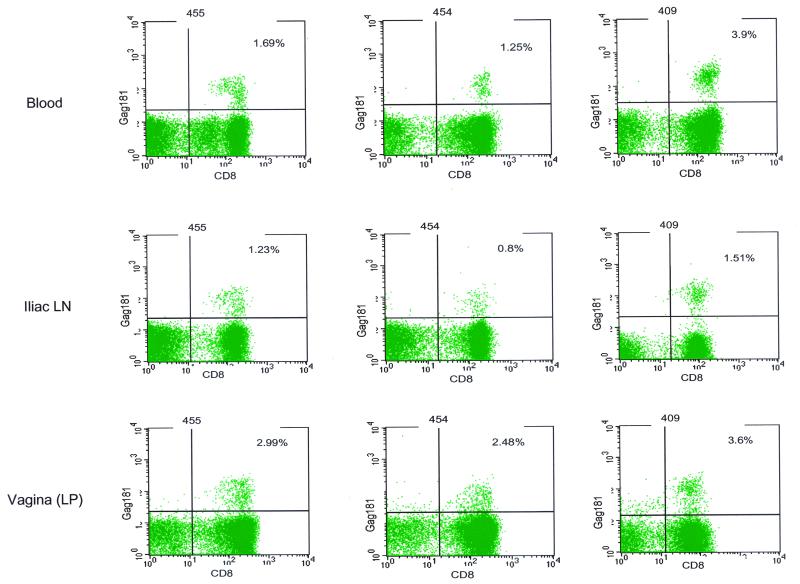
To assess whether these findings could be extended to other animals, chronically SHIVKU2 infected macaques were also studied. Interestingly, a high percentage of Gag181-specific cells was found in the vaginal laminae propriae of these macaques as well, and again the frequency of this response in this compartment exceeded that found in the blood or iliac lymph node in two of the three animals (Fig. 6). Possibly, ongoing viral replication in this location may account for the high frequency of this virus-specific CD8+ T-cell response.
FIG. 6.
Frequency of Gag181 tetramer-positive CD3+ CD8+ T cells in tissues of chronically SHIVKU2 infected macaques. Flow charts show the proportions of Gag181 tetramer-positive lymphocytes isolated from the blood, iliac lymph nodes, and vaginal laminae propriae of SHIVKU2-infected macaques. Cells were analyzed as described in the legend to Fig. 5 and in Materials and Methods.
DISCUSSION
Mucosal immune responses to pathogens such as HIV and SIV are likely to be important in conferring protection against infection (20). As research in this area evolves, the establishment of immunological parameters that correlate with protection from infection in humans as well as in animal models is of great importance.
The mucosa of the vagina and ectocervix consists of stratified squamous epithelium and lamina propria whose thickness varies greatly depending on its vascularization, which in turn is related to the phase of the menstrual cycle. Lymphocytes of the vaginal mucosa are localized within the epithelial layer (IELs) and in the lamina propria. In other mucosal sites, such as the gut, these two populations have distinct properties. The IELs in the gut are predominantly of the CD8+ and memory phenotype and also express the αEβ7 marker. In the same compartment, lamina propria lymphocytes are a mixed population of CD4+ and CD8+ lymphocytes of both naive and memory phenotypes that express a lower level of αEβ7 (22, 35, 50). Immunohistochemical studies with macaques have demonstrated that the vaginal mucosa and the ectocervix contain large numbers of CD8+ T cells and fairly small portions of CD4+ T cells (44). In addition, cytolytic CD8+ T-cell lines derived from the vaginal epithelium were shown to be αEβ7 positive and L-selectin negative (42). However, due to the difficulties in the isolation procedure and the low yield of cells, direct staining and phenotypic characterization of ex vivo lymphocytes are usually not performed.
In this study, we quantified the proportion of CD4+ and CD8+ T cells in the cervicovaginal mucosa and assessed the expression of αEβ7, L-selectin, and CD45RA in the predominant CD8+ T-cell population. Our results show that a high proportion of vagina lamina propria lymphocytes are CD8+, approximately one-third express αEβ7, and, as expected, a small percentage express L-selectin. About a third of vaginal lamina propria CD8+ T cells also express the CD45RA marker.
The IEL marker αEβ7 was demonstrated to mediate T-cell adhesion to epithelial cells (7) through its binding to E-cadherin, which is expressed on epithelial cells of mucosal surfaces (8, 19). Recently, it was established that αEβ7 also interacts with human endothelial cells of the intestine (59). In mice, αEβ7 is expressed on more than 90% of intestinal IELs and on 45 to 50% of lamina propria T lymphocytes (22, 50). Integrin αEβ7 knockout mice have reduced numbers of mucosal T lymphocytes, especially of the CD8α/β+ T-cell type, particularly following bacterial colonization of mucosal surfaces (53). In mice exposed to Mycobacterium tuberculosis (12), the proportion of αEβ7 CD8+ T cells decreases significantly in the lungs, and blocking αEβ7 antibodies prevent and ameliorate immunization-induced colitis in interleukin-2−/− mice (34). As the function of αEβ7 still remains largely unknown, these findings indicate that αEβ7 may play a significant role in the recruitment and retention of intraepithelial mucosal lymphocytes.
In humans, this marker is expressed in 60 to 80% of intestinal IELs and in approximately 40% of intestinal lamina propria lymphocytes (35). In AIDS patients, however, the expression of CD103 by GALT-derived mononuclear cells was reportedly decreased from a mean of 41% in controls (range, 31 to 54%) to a mean of 22% (range, 16 to 59%) (52). Recent studies with human biopsy specimens have shown that, in HIV patients, CD103 was expressed in 5.2 to 43.7% of CD8+ GALT-derived mononuclear cells compared to 0.9 to 1.6% of CD8+ peripheral blood monocytes (55).
For macaques, we have observed that the expression of αEβ7 in intestinal tissues is similar to that for humans and mice and that this marker is also expressed in vaginal tissue at a level comparable to that in GALT. Decreased levels of αEβ7 have been observed in human HIV-1-infected colonic tissue (55) and in mice following pulmonary M. tuberculosis infection (12). Within the limitations of the relatively small number of infected animals examined for αEβ7 expression in this study, a similar phenomenon may occur in macaques infected with SIVmac251, and mucosally acquired infection may be associated with a lower level of expression of this marker. As also implied in studies by others, systemic SIVmac251 infection does not appear to alter αEβ7 expression in the gut (29).
CTLs play an important role in containing HIV and SIV replication (6, 17, 25, 38, 47, 51). In blood, emergence of virus-specific CTLs is associated with containment of viral replication during primary infection (25, 51). Similarly, mucosal lamina propria lymphocytes have been shown to have virus-specific cytolytic activity, and the frequency of antigen-specific CD8+ CTLs in the lamina propria of the gut has correlated with the degree of protection from viral challenge in some cases (3, 39, 45; I. M. Belyakov, Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky, unpublished data).
The presence of HIV-specific CTLs in the cervices of HIV-exposed sex workers apparently resistant to infection further suggests the importance of cellular responses in the genital tract in the prevention of infection (20). Cytotoxic activity against viral antigens has been readily demonstrated in cell lines derived from vaginal mucosa (15, 23, 33, 41).
Here, we have measured the frequency of Gag-specific responses in the cervicovaginal mucosae of genetically defined macaques (Mamu-A*01 positive) using a tetramer complex that specifically binds to CD8+ T cells that recognize a Gag dominant epitope (24). Our data indicate that the frequency of this virus-specific CD8+ T-cell response is surprisingly high in this compartment, exceeding that in systemic compartments for several macaques.
Studies by others suggest that the frequency of virus-specific CD8+ T-cell responses may depend on the level of viral replication in mucosal tissues (48). It is possible that the high numbers of tetramer-positive cells in the vaginal and cervical tissues of chronically infected macaques reflect high viral replication at these sites. Because of its relevance to viral transmission, further investigations are needed to establish if the cervicovaginal mucosa is a site of active replication of SIV or HIV.
The finding of a high frequency of virus-specific CD8+ T cells in the cervicovaginal compartment, coupled with the notion that mucosal immunization may provide better protection against mucosal challenge than systemic immunization (3, 4, 30, 31), has important implications for the development of vaccine modalities (57) that can target mucosal sites in order to prevent HIV-1 infection.
Acknowledgments
We thank John Altman and David Watkins for providing peptides and the tetrameric complexes, Ruth Woodward for performing the vaginal biopsies in macaques, Nancy Miller and Ranajit Pal for providing macaque tissues, and Steven Snodgrass for editorial assistance.
REFERENCES
- 1.Allen, T. M., D. H. O’Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386–390. [DOI] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94–96. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, and C. Natpratan. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J. Infect. Dis. 179:59–67. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cepek, K. L., C. M. Parker, J. L. Madara, and M. B. Brenner. 1993. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J. Immunol. 150:3459–3470. [PubMed] [Google Scholar]
- 8.Cepek, K. L., S. K. Shaw, C. M. Parker, G. J. Russell, J. S. Morrow, D. L. Rimm, and M. B. Brenner. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372:190–193. [DOI] [PubMed] [Google Scholar]
- 9.Cromwell, M. A., R. S. Veazey, J. D. Altman, K. G. Mansfield, R. Glickman, T. M. Allen, D. I. Watkins, A. A. Lackner, and R. P. Johnson. 2000. Induction of mucosal homing virus-specific CD8+ T lymphocytes by attenuated simian immunodeficiency virus. J. Virol. 74:8762–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desrosiers, R. C. 1990. The simian immunodeficiency viruses. Annu. Rev. Immunol. 8:557–578. [DOI] [PubMed] [Google Scholar]
- 11.Dietz, S. B., D. Whitaker-Menezes, and S. R. Lessin. 1996. The role of αEβ7 integrin (CD103) and E-cadherin in epidermotropism in cutaneous T-cell lymphoma. J. Cutan. Pathol. 23:312–318. [DOI] [PubMed] [Google Scholar]
- 12.Feng, C. G., W. J. Britton, U. Palendira, N. L. Groat, H. Briscoe, and A. G. Bean. 2000. Up-regulation of VCAM-1 and differential expansion of beta integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J. Immunol. 164:4853–4860. [DOI] [PubMed] [Google Scholar]
- 13.Hadley, G. A., S. T. Bartlett, C. S. Via, E. A. Rostapshova, and S. Moainie. 1997. The epithelial cell-specific integrin, CD103 (αE integrin), defines a novel subset of alloreactive CD8+ CTL. J. Immunol. 159:3748–3756. [PubMed] [Google Scholar]
- 14.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6:1140–1146. [DOI] [PubMed] [Google Scholar]
- 14a.Hel, Z., J. Nacsa, B. Kelsall, W.-P. Tsai, N. Letvin, R. Washington Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483–11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaoka, K., C. J. Miller, M. Kubota, M. B. McChesney, B. Lohman, M. Yamamoto, K. Fujihashi, K. Someya, M. Honda, J. R. McGhee, and H. Kiyono. 1998. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus-specific immune responses in reproductive tissues. J. Immunol. 161:5952–5958. [PubMed] [Google Scholar]
- 16.Inoue, M., H. Ogawa, M. Miyata, H. Shiozaki, and O. Tanizawa. 1992. Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. Am. J. Clin. Pathol. 98:76–80. [DOI] [PubMed] [Google Scholar]
- 17.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joag, S. V., Z. Li, C. Wang, F. Jia, L. Foresman, I. Adany, D. M. Pinson, E. B. Stephens, and O. Narayan. 1998. Chimeric SHIV that causes CD4+ T cell loss and AIDS in rhesus macaques. J. Med. Primatol. 27:59–64. [DOI] [PubMed] [Google Scholar]
- 19.Karecla, P. I., S. J. Bowden, S. J. Green, and P. J. Kilshaw. 1995. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290β7 (αEβ7). Eur. J. Immunol. 25:852–856. [DOI] [PubMed] [Google Scholar]
- 20.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602–1611. [DOI] [PubMed] [Google Scholar]
- 21.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23–29. [DOI] [PubMed] [Google Scholar]
- 22.Kilshaw, P. J., and K. C. Baker. 1988. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol. Lett. 18:149–154. [DOI] [PubMed] [Google Scholar]
- 23.Klavinskis, L. S., L. A. Bergmeier, L. Gao, E. Mitchell, R. G. Ward, G. Layton, R. Brookes, N. J. Meyers, and T. Lehner. 1996. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J. Immunol. 157:2521–2527. [PubMed] [Google Scholar]
- 24.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127–5133. [PubMed] [Google Scholar]
- 26.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 73:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda, M. J., J. E. Schmitz, A. Seth, R. S. Veazey, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys Blood 96:1474–1479. [PubMed]
- 28.Lackner, A. A. 1994. Pathology of simian immunodeficiency virus induced disease. Curr. Top. Microbiol. Immunol. 188:35–64. [DOI] [PubMed] [Google Scholar]
- 29.Lefrancois, L., C. M. Parker, S. Olson, W. Muller, N. Wagner, M. P. Schon, and L. Puddington. 1999. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehner, T., L. Tao, C. Panagiotidi, L. S. Klavinskis, R. Brookes, L. Hussain, N. Meyers, S. E. Adams, A. J. Gearing, and L. A. Bergmeier. 1994. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J. Virol. 68:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehner, T., Y. Wang, L. Ping, L. Bergmeier, E. Mitchell, M. Cranage, G. Hall, M. Dennis, N. Cook, C. Doyle, and I. Jones. 1999. The effect of route of immunization on mucosal immunity and protection. J. Infect. Dis. 179(Suppl. 3):S489–S492. [DOI] [PubMed] [Google Scholar]
- 32.Letvin, N. L., and N. W. King. 1990. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Acquir. Immune Defic. Syndr. 3:1023–1040. [PubMed] [Google Scholar]
- 33.Lohman, B. L., C. J. Miller, and M. B. McChesney. 1995. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J. Immunol. 155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 34.Ludviksson, B. R., W. Strober, R. Nishikomori, S. K. Hasan, and R. O. Ehrhardt. 1999. Administration of mAb against αEβ7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J. Immunol. 162:4975–4982. [PubMed] [Google Scholar]
- 35.Lundqvist, C., V. Baranov, S. Hammarstrom, L. Athlin, and M. L. Hammarstrom. 1995. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int. Immunol. 7:1473–1487. [DOI] [PubMed] [Google Scholar]
- 36.Ma, Z., F. X. Lu, M. Torten, and C. J. Miller. 2001. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin. Immunol. 100:240–249. [DOI] [PubMed] [Google Scholar]
- 37.Margolis, L., S. Glushakova, C. Chougnet, G. Shearer, P. Markham, M. Robert-Guroff, R. Benveniste, C. J. Miller, M. Cranage, V. Hirsch, and G. Franchini. 2000. Replication of simian immunodeficiency virus (SIV) in ex vivo lymph nodes as a means to assess susceptibility of macaques in vivo. Virology 275:391–397. [DOI] [PubMed] [Google Scholar]
- 38.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattapallil, J. J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72:6421–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250–1257. [DOI] [PubMed] [Google Scholar]
- 41.McChesney, M. B., J. R. Collins, D. Lu, X. Lu, J. Torten, R. L. Ashley, M. W. Cloyd, and C. J. Miller. 1998. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+ T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J. Virol. 72:10029–10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McChesney, M. B., J. R. Collins, and C. J. Miller. 1998. Mucosal phenotype of antiviral cytotoxic T lymphocytes in the vaginal mucosa of SIV-infected rhesus macaques. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S63–S66. [PubMed] [Google Scholar]
- 43.McFarland, R. D., D. C. Douek, R. A. Koup, and L. J. Picker. 2000. Identification of a human recent thymic emigrant phenotype. Proc. Natl. Acad. Sci. USA 97:4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, C. J., M. McChesney, and P. F. Moore. 1992. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab. Investig. 67:628–634. [PubMed] [Google Scholar]
- 45.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540–549. [PubMed] [Google Scholar]
- 45a.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. Vancott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2001. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picker, L. J., L. W. Terstappen, L. S. Rott, P. R. Streeter, H. Stein, and E. C. Butcher. 1990. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J. Immunol. 145:3247–3255. [PubMed] [Google Scholar]
- 47.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, et al. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg, Y. J., A. Cafaro, T. Brennan, J. G. Greenhouse, K. McKinnon, S. Bellah, J. Yalley-Ogunro, S. Gartner, and M. G. Lewis. 1997. Characteristics of the CD8+ lymphocytosis during primary simian immunodeficiency virus infections. AIDS 11:959–968. [DOI] [PubMed] [Google Scholar]
- 49.Rowland-Jones, S. L., and A. McMichael. 1995. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr. Opin. Immunol. 7:448–455. [DOI] [PubMed] [Google Scholar]
- 50.Russell, G. J., C. M. Parker, K. L. Cepek, D. A. Mandelbrot, A. Sood, E. Mizoguchi, E. C. Ebert, M. B. Brenner, and A. K. Bhan. 1994. Distinct structural and functional epitopes of the αEβ7 integrin. Eur. J. Immunol. 24:2832–2841. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. [DOI] [PubMed] [Google Scholar]
- 52.Schneider, T., R. Ullrich, C. Bergs, W. Schmidt, E. O. Riecken, and M. Zeitz. 1994. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin. Exp. Immunol. 95:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schon, M. P., A. Arya, E. A. Murphy, C. M. Adams, U. G. Strauch, W. W. Agace, J. Marsal, J. P. Donohue, H. Her, D. R. Beier, S. Olson, L. Lefrancois, M. B. Brenner, M. J. Grusby, and C. M. Parker. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162:6641–6649. [PubMed] [Google Scholar]
- 54.Schon, M. P., M. Schon, H. B. Warren, J. P. Donohue, and C. M. Parker. 2000. Cutaneous inflammatory disorder in integrin αE (CD103)-deficient mice. J. Immunol. 165:6583–6589. [DOI] [PubMed] [Google Scholar]
- 55.Shacklett, B. L., T. J. Beadle, P. A. Pacheco, J. H. Grendell, P. A. Haslett, A. S. King, G. S. Ogg, P. M. Basuk, and D. F. Nixon. 2000. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology 270:317–327. [DOI] [PubMed] [Google Scholar]
- 56.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevceva, L., A. G. Abimiku, and G. Franchini. 2000. Targeting the mucosa: genetically engineered vaccines and mucosal immune responses. Genes Immun. 1:308–315. [DOI] [PubMed] [Google Scholar]
- 58.Stevceva, L., E. Tryniszewska, Z. Hel, J. Nacsa, B. Kelsall, R. Washington Parks, and G. Franchini. 2001. Differences in time of virus appearance in the blood and virus-specific immune responses in intravenous and intrarectal primary SIVmac251 infection of rhesus macaques; a pilot study. BMC Infect. Dis. 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauch, U. G., R. C. Mueller, X. Y. Li, M. Cernadas, J. M. Higgins, D. G. Binion, and C. M. Parker. 2001. Integrin αE (CD103) β7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J. Immunol. 166:3506–3514. [DOI] [PubMed] [Google Scholar]
- 60.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431. [DOI] [PubMed] [Google Scholar]