Abstract
Among 65 samples obtained from a primate rescue center located in Cameroon, two female adult red-capped mangabeys (Cercocebus torquatus) (CTO-602 and CTO-604), of wild-caught origin, had a peculiar human T-cell lymphotropic virus type 2 (HTLV-2)-like Western blot seroreactivity (p24, RGD21, +/-K55). Analyses of the simian T-cell lymphotropic virus type 3 (STLV-3)/CTO-604 complete proviral sequence (8,919 bp) indicated that this novel strain was highly divergent from HTLV-1 (60% nucleotide similarity), HTLV-2 (62%), or STLV-2 (62%) prototypes. It was, however, related to STLV-3/PH-969 (87%), a divergent STLV strain previously isolated from an Eritrean baboon. The STLV-3/CTO-604 sequence possesses the major open reading frames corresponding to the structural, enzymatic, and regulatory proteins. However, its long terminal repeat is shorter, with only two 21-bp repeats. Furthermore, as demonstrated by reverse transcriptase PCR, this new STLV exhibits significant differences from STLV-3/PH-969 at the mRNA splice junction position level. In all phylogenetic analyses, STLV-3/CTO-604 and STLV-3/PH-969 clustered in a highly supported single clade, indicating an evolutionary lineage independent from primate T-lymphotropic virus type 1 (PTLV-1) and PTLV-2. Nevertheless, the nucleotide divergence between STLV-3/PH-969 and STLV-3/CTO-604 is equivalent to or higher than the divergence observed between the different HTLV-1 or HTLV-2 subtypes. Thus, the STLV-3/CTO-604 strain can be considered the prototype of a second subtype in the PTLV-3 type. The presence of two related viruses in evolutionarily distantly related African monkeys species, living in two opposite ecosystems (rain forest versus desert), reinforces the possible African origin of PTLV and opens new avenues regarding the search for a possible human counterpart of these viruses in individuals exhibiting such HTLV-2-like seroreactivities.
The primate T-lymphotropic viruses (PTLVs), which include human T-cell lymphotropic virus type 1 (HTLV-1) (25), simian T-cell lymphotropic virus type 1 (STLV-1) (22), HTLV-2 (10), STLV-2 (8, 33), and PTLV-L (9), constitute a group of related human and simian retroviruses sharing common biological and molecular features. Nevertheless, their origin and evolutionary relationship, as well as their modes of dissemination, are still unclear and a matter of discussion (5, 6, 11, 26, 29).
Most of the viruses belonging to the PTLV-1 lineage, which comprises HTLV-1 and STLV-1, cannot be separated into distinct phylogenetic lineages according to their species of origin. Their phylogenetic intermixing has been interpreted as evidence for past and recent interspecies transmission episodes (5, 6, 24, 29, 34). However, with regard to the viral transmission from monkeys to humans, this hypothesis is supported by a still-limited but increasing number of observations (13, 14, 17, 35, 37–39). The situation for PTLV-2, which comprises HTLV-2 and STLV-2, is different, since HTLV-2 and STLV-2, while clustering in the same large phylogenetic clade, are distantly related, with no evidence for recent interspecies transmissions (32). Regarding PTLV-L, the only known strain (STLV-L/PH-969) was isolated in 1994 from an Eritrean baboon (Papio hamadryas) kept in a captive colony in Leuven, Belgium. STLV-3/PH-969 (formerly STLV-L/PH-969), which remains the unique prototype of its type, exhibits 40 and 38% divergence at the nucleotide level from HTLV-1 and HTLV-2, respectively (30). The goals of this study were to search for highly divergent PTLV strains among African primates in order to gain new insights into the origin, evolution, and modes of dissemination of such viruses and their hosts.
We report here the isolation, molecular characterization (complete nucleotide sequence), and phylogenetic analysis of a novel STLV subtype infecting two wild-caught red-capped mangabeys (Cercocebus torquatus torquatus) (CTO-604 and CTO-602) originating from southern Cameroon. These viral strains, named STLV-3/CTO-604 and STLV-3/CTO-602, which elicit in their host an HTLV-2-like serology as determined by Western blotting, are genetically highly divergent from the PTLV-1 and PTLV-2 strains and distantly related to STLV-3/PH-969. The finding of this viral subtype in Cameroon greatly enlarges the geographical distribution of this PTLV type in the African continent. In addition, the presence of two highly divergent (compared to PTLV-1 and PTLV-2) but related viruses in two evolutionarily distantly related African monkeys species (i.e., mangabeys and Eritrean baboons) living in two opposite ecosystems (rain forest versus desert) reinforces the possible African origin of PTLV. It also opens new avenues regarding the search for a possible human counterpart of these viruses in individuals exhibiting such HTLV-2-like seroreactivities, especially in the African continent.
MATERIALS AND METHODS
Population of animals studied.
In 1997, blood samples were obtained from 65 monkeys living in a wildlife rescue center located in the southern region of Cameroon. All of these animals were wild-born monkeys originating from different parts of Cameroon (mostly southern), where they were originally kept as pets after their mothers had been killed by hunters (21).
Serological tests.
Serum or plasma samples were screened for the presence of HTLV and STLV antibodies by enzyme-linked immunosorbent assay (Sanofi Diagnostics Pasteur, France) and by particle agglutination (PA) tests (Fujirebio Japan), as well as with an in-house immunofluorescence assay (IFA) using HTLV-1-producing (MT2) or HTLV-2-producing (C19) cell lines (16). IFA and PA tests were also used to determine the titers of HTLV and STLV antibodies. All positive or borderline samples were then tested with a Western blot assay (HTLV 2.4; Diagnostic Biotechnology, Singapore), which contains HTLV-1-purified virions enriched with a gp21 recombinant protein (RGD21) that reacts with sera containing HTLV-1 or HTLV-2 antibodies and two gp46 Env synthetic peptides specific either for HTLV-1 (MTA1) or HTLV-2 (K55) (36).
Cell culture and virus isolation.
Heparinized blood specimens were drawn from the two animals (CTO-602 and CTO-604) identified as having HTLV-2-like seropositivity in the pilot survey and then rushed to our unit, where the peripheral blood mononuclear cells (PBMCs) were separated with Ficoll-Hypaque (Eurobio, Les Ulis, France). Ten million cells were placed in culture in RPMI 1640 medium with 20% heat-inactivated fetal calf serum, 1% L-glutamine, and 1% penicillin-streptomycin. During the subsequent 3 days, the cells were stimulated with phytohemagglutinin (Difco, Detroit, Mich.) at 2 μg/106 cells. The cells were then cultivated in a humidified 5% CO2 atmosphere in the same medium as described above in the presence of 10% interleukin-2 (Boehringer, Mannheim, Germany); the medium was changed twice per week.
Indirect immunofluorescence and antigen detection.
Indirect immunofluorescence was performed on cultured cells in order to detect the expression of viral antigens, using either mouse monoclonal antibodies directed against HTLV-1 p19 or p24 (Cambridge Biotech, Cambridge, Mass.) or human HTLV-1 and HTLV-2 polyclonal sera. Sera from the two animals studied (CTO/604 and CTO/602) were also used.
PCR.
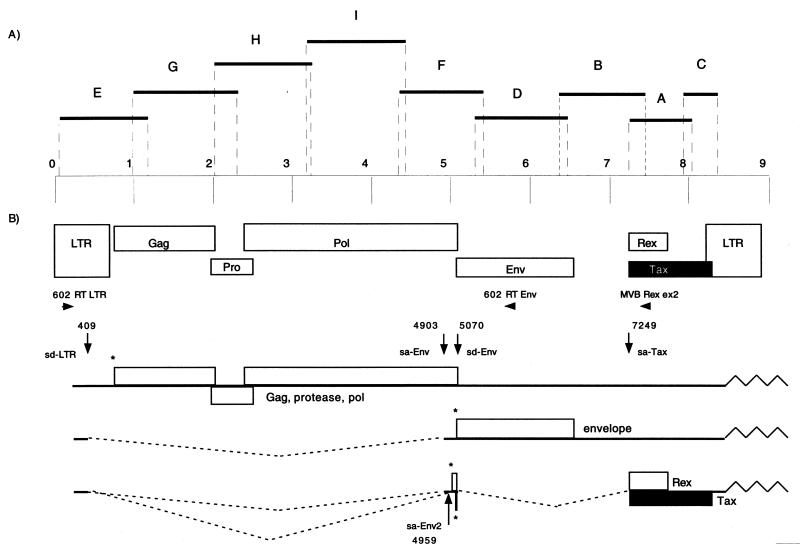
PCR was implemented using previously described conditions (7) on high-molecular-weight DNA extracted from PBMCs before and after 2 months of culture. A first round of PCR was performed with the primer sets GAG1-GAG2 (gag region of HTLV-1), SK110-SK111 (pol region of HTLV-1 and HTLV-2), and KKPX1-KKPX2, SK43-SK44, and TR101-TR102 (tax region of HTLV-1 and HTLV-2) as described previously (17, 21). In order to obtain the complete sequence of this novel isolate, successive PCRs were performed using nine different primers sets designated A to I (Fig. 1). For each set, the first primer was specific for the previously obtained sequence and the second primer was a consensus sequence of all previously known HTLV and STLV prototype strains (Fig. 1 and Table 1). For all of the PCRs, the amplification conditions were as follows: denaturation at 94°C for 9 min and then 40 cycles of 94°C for 30 s, annealing at a primer-specific temperature (Table 1) for 30 s, and extension at 72°C for 30 s per 500 bp. An extension of 10 min was performed after the last cycle. Reaction tubes, prepared in a room physically separate from the laboratory, contained 1 μg of DNA, 0.2 mM deoxynucleoside triphosphate mix (Boehringer), 5 μl of 10× reaction buffer, 1.5 to 2.5 mM MgCl2, and 2.5 U of Taq Gold DNA polymerase (Perkin-Elmer) in a total volume of 50 μl. All PCR products were purified on a 1% agarose gel by gel extraction using the QIAquick gel extraction kit (Qiagen GmbH, Hilden, Germany). Purified DNA was then cloned in the pCR2.1 vector of the TA cloning kit (Invitrogen, Carlsbad, Calif.), sequenced, and verified on both DNA strains.
FIG. 1.
(A) PCR strategy for amplifying the entire STLV-3/CTO-604 proviral genome. The nine proviral fragments which were amplified by PCR, cloned, and sequenced are shown (black bars). (B) Schematic representation of the STLV-3/CTO-604 proviral genome (top) and of the resulting viral messengers (bottom). The start codon used for the translation of the precursor protein (asterisks), the primers used for detection of singly spliced or doubly spliced messengers (horizontal arrows), and the positions and designations of the spliced sites (vertical arrows) identified in the STLV-3/CTO-604 genome are indicated. Nucleotide numbering is according to the STLV-3/CTO604 proviral genome. sa, splice acceptor; sd, splice donor.
TABLE 1.
Sequences of primer sets used for amplifying the complete proviral genome of STLV-3/CTO-604
| Fragment | Sense primer
|
Antisense primer
|
PCR product size (bp) | Optimal annealing temp (°C) | ||
|---|---|---|---|---|---|---|
| Name | Primer sequence (5′→3′)a | Name | Primer sequence (5′→3′)a | |||
| A | KKPX1 | 7249-CCCACTTCCCAGGGTTTGGACAGAGT-7274 | 604 AS 8260 | 8035-TCYTTGGGGCARGGMCCGGAAATC-8012 | 787 | 62 |
| B | 604 S 6617 | 6378-CTCTCMCAATGGGCYCGAGARGCC-6401 | KKPX2 | 7450-TGTAGAGCCGAGCTGACAACG-7430 | 1,073 | 56.2 |
| C | 604 pxS | 7930-CCTGGCACACGGGCCTACTCCC-7951 | 604 LTR AS 134 | 8359-GYAGGGRGARACGTCAGAGCC-8339 | 430 | 54.8 |
| D | 604 env S | 5300-GCCCTACTCCCTRTATSTATTCCC-5323 | 604 env AS 1403 | 6469-CGTATGACACAGGGCCCTACGAC-6447 | 1,170 | 53.3 |
| E | 604 LTR S | 59-GAATCATCCGTCTGAGGGCCG-79 | 604 GAG AS 1 | 1173-CATYTGCCAAGGGCKRTGAGC-1153 | 1,115 | 59.9 |
| F | 604 POL 1S | 4352-CCGAATATCACCTGGCAAGG-4371 | 604 env AS | 5402-GCATTGTATAGCGCAGGGGTC-5382 | 1,051 | 55 |
| G | 604 P19 S | 989-GGGTCCATGAAATAGTAGCCATCC-1012 | 604 PRO 2304 | 2300-GGTTACTGGCTGCCTTCGGAAGGG-2277 | 1,312 | 58.9 |
| H | 604 2015 S | 2011-GGGGAGGACTAACCTGCCACCG-2032 | 604 3324 AS | 3242-GCTGGTGTAGGGAGGAACGGAGG-3220 | 1,232 | 58.5 |
| I | 604 3189 S | 3185-GGGGAACTGCAATGGGTCTCCAAGGGCAC-3213 | 604 4143 AS | 4437-GAGTAAGTATCCACCCATACCAAGAGGC-4410 | 1,253 | 58.1 |
The positions of the primers are given according to the STLV-3/CTO-604 proviral geneme. Y, C or T; R, A or G; M, A or C; K, G or T.
RT-PCR.
Total RNA was extracted with the Rneasy Mini Kit (Qiagen), and 0.5 μg of total RNA was used as a matrix for reverse transcriptase PCR (RT-PCR) according to the instructions with the OneStep RT-PCR kit (Qiagen). The cDNA was amplified with two primer sets: 602 RT LTR (sense, 283-CCTTGCCTACCCCTCCCTCG-303) and either 602 RT Env (antisense, 5761-GAGCGATCGACACAGACC-5743) or 602 MVB Rex ex2 (antisense, 7414-TCCCAGGTAATCTGATGTT-7395) (Fig. 1). Reverse transcription was performed for 30 min at 50°C, followed by a HotStar Taq PCR activation step for 15 min at 94°C (which inactivates the RT). A total of 40 cycles (94°C for 30 s, 53°C for 30 s, and 72°C for 30 s) were performed before a final extension of 10 min at 72°C. The amplified products were purified on a 2% agarose gel and cloned in the pCR2.1 vector from the TA cloning kit (Invitrogen).
Phylogenetic analyses.
Multiple nucleotide and amino acid sequence alignments were performed with the ClustalW algorithm implemented in MacVector 6.5 (Oxford Molecular). The 16 PTLV Tax amino acid sequences (331 amino acids [aa]; MO Tax aa 1 to 331) were aligned using a PAM 250 matrix, and the 16 Env polyprotein PTLV nucleotide sequences were aligned to the ATK Env polyprotein sequence (nucleotides [nt] 1 to 1467). Phylogenetic analyses were performed using the PHYLIP package with two different methods: neighbor joining (NJ) and maximum parsimony (MP). The SEQBOOT program generated 1,000 data sets that are randomly resampled versions of the aligned sequences. A distance matrix was calculated for each data set using the PROTDIST and DNADIST programs with the Kimura two-parameter model, and an empirical transition/transversion ratio for the Env polyprotein (2.23) was used. This ratio was estimated from the data set with the Treepuzzle 5.0 program. The NEIGHBOR program generated a tree for each data set, and a consensus tree was constructed by using the CONSENSE program with the majority-rule criteria. The same data sets were examined with the PROTPARS and DNAPARS programs, based on the MP method, with the same parameters used in NJ to test the robustness of the phylogeny.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences determined in this study are AF391797 for the complete STLV-3/CTO-604 sequence and AF391796 for the complete tax sequence of STLV-3 CTO/602.
RESULTS
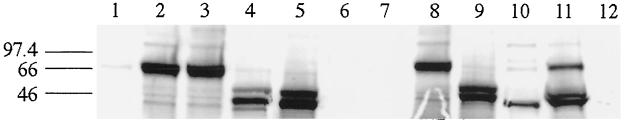
Among the 65 plasma samples obtained from the various monkey species, 9 scored positive with the screening assays (IFA, PA, and enzyme-linked immunosorbent assay), with 7 of them exhibiting a typical HTLV-1 seroreactivity by Western blotting (data not shown and reference 21). Strikingly, two plasma samples had an HTLV-2-like seroreactivity as detected by Western blotting (Fig. 2). One of them exhibited a typical HTLV-2 pattern with strong reactivities against p24, RGD21, and K55, while the second exhibited reactivities only against p24 and RGD21 (Fig. 2). For both animals, these Western blot patterns remained unchanged over a 6-month period. These monkeys, designated CTO-602 and CTO-604, were adult (>7 years old) female red-capped mangabeys (C. torquatus torquatus) which had been kept in the wildlife rescue center since 1994. They were both wild born, originating from southern Cameroon, and were kept initially as pets in the neighborhood before their arrival at the center. The two plasma samples exhibited high titers on MT2 (1/160 and 1/320) and C19 (1/160 and 1/1,280) cells and reacted strongly in the PA assay (1/2,048 and 1/512).
FIG. 2.
Western blot serological patterns of the two C. torquatus animals from Cameroon, using the Western blot from Diagnostic Biotechnology (HTLV blot version 2.4). Lane 1, HTLV-1 positive control; lane 2, HTLV-2 positive control; lane 3, HTLV-1 and -2 negative control; lane 4, plasma from CTO-604; lane 5, plasma from CTO-602.
PBMCs obtained from both animals (CTO-602 and CTO-604) were kept in culture in the presence of interleukin-2 for 2 and 3 months, respectively, with a low growth rate, but no long-term culture could be established. No fluorescent cells could be detected in these cultured cells by IFA using different polyclonal antibodies (HTLV-1, HTLV-2, and plasma of the infected animals) or monoclonal antibodies directed against HTLV-1 p19 and p24 antigens.
In order to confirm the presence of HTLV-related viruses in these two animals, high-molecular-weight DNA, extracted from uncultured and cultured PBMCs, was subjected to PCR screening with gag, pol, and tax primer pairs. The amplified products were analyzed by Southern blotting with HTLV-1-specific (gag, pol, and tax), HTLV-2-specific (pol), or both HTLV-1- and -2-specific (tax) γ-32P-radiolabeled probes. The CTO-602 and CTO-604 DNA samples scored positive only with a highly conserved tax probe (SK45) that can detect both HTLV-1 and HTLV-2. Such data strongly suggested that the two red-capped mangabeys were infected with a highly divergent STLV strain. Using highly conserved primers (KKPX1-MacArc4S) (18), a 433-bp sequence (tax region) was obtained and found to be nearly identical (only one nucleotide difference) for the four uncultured and cultured CTO-602 and CTO-604 samples. Comparison with all of the available PTLV prototype sequences indicated that this 433-bp fragment exhibited a high similarity (95%) to the STLV-3/PH-969 sequence, while it was much more divergent but roughly equidistant from all of the other PTLV prototypes, including HTLV-1 ATK (78%), HTLV-2 MO (77%), and STLV-2 PP1664 (78%).
The complete sequence of one of these two strains (STLV-3/CTO-604) was then obtained by cloning and sequencing nine proviral fragments amplified by successive PCR on cellular DNA from cultured PBMCs of the CTO-604 animal (Fig. 1). The comparison of this complete sequence (8,919 bp) with the other PTLV prototypes indicated that although it was unique, this new strain was more related to STLV-3/PH-969 (87% similarity for the complete sequence) than to HTLV-1 (60%), HTLV-2 (62%), or STLV-2 (62%) prototypes (Table 2). Furthermore, the complete STLV-3/CTO-602 tax cDNA sequence obtained from short-term-cultured PBMCs demonstrated that these two novel strains were almost identical (99.9% for a 1,053-bp tax sequence).
TABLE 2.
Nucleotide sequence comparisons between the PTLV prototype subtype strains and STLV-3/CTO-604
| Comparison | % Similarity
|
||||
|---|---|---|---|---|---|
| General | Gag | Pol | Env | Tax | |
| STLV-3/CTO-604 vs STLV-3/ PH-969 | 87 | 87 | 88 | 85 | 92 |
| STLV-3/CTO-604 vs STLV-2 PP1664 | 62 | 69 | 63 | 66 | 73 |
| STLV-3/CTO-604 vs HTLV-2 MO | 62 | 68 | 63 | 68 | 70 |
| STLV-3/CTO-604 vs HTLV-1 ATK | 60 | 67 | 62 | 64 | 74 |
| HTLV-1 ATK vs HTLV-2 MO | 62 | 69 | 64 | 66 | 71 |
| HTLV-2A MO vs HTLV-2D EFE2 | 92 | 93 | 93 | 93 | 92 |
| HTLV-1A ATK vs HTLV-1C MEL5 | 91 | 92 | 93 | 92 | 92 |
| STLV-2 PP1664 vs STLV-2 PanP | 93 | 93 | 93 | 94 | 91 |
Sequence alignment showed that, despite being quite divergent from them, the overall genetic organization of the new STLV-3/CTO-604 provirus was similar to that of the other PTLVs, with the existence of the major open reading frames (ORFs) that encode the structural Gag (nt 754 to 2022) and envelope (nt 5067 to 6548) polyproteins and the third exon of the regulatory proteins Tax (nt 7249 to 8297) and Rex (nt 7249 to 7734). As described for HTLV-1 and -2, the protease and polymerase proteins of STLV-3/CTO-604 are encoded by two overlapping ORFs (nt 1974 to 2507 and nt 2393 to 5074) translated in a precursor Gag-Pro or Gag-Pro-Pol fusion protein via one or two successive −1 ribosomal frameshifts that align the different ORFs. Indeed, the consensus sequences needed for the frameshift mechanism are highly conserved among PTLVs, as is the slippage sequence 6(A)-8nt-6(G)-11nt-6(C) in the Gag-Pro overlap (27). In the Pro-Pol overlap slippage sequence of STLV-3/CTO-604, only one mutation exists (GTTAAAC instead of TTTAAAC in HTLV-1 and -2), and it does not affect the asparagine codon (AAC) involved in the mechanism (23).
Interestingly, the long terminal repeat (LTR) of STLV-3/CTO-604 has high similarity (86.2%) with the STLV-3/PH-969 LTR and a similar length (694 and 695 bp, respectively). Its overall organization is also identical to the one present in the HTLV-bovine leukemia virus genus, with highly conserved regions (positions of U3-R and R-U5 boundaries, TATA box, polyadenylation site and signal, and potential splice donor site). Interestingly, the STLV-3/CTO-604 LTR was shorter than the HTLV-1 (756 bp) and HTLV-2 (764 bp) LTRs. This was mainly due to the presence of only two 21-bp repeats (the middle and the proximal ones) in the STLV-3/CTO-604 U3 region. This was also previously reported for STLV-3/PH-969 (30).
Although the STLV-3/CTO-604 sequence was related to STLV-3/PH-969, the overall divergence between the two strains in the major ORFs was equivalent to or higher than (8 to 13%) those between the different subtypes of HTLV-1 (A, B, C, and D) or HTLV-2 (A, B, and D) (7 to 9%) (Table 2).
A comparison of the protein sequences of the different PTLV prototypes is given in Table 3. p19, p24, and p15, in analogy to the HTLV-1 proteins, were defined in the 422-aa Gag precursor. The p24 protein is the most conserved among the different types (around 85% similarity) and subtypes (range, 96 to 98%). This is in agreement with the strong reactivities observed against the conformational p24 epitope by Western blotting. The highest divergence among the Gag proteins is observed in p19, with an important deletion in the carboxy-terminal part of the protein in the new STLV-3/CTO-604. Thus, the p19 immunodominant epitope in HTLV-1 ATK (residues 102 to 117, PPSSPTHDPPDSDPQI) shows only 18% amino acid similarity with STLV-3/CTO-604.
TABLE 3.
Protein sequence comparisons between the PTLV prototype subtype strains and STLV-3/CTO-604
| Comparison | % Similarity
|
||||||
|---|---|---|---|---|---|---|---|
| Gag
|
Env
|
Px
|
|||||
| p19 | p24 | POL | SU | TM | Tax | Rex | |
| STLV-3/CTO-604 vs STLV-3/PH-969 | 94 | 96 | 92 | 88 | 98 | 95 | 87 |
| STLV-3/CTO-604 vs STLV-2 PP1664 | 56 | 85 | 63 | 60 | 84 | 73 | 56 |
| STLV-3/CTO-604 vs HTLV-2 MO | 55 | 85 | 64 | 63 | 85 | 73 | 53 |
| STLV-3/CTO-604 vs HTLV-1 ATK | 60 | 85 | 59 | 60 | 72 | 74 | 53 |
| HTLV-1 ATK vs HTLV-2 MO | 58 | 85 | 62 | 62 | 78 | 72 | 56 |
| HTLV-2A MO vs HTLV-2D EFE2 | 96 | 98 | 96 | 95 | 99 | 94 | 91 |
| HTLV-1A ATK vs HTLV-1C MEL5 | 89 | 96 | 95 | 94 | 94 | 91 | 87 |
| STLV-2 PP1664 vs STLV-2 PanP | 95 | 96 | 89 | 94 | 100 | 87 | 86 |
The sequence of the transmembrane (TM) Env protein (178 aa) is highly conserved among the different PTLVs (Table 3). This fits with the Western blot reactivities observed against the recombinant protein GD21 with the two C. torquatus sera. The Env surface (SU) protein (315 aa) is more divergent, but there is no clear correlation with the immunoblot pattern. Indeed, the HTLV-specific peptides K55 (HTLV-2 aa 162 to 205) and MTA-1 (HTLV-1 aa 162 to 209) show 59 and 62.5% similarity with STLV-3/CTO-604, respectively, but only one of the two C. torquatus sera has antibodies that cross-react with K55 on a Western blot.
Although previous studies (30) have shown the existence of four putative ORFs in the pX region of STLV3-PH969, only three alternatively spliced messengers could be detected by RT-PCR. These doubly spliced messengers encoded the Tax and Rex proteins and a putative protein called RORFI. This protein is related at the amino acid level to p12I and p10I of HTLV-1 and HTLV-2, respectively. The sequence analyses of the new STLV-3/CTO-604 and CTO-602 Px regions show only two ORFs that correspond to Tax and Rex ORFs (Table 3). Indeed, regarding the possible equivalent of the STLV-3/PH-969 RORFI sequence in the novel strain, there are three mutations that replace two glutamines (CAG) with stop codons (TAG and TAA), and another mutation in the splice acceptor site replacing AG with AA, that probably eliminate the splice junction.
To gain new insights into the genomic organization of the Px region and to search for the presence of singly or doubly spliced viral messengers previously described for the other HTLV and STLV retroviruses, we performed a series of RT-PCR experiments on the total RNA extracted from a 2-month culture of the CTO-602 PBMCs. RT-PCR using primer pair 602 RT LTR and 602 RT env (Fig. 1) amplified a cDNA (950 bp) that encodes the major Env polyprotein, consisting of exon 1 (splice donor [sd]-LTR at nt 409) spliced directly to exon 2 (splice acceptor [sa]-env at nt 4903). A doubly spliced mRNA (424 bp long) was also detected using primer pair 602 RT LTR and MVB Rex ex2 with a second splicing event between the exon 2 env splice donor (sd-env nt 5070) and exon 3 Tax-Rex splice acceptor (sa-T/R at nt 7249). The splice junction between exon 2 and exon 3 is located at a nucleotide position very similar to that found in STLV-3/PH-969, while the one located between exon 1 and exon 2 occurs 16 nt downstream from the equivalent splice of the STLV-3/PH-969 strain. As previously described for HTLV-1 (3), we found at least one alternative splice acceptor for exon 2 (sa-env2 nt 4959) that could be used to generate the env and all doubly spliced mRNAs.
To detect other potential doubly spliced viral messengers, we hybridized the RT-PCR products with two specific γ-32P-labeled probes, one in the LTR (604 RT 328S) and the other in the Px proximal region (604 RT ORF1). None of the several sequenced clones that doubly hybridized correspond to a new cDNA.
Although the MVB Rex ex2 primer could amplify all of the potential singly and doubly spliced mRNAs, our method probably lacks sensitivity. This results in the preferential amplification of the Tax-Rex doubly spliced mRNA. To improve our RT-PCR sensitivity, we used two primers specific for the proximal Px region: 602 RT-ORFI (antisense, 6900-GCTAAGCTATTGGCGAGAGCG-6880) and 602 RT Px2 (antisense, 7025-GATCAGGTGGACATGCTTCAG-7005). We did not obtain any single or doubly spliced mRNAs.
Phylogenetic analyses were performed with the STLV-3/CTO-604 strain compared to representatives of the PTLV-1 and PTLV-2 subtypes and to STLV-3/PH-969. Due to the high sequence divergence observed between the PTLV sequences in noncoding regions, we aligned both the Tax amino acid sequence and the Env polyprotein nucleotide sequence rather than the complete genomes. Unrooted trees generated three distinct PTLV groups, supported by high bootstrap values (100% by NJ and MP), in both env nucleotide and Tax amino acid analyses (Fig. 3 and 4). Two of them, i.e., PTLV-1 and PTLV-2, comprised human and simian viral strains, while the third one included only the new simian strains (STLV-3/CTO-604 and STLV-3/CTO-602) described in this study and the previously described STLV-L/PH-969. Phylogenetic clustering of all of these strains in three clearly distinct groups strongly suggests a long independent evolution of these viruses.
FIG. 3.
Unrooted phylogenetic tree generated by the NJ method on the Tax amino acid sequences (aa 1 to 331 of the HTLV-2A MO prototype sequence). Bootstrap support (1,000 replicates) for the NJ tree is noted on the branches of the tree. The STLV-3/CTO-604 and STLV-3/CTO-602 sequences were analyzed with HTLV and STLV prototype sequences available from the GenBank database (26). Branch lengths are proportional to the evolutionary distance (scale bar) between the taxa.
FIG. 4.
Unrooted phylogenetic tree generated by the NJ method on the Env polyprotein nucleotide sequence (nt 1 to 1467 of the HTLV-1A ATK prototype sequence). Bootstrap support (1,000 replicates) for the NJ tree is noted on the branches of the tree. The STLV-3/CTO-604 strain was analyzed with HTLV and STLV prototype sequences available from the GenBank database (26). Branch lengths are proportional to the evolutionary distance (scale bar) between the taxa.
DISCUSSION
In this paper, we report the detection, complete nucleotide sequence, and genomic organization of a novel highly divergent STLV strain that is related to but distinct from the STLV-3/PH-969 isolate, the still-unique strain of the PTLV-L type (9, 30, 31). Based not only on sequence comparison analyses but also on the clustering of the strains STLV-3/PH-969, STLV-3/CTO-604, and STLV-3/CTO-602 in a phylogenetically highly supported single clade, it is clear that the two new strains (STLV-3/CTO-604 and STLV-3/CTO-602) represent the second and third isolates of this retroviral type. It is worthwhile to note, however, that the nucleotide divergence between the two strains STLV-3/PH-969 and STLV-3/CTO-604 is equivalent to or even higher than (depending on the gene studied) the divergence observed between different subtypes of HTLV-1 (A, B, C, and D) and HTLV-2 (A, B, and D) (Table 2). Thus, the STLV-3/CTO-604 strain could be considered the prototype of a second subtype of the PTLV-L type.
Originally, this third type of PTLV was provisionally designated L, which stands for Leuven (9). From now on, we propose to designate this third type PTLV-3. Thus, PTLV-3 would provisionally comprise two subtypes designated STLV-3 subtype A, with STLV-3/PH-969 as the prototype, and STLV-3 subtype B, with STLV-3/CTO-604 as the prototype and STLV-3/CTO-602 as a second strain of subtype B.
Recent data (26) based on molecular clock analysis of PTLV evolution, using the third codon position, estimated the evolutionary rate of PTLV to be not higher than 1.67 × 10−6 ± 0.17 × 10−6 nucleotide substitution per site per year. The clock was calibrated by employing the earliest human migration from Asia to Melanesia 60,000 years ago as the lower limit for the node separating HTLV-1 Melanesian subtype C from the other HTLV-1 subtypes. The separation between the ancient African HTLV-2 subtype D and HTLV-2 subtypes A and B was also estimated to occur around 58,000 years ago, and that between the STLV-3/PH969 and PTLV-2 strains was estimated to occur around 1,026,000 ± 110,000 years ago. Assuming that PTLV-3 evolves at the same evolutionary rate as other PTLVs, we tested the molecular clock hypothesis for PTLV (without the IIA and IIB drug user strains) on the third codon position of the Env polyprotein and Env gp21. In both cases the clock is indeed valid, and we calibrated the evolutionary rate at around 1.9 × 10−6 ± 0.1 × 10−6 nucleotide substitution per site per year. Using this value, we estimated that the separation between STLV-3/PH-969 and STLV-3/CTO-604 occurred 200,000 ± 30,000 years ago. These provisional values would probably be modified with the characterization of other PTLV-3 strains.
At the molecular level, HTLV-1 and HTLV-2 promoters consist of three 21-bp repeated elements which contain a core element essential for the LTR transcriptional activation (28). The analysis of the STLV-3/CTO-604 LTR showed a U3 region smaller than that of HTLV-1 or HTLV-2, due to the deletion of the TATA-distal 21-bp repeat. These HTLV-1 and -2 promoter elements play an important role in basal transcription in absence of Tax, but mutating one of them unequally reduces the basal HTLV-1 transcription level (1). Although only two 21-bp repeats seem to be sufficient for a high level of Tax activation in HTLV-1-infected cells (2), the absence of one 21-bp repeat may suggest that the STLV-3/CTO-604 LTR transactivation mediated by Tax could be different than that in HTLV-1 or -2. Recent studies demonstrated that HTLV-1 and HTLV-2 subtype A Tax proteins repress the p53 transcriptional activity differently (19). These data suggest that the transactivations mediated by different PTLV Tax proteins are nonequivalent. Therefore, the potential transformant capacity of STLV-3/CTO-604 Tax is now under investigation.
These two new strains (STLV-3/CTO-604 and CTO-602) were present in two red-capped mangabeys (C. torquatus torquatus), small primates whose habitat is mostly restricted to valley forests and swamps of the western part of Central Africa (12). When we initiated the study, we did not detect any other monkeys (all of wild-caught origin) infected by such a variant strain in the wildlife center studied. Indeed, among the 9 HTLV- or STLV-seropositive monkeys out the 65 animals tested, 7 were infected by a classical African STLV-1 strain (15, 21), while the two remaining HTLV-seropositive animals were CTO-602 and the CTO-604. These data suggest that these novel viruses naturally infect C. torquatus torquatus in the wild. Furthermore, to our knowledge there are no other data reporting HTLV or STLV infection in C. torquatus torquatus.
HTLV-2-like serology (strong p24 associated with RGD21 and low or no p19 seroreactivities) seems to be very rare in African monkeys. It has been reported for only a few Pan paniscus animals infected by STLV-2 strains (8, 33), in rare Papio hamadryas animals infected by STLV-L strains (9), and in one Papio anubis animal (Bab 503) infected with an African STLV-1 strain (15). It is worthwhile to note, however, that most of the seroepidemiological surveys conducted with monkeys cannot be considered representative of the situation in the wild, since most of the samples were obtained from captive animals. The biodiversity of such viruses in the wild, especially in central Africa, is thus far from being known. The recent findings of several new and highly divergent simian immunodeficiency viruses illustrate this fact well (4, 20, 40).
Multiple episodes of interspecies transmission of PTLV-1 (STLV-1 or HTLV-1) have occurred between different primates, including humans, in Central Africa (13, 14, 17, 34). It is thus tempting to speculate that some other STLV-3 strains or related viruses may exist in other monkeys species but also that HTLV strains related to STLV-3 may exist in human populations living in such areas. Searches for a possible human counterpart (possible HTLV-3) using specific consensus or degenerated primers are ongoing, especially in individuals living in the same rain forest areas as monkeys infected by STLV-3 and whose sera exhibit these HTLV-2-like seroreactivities.
Regarding the name of the new PTLV subtype described in this paper, we have tentatively and provisionally named it STLV-3 subtype B. However, among the specialists in the field, a new proposal for PTLV nomenclature is being discussed and debated in order to clarify the situation regarding these new strains and other recent findings (W. M. Switzer, V. Shanmugam, S. Van Dooren, A.-M. Vandamme, V. Bhullar, B. Parekh, and W. Heneine, Abstr. 10th Int. Conf. Hum. Retroviruses [AIDS Res. Hum. Retroviruses, Suppl. 17], abstr. O-26, 2001; S. Van Dooren, X. Pourrut, M. Peeters, E. Delaporte, and A.-.M Vandamme, Abstr. 10th Int. Conf. Hum. Retroviruses [AIDS Res. Hum. Retroviruses, Suppl. 17], abstr. P-045, 2001; T. Takemura, M. Yamashita, M. K. Shimada, T. Shotake, T. Miura, and M. Hayami, Abstr. 10th Int. Conf. Hum. Retroviruses [AIDS Res. Hum. Retroviruses, Suppl. 17], P-Add-10, 2001). When new names are approved by the consensus of such specialist groups, we will, of course, modify the names of these new PTLVs in our papers.
Acknowledgments
This study was financially supported by the Agence Nationale de Recherches contre le SIDA (ANRS). Laurent Meertens is supported by a fellowship from the Ministère de la Recherche, and Renaud Mahieux is supported by a Bourse Roux fellowship (Institut Pasteur).
We acknowledge the Pandrillus (Cameroon/Nigeria) directors, Elizabeth Gadsby and Peter Jenkins, for their great help in obtaining the blood samples studied. We thank Marco Salemi for helpful discussion.
REFERENCES
- 1.Barnhart, M. K., L. M. Connor, and S. J. Marriott. 1997. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J. Virol. 71:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady, J., K. T. Jeang, J. Duvall, and G. Khoury. 1987. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J. Virol. 61:2175–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. K. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 66:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall, K. A. 1996. Multiple interspecies transmissions of human and simian T-cell leukemia/lymphoma virus type I sequences. Mol. Biol. Evol. 13:115–131. [DOI] [PubMed] [Google Scholar]
- 6.Gessain, A., and R. Mahieux. 1999. Genetic diversity and molecular epidemiology of primate T cell lymphotrophic viruses: human T cell leukaemia/lymphoma viruses types 1 and 2 and related simian retroviruses (STLV-1, STLV-2 pan-p and PTLV-L), p. 281–327. In A. Press (ed.), HIV and the new viruses. Harcourt Brace & Company, Publishers, London, United Kingdom.
- 7.Gessain, A., P. Mauclere, A. Froment, M. Biglione, J. Y. Le Hesran, F. Tekaia, J. Millan, and G. de The. 1995. Isolation and molecular characterization of a human T-cell lymphotropic virus type II (HTLV-II), subtype B, from a healthy Pygmy living in a remote area of Cameroon: an ancient origin for HTLV-II in Africa. Proc. Natl. Acad. Sci. USA 92:4041–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giri, A., P. Markham, L. Digilio, G. Hurteau, R. C. Gallo, and G. Franchini. 1994. Isolation of a novel simian T-cell lymphotropic virus from Pan paniscus that is distantly related to the human T-cell leukemia/lymphotropic virus types I and II. J. Virol. 68:8392–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goubau, P., M. Van Brussel, A. M. Vandamme, H. F. Liu, and J. Desmyter. 1994. A primate T-lymphotropic virus, PTLV-L, different from human T-lymphotropic viruses types I and II, in a wild-caught baboon (Papio hamadryas). Proc. Natl. Acad. Sci. USA 91:2848–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyanaraman, V. S., M. G. Sarngadharan, M. Robert-Guroff, I. Miyoshi, D. Golde, and R. C. Gallo. 1982. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 218:571–573. [DOI] [PubMed] [Google Scholar]
- 11.Kelsey, C. R., K. A. Crandall, and A. F. Voevodin. 1999. Different models, different trees: the geographic origin of PTLV-I. Mol. Phylogenet. Evol. 13:336–347. [DOI] [PubMed] [Google Scholar]
- 12.Kingdom, J. 1997. The Kingdom field guide to African mammals. Academic Press, San Diego, Calif.
- 13.Koralnik, I. J., E. Boeri, W. C. Saxinger, A. L. Monico, J. Fullen, A. Gessain, H. G. Guo, R. C. Gallo, P. Markham, V. Kalyanaraman, et al. 1994. Phylogenetic associations of human and simian T-cell leukemia/lymphotropic virus type I strains: evidence for interspecies transmission. J. Virol. 68:2693–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahieux, R., C. Chappey, M. C. Georges-Courbot, G. Dubreuil, P. Mauclere, A. Georges, and A. Gessain. 1998. Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J. Virol. 72:10316–10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahieux, R., C. Chappey, L. Meertens, P. Mauclere, J. Lewis, and A. Gessain. 2000. Molecular characterization and phylogenetic analyses of a new simian T cell lymphotropic virus type 1 in a wild-caught African baboon (Papio anubis) with an indeterminate STLV type 2-like serology. AIDS Res. Hum. Retroviruses 16:2043–2048. [DOI] [PubMed] [Google Scholar]
- 16.Mahieux, R., P. Horal, P. Mauclere, O. Mercereau-Puijalon, M. Guillotte, L. Meertens, E. Murphy, and A. Gessain. 2000. Human T-cell lymphotropic virus type 1 Gag indeterminate Western blot patterns in Central Africa: relationship to Plasmodium falciparum infection. J. Clin. Microbiol. 38:4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahieux, R., J. Pecon-Slattery, G. M. Chen, and A. Gessain. 1998. Evolutionary inferences of novel simian T lymphotropic virus type 1 from wild-caught chacma (Papio ursinus) and olive baboons (Papio anubis). Virology 251:71–84. [DOI] [PubMed] [Google Scholar]
- 18.Mahieux, R., J. Pecon-Slattery, and A. Gessain. 1997. Molecular characterization and phylogenetic analyses of a new, highly divergent simian T-cell lymphotropic virus type 1 (STLV-1marc1) in Macaca arctoides. J. Virol. 71:6253–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahieux, R., C. A. Pise-Masison, P. F. Lambert, C. Nicot, L. De Marchis, A. Gessain, P. Green, W. Hall, and J. N. Brady. 2000. Differences in the ability of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 Tax to inhibit p53 function. J. Virol. 74:6866–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauclere, P. 2000. HIV-1 group N in Cameroon and apparent viruses in the chimpanzee. Bull. Soc. Pathol. Exot. 93:162. [PubMed] [Google Scholar]
- 21.Meertens, L., J. Rigoulet, P. Mauclere, M. Van Beveren, G. M. Chen, O. Diop, G. Dubreuil, M. C. Georges-Goubot, J. L. Berthier, J. Lewis, and A. Gessain. 2001. Molecular and phylogenetic analyses of 16 novel simian T cell leukemia virus type 1 from Africa: close relationship of STLV-1 from Allenopithecus nigroviridis to HTLV-1 subtype B strains. Virology 287:275–285. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi, I., S. Yoshimoto, M. Fujishita, H. Taguchi, I. Kubonishi, K. Niiya, and M. Minezawa. 1982. Natural adult T-cell leukemia virus infection in Japanese monkeys. Lancet ii:658. [DOI] [PubMed] [Google Scholar]
- 23.Nam, S. H., T. D. Copeland, M. Hatanaka, and S. Oroszlan. 1993. Characterization of ribosomal frameshifting for expression of pol gene products of human T-cell leukemia virus type I. J. Virol. 67:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nerrienet, E., L. Meertens, A. Kfutwah, Y. Foupouapouognigni, and A. Gessain. Molecular epidemiology of simian T cell lymphotropic virus in wild-caught monkeys from Cameroon. A new STLV-1, related to HTLV-subtype F, in a Cercocebus agilis. J. Gen. Virol., in press. [DOI] [PubMed]
- 25.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salemi, M., J. Desmyter, and A. M. Vandamme. 2000. Tempo and mode of human and simian T-lymphotropic virus (HTLV/STLV) evolution revealed by analyses of full-genome sequences. Mol. Biol. Evol. 17:374–386. [DOI] [PubMed] [Google Scholar]
- 27.Shimotohno, K., Y. Takahashi, N. Shimizu, T. Gojobori, D. W. Golde, I. S. Chen, M. Miwa, and T. Sugimura. 1985. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. USA 82:3101–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimotohno, K., M. Takano, T. Teruuchi, and M. Miwa. 1986. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc. Natl. Acad. Sci. USA 83:8112–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery, J. P., G. Franchini, and A. Gessain. 1999. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 9:525–540. [PubMed] [Google Scholar]
- 30.Van Brussel, M., P. Goubau, R. Rousseau, J. Desmyter, and A. M. Vandamme. 1997. Complete nucleotide sequence of the new simian T-lymphotropic virus STLV-PH969 from a Hamadryas baboon and unusual features of its long terminal repeat. J. Virol. 71:5464–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Brussel, M., P. Goubau, R. Rousseau, J. Desmyter, and A. M. Vandamme. 1996. The genomic structure of a new simian T-lymphotropic virus, STLV-PH969, differs from that of human T-lymphotropic virus types I and II. J. Gen. Virol. 77:347–358. [DOI] [PubMed] [Google Scholar]
- 32.Van Brussel, M., M. Salemi, H. F. Liu, P. Goubau, J. Desmyter, and A. M. Vandamme. 1999. The discovery of two new divergent STLVs has implications for the evolution and epidemiology of HTLVs. Rev. Med. Virol. 9:155–170. [DOI] [PubMed] [Google Scholar]
- 33.Vandamme, A. M., H. F. Liu, M. Van Brussel, W. De Meurichy, J. Desmyter, and P. Goubau. 1996. The presence of a divergent T-lymphotropic virus in a wild-caught pygmy chimpanzee (Pan paniscus) supports an African origin for the human T-lymphotropic/simian T-lymphotropic group of viruses. J. Gen. Virol. 77:1089–1099. [DOI] [PubMed] [Google Scholar]
- 34.Vandamme, A. M., M. Salemi, and J. Desmyter. 1998. The simian origins of the pathogenic human T-cell lymphotropic virus type I. Trends Microbiol. 6:477–483. [DOI] [PubMed] [Google Scholar]
- 35.Van Dooren, S., M. Salemi, and A. M. Vandamme. 2001. Dating the origin of the African human T-cell lymphotropic virus type-I (HTLV-I) subtypes. Mol. Biol Evol. 18:661–671. [DOI] [PubMed] [Google Scholar]
- 36.Varma, M., D. L. Rudolph, M. Knuchel, W. M. Switzer, K. G. Hadlock, M. Velligan, L. Chan, S. K. Foung, and R. B. Lal. 1995. Enhanced specificity of truncated transmembrane protein for serologic confirmation of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 infections by Western blot (immunoblot) assay containing recombinant envelope glycoproteins. J. Clin. Microbiol. 33:3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voevodin, A. F., B. K. Johnson, E. I. Samilchuk, G. A. Stone, R. Druilhet, W. J. Greer, and C. J. Gibbs, Jr. 1997. Phylogenetic analysis of simian T-lymphotropic virus type I (STLV-I) in common chimpanzees (Pan troglodytes): evidence for interspecies transmission of the virus between chimpanzees and humans in Central Africa. Virology 238:212–220. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe, T., M. Seiki, Y. Hirayama, and M. Yoshida. 1986. Human T-cell leukemia virus type I is a member of the African subtype of simian viruses (STLV). Virology 148:385–388. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe, T., M. Seiki, H. Tsujimoto, I. Miyoshi, M. Hayami, and M. Yoshida. 1985. Sequence homology of the simian retrovirus genome with human T-cell leukemia virus type I. Virology 144:59–65. [DOI] [PubMed] [Google Scholar]
- 40.Yang, C., B. C. Dash, F. Simon, G. van der Groen, D. Pieniazek, F. Gao, B. H. Hahn, and R. B. Lal. 2000. Detection of diverse variants of human immunodeficiency virus-1 groups M, N, and O and simian immunodeficiency viruses from chimpanzees by using generic pol and env primer pairs. J. Infect. Dis. 181:1791–1795. [DOI] [PubMed] [Google Scholar]