Abstract
It is known that both animal and human retroviruses typically cause immunosuppression in their respective hosts, but the mechanisms by which this occurs are poorly understood. The present study uses Friend virus (FV) infections of mice as a model to determine how major histocompatibility complex (MHC) genes influence immunosuppression. Previously, MHC-I genes were shown to influence antibody responses to potent antigenic challenges given during acute FV infection. The mapping of an immune response to an MHC-I gene implicated CD8+ T cells in the mechanism, so we directly tested for their role by using in vivo CD8+ T-cell depletions. Mice resistant to FV-induced immunosuppression became susceptible when they were depleted of CD8+ T cells. Resistance also required gamma interferon (IFN-γ), as in vivo neutralization of IFN-γ converted mice from a resistant to susceptible phenotype. On the other hand, susceptibility to FV-induced immunosuppression was dependent on the immunosuppressive cytokine, interleukin-10 (IL-10), as antibody responses were restored in susceptible mice when IL-10 function was blocked in vivo. Thus, FV-induced immunosuppression of antibody responses involves complex mechanisms controlled at least in part by CD8+ T cells.
Immunosuppression is a common feature of many viral infections. For example, retroviruses, such as human immunodeficiency virus (HIV) and human T-cell leukemia virus type 1 (HTLV-1), often induce severe immunosuppression in infected hosts by mechanisms that are poorly understood (7). It has been hypothesized for HIV that cytotoxic CD8+ T cells (CTL) may destroy virus-infected cells of the immune system (32). Decreases in lymphocyte numbers would then contribute to the inability of the host to respond to foreign antigens. This type of CD8+ T-cell-dependent immunosuppression has been demonstrated with lymphocytic choriomeningitis virus (LCMV)-infected mice, in which virus-specific CD8+ T cells kill infected B cells that produce neutralizing antibodies against LCMV (29). In addition, dendritic cells presenting LCMV antigens can also be destroyed by CD8+ T cells causing suppression of antigen presentation (2, 33). In that case, CD8+ T-cell-induced immunosuppression was ameliorated by depletion of CD8+ T cells in the infected mice (27).
Indirect evidence has implicated a role for CD8+ T cells in Friend retrovirus (FV)-induced immunosuppression, but it is not known whether it is a positive or a negative effect. The association between CD8+ T cells and FV-induced immunosuppression derives from findings that suppression of antibody responses in FV-infected mice maps to the major histocompatibility complex class I (MHC-I) gene region (25), the antigen presentation molecules for CD8+ T cells. However, the mechanism by which CD8+ T cells might influence B-cell responses in this model is not understood. The present studies directly assess the involvement of CD8+ T cells in FV-induced immunosuppression and investigate the role of cytokines in the mechanism.
FV is a complex of two retroviruses: replication-competent Friend murine leukemia virus (F-MuLV), a helper virus that itself is nonpathogenic in adult mice, and replication-defective, but pathogenic, spleen focus-forming virus (SFFV) (10, 20). Coinfection of cells by the two viruses allows SFFV to spread by being packaged into F-MuLV-encoded virus particles. FV infection of susceptible adult mice induces polyclonal proliferation of erythroid precursor cells causing massive splenomegaly. This proliferation is caused by binding of SFFV gp55 envelope glycoproteins to the erythropoietin receptors of nucleated erythroid cells (18, 22). Spleen weights can increase 10 to 20 times normal within the first 2 weeks after infection (16). In susceptible mice that fail to mount protective immune responses, infection eventually leads to fully malignant erythroleukemias (17).
In addition to erythroleukemia, certain strains of mice develop a severe FV-induced immunosuppression characterized by impaired antibody responses to potent antigenic stimuli, such as injections of sheep red blood cells (SRBC) (3, 9, 26). Resistance to FV-induced immunosuppression of the antibody response does not directly correlate with recovery, since some mouse strains are resistant to FV-induced immunosuppression but still die from FV-induced erythroleukemia. It was previously demonstrated that the severity of the suppression of the anti-SRBC response was strongly influenced by the H-2D class I gene region of the MHC. Experiments with MHC recombinant mice showed that mice with at least one b allele at the H-2D region were generally able to mount anti-SRBC responses during FV infection, while mice with only a alleles could not (25). On the other hand, two b alleles at the H-2D region allow for both resistance to immunosuppression and recovery from FV infection. The present experiments focus on FV-induced immunosuppression of the antibody response rather than resistance to FV-induced erythroleukemia, so the term resistance will refer to resistance to immunosuppression, not resistance to FV-induced erythroleukemia.
Since MHC-I gene products function by presenting antigens to CD8+ T cells, the mapping experiments implicated CD8+ T cells in FV-induced immunosuppression but provided no direct evidence. Furthermore, since expression of a single H-2Db allele in H-2a/b heterozygotes imparted protection from severe FV-induced immunosuppression, it appeared that H-2Db-restricted CD8+ T cells had a protective rather than an inhibitory effect on this aspect of Friend disease.
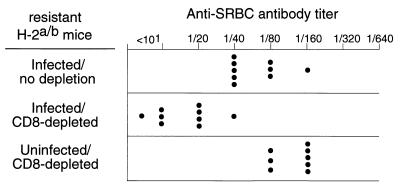
To directly test for CD8+ T-cell effects on FV-induced immunosuppression, resistant H-2a/b mice were depleted of CD8+ T cells by injections of CD8-specific monoclonal antibody (MAb) (6, 15) prior to infection with 1,500 spleen focus-forming units of the polycythemia strain of FV. At 10 days following the last injection of anti-CD8 antibody, splenic CD8+ T cells were reduced to less than 1.5% of the nucleated spleen cells, compared to an average of 14.5% in untreated mice. Some dendritic cells also express CD8, but there was no depletion of CD8+ splenic cells that coexpressed the dendritic cell markers CD11c and MHC-II (data not shown). However, we could not exclude the possibility that dendritic cells bound the anti-CD8 antibodies, which could have altered their function. At 3 weeks postinfection, the groups of mice were injected with SRBC and then tested 1 week later for antibody responses. The CD8-depleted mice had significantly suppressed antibody titers against SRBC compared to untreated, FV-infected control animals (Fig. 1). No suppression of the antibody response was observed in CD8-depleted mice not infected with FV, indicating that CD8+ T-cell depletion alone did not suppress the SRBC response. These results provided direct evidence that CD8+ T cells were important in preventing FV-induced immunosuppression in resistant mice.
FIG. 1.
Effect of CD8+ T-cell depletions on FV-induced immunosuppression. Plasma samples from H-2a/b-resistant (A.BY × B10.A)F1 mice were tested for anti-SRBC antibody titers in plasma at 28 days after infection with 1,500 spleen focus-forming units of FV (26). CD8+ T-cell depletions were performed by five intraperitoneal injections of 0.5 ml of supernatant from clone 169.4 MAb starting at the time of infection as previously described (15). The difference between geometric mean (log2) anti-SRBC titers in infected mice versus infected, CD8-depleted, mice was statistically significant (P < 0.0001 by unpaired t test).
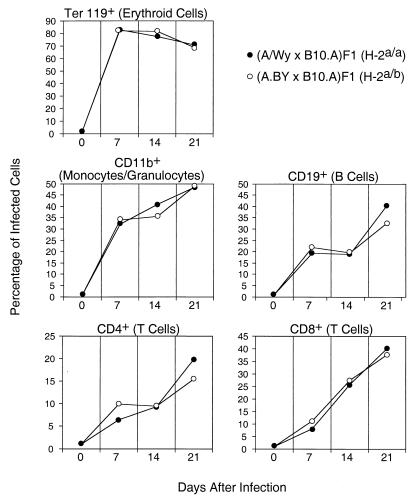
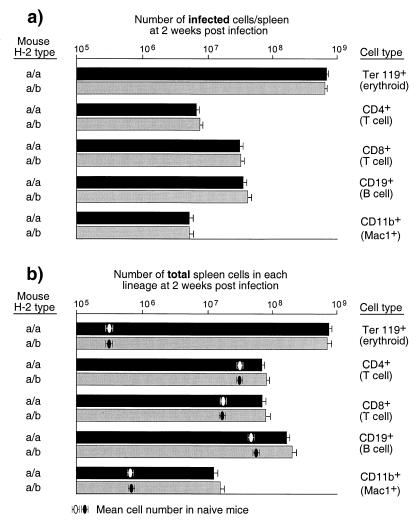
One possible explanation for the involvement of CD8+ T cells was that the CD8+ T cells from the resistant strain were better at killing infected cells than CD8+ T cells from the susceptible strain. If so, then the levels of infection would be lower in resistant H-2a/b mice than in susceptible H-2a/a mice. It might also be possible that there would be differences in the types of cells harboring infection in one strain versus the other. For example, if the susceptible strain had more infected B cells that were then killed by CD8+ T cells, they would be less able to mount antibody responses. To address these issues, we used flow cytometry to analyze spleen cells from resistant H-2a/b mice and susceptible H-2a/a mice that were dually stained for viral antigen and lineage-specific cell surface markers. To detect viral antigen, we used MAb 34 that recognizes the cell surface glycosylated Gag antigen expressed by both F-MuLV and SFFV (4, 5). The analyses were done at weekly intervals between zero and 3 weeks postinfection. As measured by cell surface expression of viral antigen, all subsets of spleen cells were infected by FV to various degrees, and there were no statistically significant differences between the two strains in their relative percentages of infected B cells, T cells, monocytes/granulocytes, or erythroid precursor cells (Fig. 2). Thus, the differential infection of a particular subset in H-2a/a versus H-2a/b mice did not account for differences in FV-induced immunosuppression. Furthermore, there were no significant differences in real numbers of infected cells between the two strains (Fig. 3a).
FIG. 2.
FV infection of different spleen cell subpopulations. Shown are mean percentages of spleen cells of the indicated lineage that stained positive for the expression of FV glycosylated Gag antigen using MAb 34 (5) as previously described (11). Analyses were performed using flow cytometry by gating on populations of cells stained positive for lineage-specific markers as previously described (14). Dead cells were excluded from analysis by propidium iodide staining. Five mice per strain per time point were analyzed, and none of the differences between the resistant and susceptible strains were statistically significant, as determined by Student’s t test (all P > 0.05).
FIG. 3.
FV effects on spleen cell subpopulations. (a) The log10 geometric mean number of infected cells in each splenic subpopulation from H-2a/a susceptible (A/WY × B10.A)F1 mice and from H-2a/b-resistant (A.BY × B10.A)F1 mice was calculated by multiplying the total number of nucleated cells per spleen times the percentage of MAb 34 positive cells of each lineage divided by 100. (b) The log10 geometric mean number of total cells of each subpopulation was calculated by multiplying the total number of nucleated cells per spleen times the percentage of lineage positive cells divided by 100. All mice were infected with 1,500 spleen focus-forming units of FV 14 days prior to analysis. The error bars represent standard errors of the mean. There were no statistical differences between the two mouse strains in either number of infected cells or total cells in each lineage when comparing the log10 geometric means by the Mann-Whitney statistical test (all P > 0.05). Results are from the same mice shown in Fig. 2.
The flow cytometric data were also analyzed to determine if FV infection differentially altered the numbers of various splenic cell subsets in the two mouse strains. Infection with FV in both strains was associated with a dramatic increase in cells of the erythroid lineage (Ter 119+), which expanded 1,000-fold by 2 weeks postinfection (Fig. 3b). The lymphoid and myeloid cells in the spleen also proliferated in response to FV infection, albeit to a much lesser extent. Proliferation of those cells likely reflects antiviral immune responses in the mice. There were no statistically significant differences between the numbers of cells in the two strains of mice for any of the cell subsets analyzed (Fig. 3b). Thus, the suppressed antibody responses in the H-2a/a mice were not due to greater overall losses of B cells than in the H-2a/b mice.
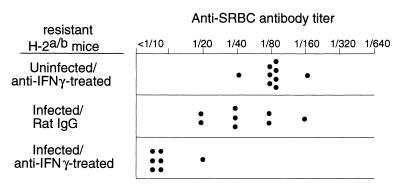
Another important function of CD8+ T cells that could potentially affect antibody responses during FV infections is the production of gamma interferon (IFN-γ) (8). Previous experiments indicated that a mutation in the H-2Db class I gene affected IFN-γ responses during FV infection (28). Thus, production of IFN-γ in the FV model is linked to the same gene as immunosuppression. To test for possible effects of IFN-γ on the anti-SRBC response in resistant H-2a/b mice, IFN-γ was neutralized by injections of anti-IFN-γ antibodies for 3 weeks post FV infection. The mice were then challenged with SRBC, and their sera were analyzed for anti-SRBC antibody titers. The neutralization of IFN-γ in infected animals significantly reduced anti-SRBC antibody titers in comparison to those of the control groups (Fig. 4). Thus, the IFN-γ response in resistant animals was an essential component in their protection from FV-induced immunosuppression.
FIG. 4.
Effects of IFN-γ on FV-induced immunosuppression. Anti-SRBC antibody titers at 28 days post FV challenge were determined for resistant H-2a/b (A.BY × B10.A)F1 mice in which IFN-γ was neutralized by intraperitoneal injections of rat MAb XMG1.2 for 3 weeks as previously described (19). The difference between the log2 geometric mean titers of infected, rat immunoglobulin G-treated control mice (5.6) versus infected, anti-IFNγ-treated mice (2.6) was statistically significant by the unpaired t test (P < 0.0001).
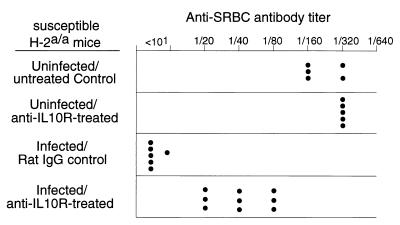
One possible explanation for the influence of IFN-γ production on the antibody response is an indirect effect, such as through CD4+ T-helper responses as has been shown in the Leishmania model (13). One CD4+ T-cell cytokine that has strong immunosuppressive effects and is upregulated in the absence of IFN-γ is IL-10. To determine if IL-10 played a role in the suppression of anti-SRBC responses in susceptible H-2a/a mice, IL-10 function was blocked in vivo by injections of anti-IL-10R antibody for 3 weeks following infection. The mice responded with significantly greater anti-SRBC responses than the control group, indicating that IL-10 was involved in the mechanism of FV-induced immunosuppression (Fig. 5).
FIG. 5.
Effects of anti-IL-10R antibody on FV-induced immunosuppression. IL-10 function was blocked in susceptible H-2a/a (A/WY × B10.A)F1 mice by intraperitoneal injections of anti-IL-10R rat MAb IB1.3a every 3 days for the first 4 weeks of infection (a gift from Robert Coffman, DNAX, Palo Alto, Calif.) (1). Anti-SRBC antibody titers were determined at 28 days postinfection. The difference between the log2 geometric mean titers of infected, rat immunoglobulin G-treated control mice (2.5) versus infected, anti-IL-10R antibody-treated mice (5.3) was statistically significant by the unpaired t test (P < 0.0001).
In conclusion, the data indicate that the influence of MHC-I genes on immunosuppression of the antibody response occurs through CD8+ T cells. The results show that the mechanism of FV-induced immunosuppression involves IL-10 and that protection from FV-induced immunosuppression requires IFN-γ. Although the in vivo relationship between the CD8+ T-cell response and the cytokine responses is not yet clear, the results suggest an indirect mechanism that may operate through CD4+ T cells.
Several other mechanisms of immunosuppression by murine retroviruses have been described, often involving alterations in the function of antigen presenting cells or alterations in cytokine secretion profiles (8, 9, 14, 23, 25). The immunosuppression that we have investigated in the present studies may be related to these other models, especially at the level where cytokine secretion is involved. However, it is a unique finding that CD8+ T cells play a role in preventing FV-induced suppression of an antibody response. Until now, T cells have mainly been described as mediators of retrovirus-induced immunosuppression, such as “veto cells” that inactivate or kill CD8+ T cells (30) or activation-induced cell death of CD4+ T cells (31). In only one retrovirus model, which is the infection of mice with a retrovirus mixture termed LP-BM5 MuLV, T cells were associated with the resistance of mice to virus-induced immunosuppression (23, 24). In this model, virus infection leads to a severe immunodeficiency in susceptible mice, which is why the disease is often called murine AIDS (MAIDS). Similar to our findings in the Friend model, resistance against MAIDS is mediated by CD8+ T cells and depletion of these cells converts the phenotype of mice from resistant to susceptible. However, in the MAIDS model, resistance to immunosuppression was associated with restriction of virus replication due to virus-specific CD8 cells (23, 24) rather then by an indirect effect of these cells as we think it occurs in the Friend model. Obviously, murine retroviruses can cause immunosuppression through numerous mechanisms involving T cells, and the elucidation of these mechanisms has led to effective treatments, such as the administration of IL-12 in model systems (12, 21). Our present finding that blocking the IL-10 receptor can also partially reverse immunosuppression provides another avenue of potential therapy.
Acknowledgments
We are grateful to Bruce Chesebro, Sue Priola, and Leonard Evans for critical review of the manuscript and to Robert Coffman for the anti-IL-10R antibody.
U.D. is supported by the Deutsche Krebsforschungszentrum Heidelberg, Nachwuchsfoerderprogramm Infektiologie. Part of the work was supported by a grant to U.D. from the Deutsche Forschungsgemainschaft (Di 714/3-1).
REFERENCES
- 1.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceglowski, W. S., and H. Friedman. 1968. Immunosuppression by leukemia viruses. I. Effect of Friend disease virus on cellular and humoral hemolysin responses of mice to a primary immunization with sheep erythrocytes. J. Immunol. 101:594–604. [PubMed] [Google Scholar]
- 4.Chesebro, B., W. Britt, L. Evans, K. Wehrly, J. Nishio, and M. Cloyd. 1983. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology 127:134–148. [DOI] [PubMed] [Google Scholar]
- 5.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 112:131–144. [DOI] [PubMed] [Google Scholar]
- 6.Cobbold, S. P., A. Jayasuriya, A. Nash, T. D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 312:548–551. [DOI] [PubMed] [Google Scholar]
- 7.Denner, J. 1998. Immunosuppression by retroviruses: implications for xenotransplantation. Ann. N. Y. Acad. Sci. 862:75–86. [DOI] [PubMed] [Google Scholar]
- 8.Dittmer, U., K. E. Peterson, R. Messer, I. M. Stromnes, B. Race, and K. J. Hasenkrug. 2001. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 75:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman, H., S. Specter, and M. Bendinelli. 1983. Influence of viruses on cells of the immune response system. Adv. Exp. Med. Biol. 162:463–474. [DOI] [PubMed] [Google Scholar]
- 10.Friend, C. 1957. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J. Exp. Med. 105:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujisawa, R., F. J. McAtee, J. H. Zirbel, and J. L. Portis. 1997. Characterization of glycosylated Gag expressed by a neurovirulent murine leukemia virus: identification of differences in processing in vitro and in vivo. J. Virol. 71:5355–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., N. A. Giese, and H. C. Morse III. 1994. In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS). J. Exp. Med. 180:2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, D. J. Fowell, R. M. Locksley, J. T. Chang, C. Y. Wu, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915–924. [DOI] [PubMed] [Google Scholar]
- 14.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasenkrug, K. J., D. M. Brooks, J. Nishio, and B. Chesebro. 1996. Differing T-cell requirements for recombinant retrovirus vaccines. J. Virol. 70:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasenkrug, K. J., D. M. Brooks, M. N. Robertson, R. V. Srinivas, and B. Chesebro. 1998. Immunoprotective determinants in Friend murine leukemia virus envelop protein. Virology 248:66–73. [DOI] [PubMed] [Google Scholar]
- 17.Hasenkrug, K. J., and B. Chesebro. 1997. Immunity to retroviral infection: the Friend virus model. Proc. Natl. Acad. Sci. USA 94:7811–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoatlin, M. E., and D. Kabat. 1995. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 3:51–57. [DOI] [PubMed] [Google Scholar]
- 19.Iwashiro, M., K. Peterson, R. J. Messer, I. M. Stromnes, and K. J. Hasenkrug. 2001. CD4+ T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J. Virol. 75:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1–42. [DOI] [PubMed] [Google Scholar]
- 21.Kelleher, P., N. J. Williams, and S. C. Knight. 1999. Interleukin-12 administration in retroviral infection of mice increases the potential to produce functional dendritic cells from bone marrow stem cells. Immunol. Lett. 65:51–54. [DOI] [PubMed] [Google Scholar]
- 22.Li, J.-P., A. D. D’Andrea, H. F. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature (London) 343:762–764. [DOI] [PubMed] [Google Scholar]
- 23.Makino, M., S. K. Chattopadhyay, J. W. Hartley, and H. C. Morse III. 1992. Analysis of role of CD8+ T cells in resistance to murine AIDS in A/J mice. J. Immunol. 149:1702–1706. [PubMed] [Google Scholar]
- 24.Mayrand, S. M., P. A. Healy, B. E. Torbett, and W. R. Green. 2000. Anti-Gag cytolytic T lymphocytes specific for an alternative translational reading frame-derived epitope and resistance versus susceptibility to retrovirus-induced murine AIDS in F1 mice. Virology 272:438–449. [DOI] [PubMed] [Google Scholar]
- 25.Morrison, R. P., P. L. Earl, J. Nishio, D. L. Lodmell, B. Moss, and B. Chesebro. 1987. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature (London) 329:729–732. [DOI] [PubMed] [Google Scholar]
- 26.Morrison, R. P., J. Nishio, and B. Chesebro. 1986. Influence of the murine MHC (H-2) on Friend leukemia virus-induced immunosuppression. J. Exp. Med. 163:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskophidis, D., H. Pircher, I. Ciernik, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1992. Suppression of virus-specific antibody production by CD8+ class I-restricted antiviral cytotoxic T cells in vivo. J. Virol. 66:3661–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson, K. E., M. Iwashiro, K. J. Hasenkrug, and B. Chesebro. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from Friend retrovirus-induced leukemia. J. Virol. 74:5363–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planz, O., P. Seiler, H. Hengartner, and R. M. Zinkernagel. 1996. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies. Nature (London) 382:726–729. [DOI] [PubMed] [Google Scholar]
- 30.Rich, R. F., and W. R. Green. 2000. Inhibition of antiviral CTL responses by virus-infected cells: line item veto (cells) revisited. Virology 272:237–243. [DOI] [PubMed] [Google Scholar]
- 31.Saha, K., P. H. Yuen, and P. K. Wong. 1994. Murine retrovirus-induced depletion of T cells is mediated through activation-induced death by apoptosis. J. Virol. 68:2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinkernagel, R. M., and H. Hengartner. 1994. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol. Today 15:262–268. [DOI] [PubMed] [Google Scholar]
- 33.Zinkernagel, R. M., O. Planz, S. Ehl, M. Battegay, B. Odermatt, P. Klenerman, and H. Hengartner. 1999. General and specific immunosuppression caused by antiviral T-cell responses. Immunol Rev. 168:305–315. [DOI] [PubMed] [Google Scholar]