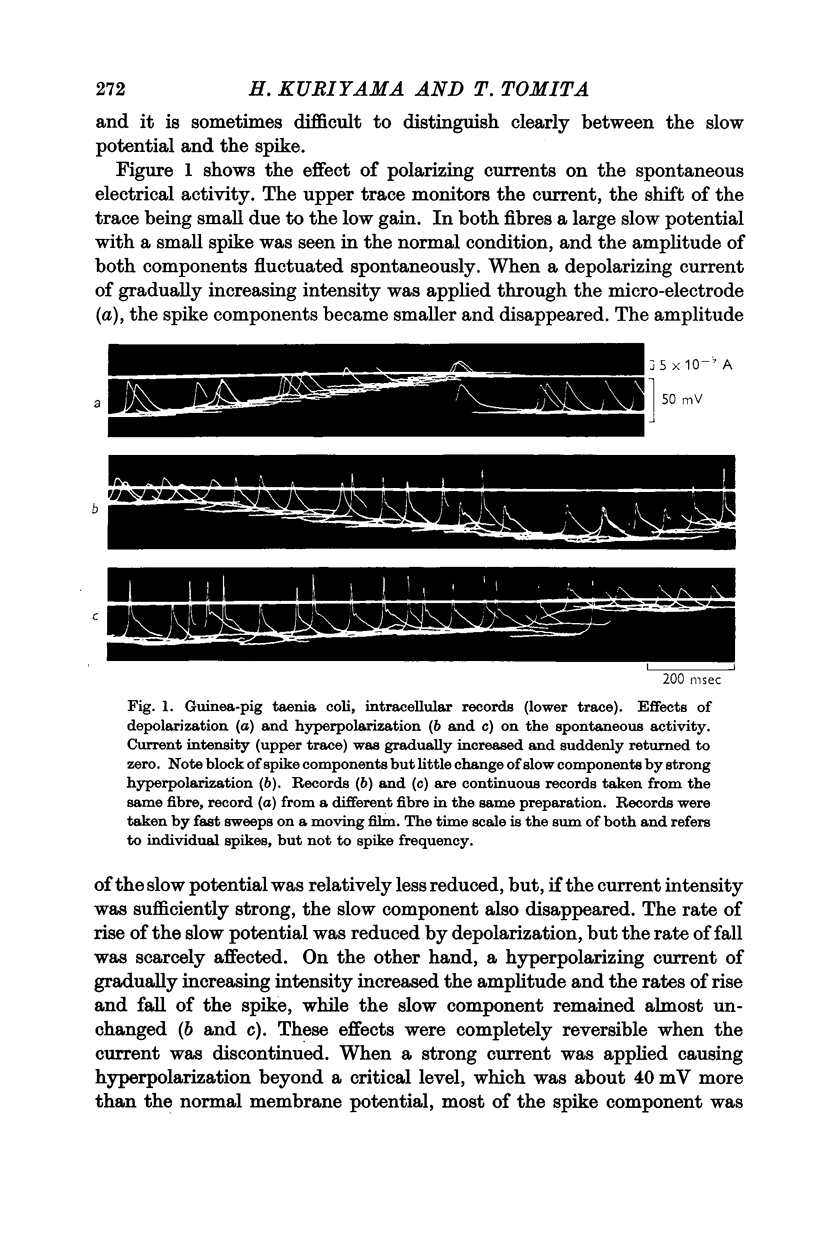
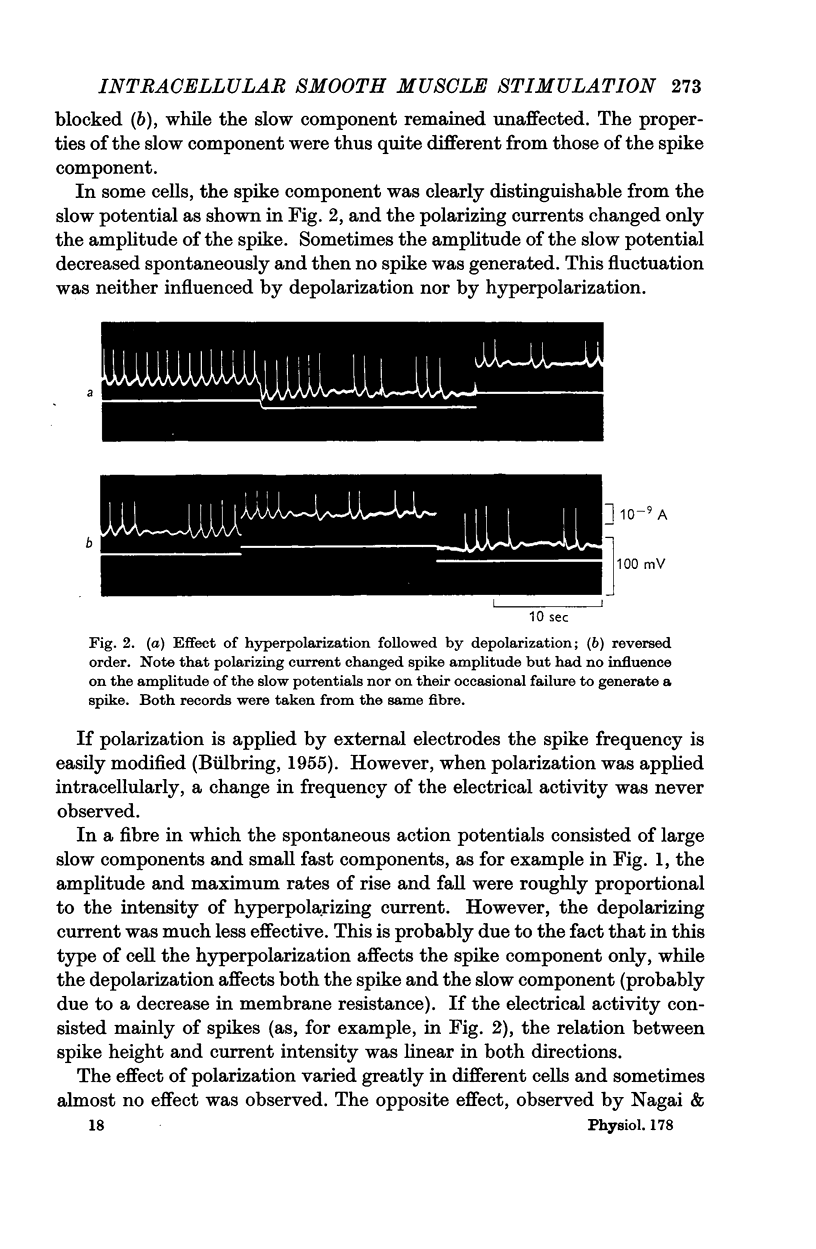
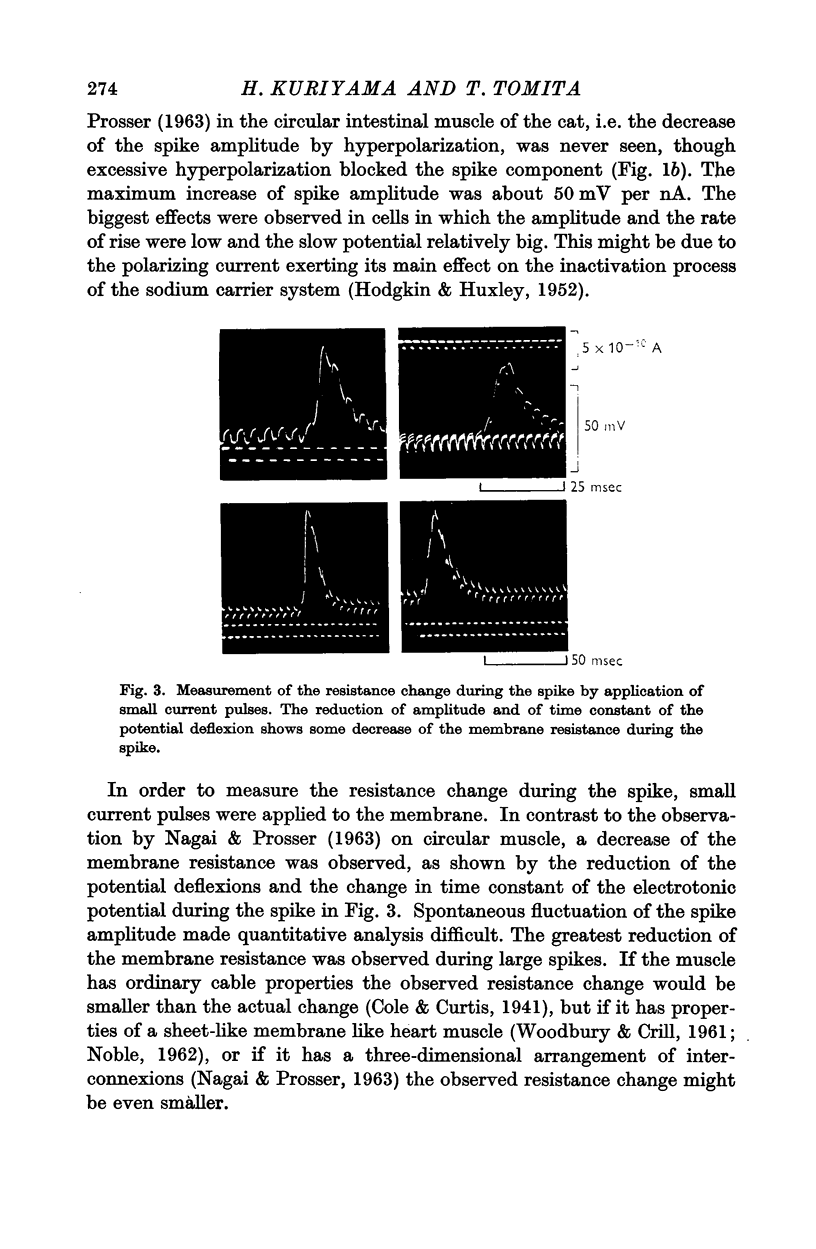
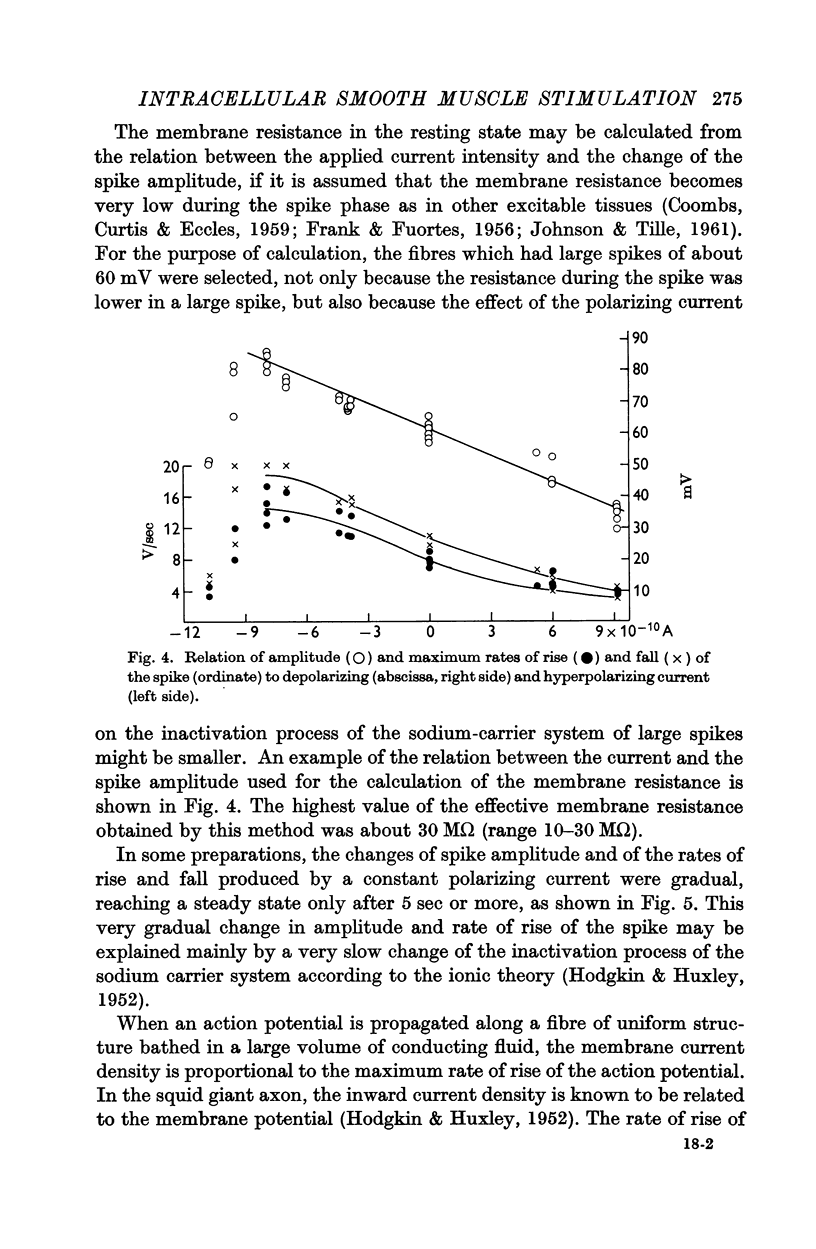
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Electrical activity in intestinal smooth muscle. Physiol Rev Suppl. 1962 Jul;5:160–178. [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The electrical constants of the motoneurone membrane. J Physiol. 1959 Mar 12;145(3):505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL E. E., SINGH H. The electrical properties of the smooth muscle cell membrane. Can J Biochem Physiol. 1958 Sep;36(9):959–975. [PubMed] [Google Scholar]
- EDWARDS C., OTTOSON D. The site of impulse initiation in a nerve cell of a crustacean stretch receptor. J Physiol. 1958 Aug 29;143(1):138–148. doi: 10.1113/jphysiol.1958.sp006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, FRANK K. Extracellular potentials from single spinal motoneurons. J Gen Physiol. 1959 Mar 20;42(4):749–760. doi: 10.1085/jgp.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H. Ionic mechanisms in electrogenesis. Ann N Y Acad Sci. 1961 Sep 6;94:405–457. doi: 10.1111/j.1749-6632.1961.tb35554.x. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. A., TILLE J. Investigations of the electrical properties of cardiac muscle fibres with the aid of intracellular double-barrelled electrodes. J Gen Physiol. 1961 Jan;44:443–467. doi: 10.1085/jgp.44.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K., KOYAMA I. Membrane responses of frog's spinal ganglion cells in calcium-free solutions. J Physiol. 1962 Aug;163:1–12. doi: 10.1113/jphysiol.1962.sp006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W. Excitation of the squid axon membrane in isosmotic potassium chloride. Nature. 1959 Jan 24;183(4656):265–266. doi: 10.1038/183265b0. [DOI] [PubMed] [Google Scholar]
- NAGAI T., PROSSER C. L. Electrical parameters of smooth muscle cells. Am J Physiol. 1963 May;204:915–924. doi: 10.1152/ajplegacy.1963.204.5.915. [DOI] [PubMed] [Google Scholar]
- NOBLE D. The voltage dependence of the cardiac membrane conductance. Biophys J. 1962 Sep;2:381–393. doi: 10.1016/s0006-3495(62)86862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROSSER C. L., BURNSTOCK G., KAHN J. Conduction in smooth muscle: comparative structural properties. Am J Physiol. 1960 Sep;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- REUBEN J. P., WERMAN R., GRUNDFEST H. The ionic mechanisms of hyperpolarizing responses in lobster muscle fibers. J Gen Physiol. 1961 Nov;45:243–265. doi: 10.1085/jgp.45.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL J. R. An anodal threshold phenomenon in the squid giant axon. Nature. 1958 Nov 15;182(4646):1370–1370. doi: 10.1038/1821370a0. [DOI] [PubMed] [Google Scholar]
- SPERELAKIS N., LEHMKUHL D. EFFECT OF CURRENT ON TRANSMEMBRANE POTENTIALS IN CULTURED CHICK HEART CELLS. J Gen Physiol. 1964 May;47:895–927. doi: 10.1085/jgp.47.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMPFLI R. Die Strom-Spannungs-Charakteristik der erregbaren Membran eines einzelnen Schnürrings und ihre Abhängigkeit von der Ionenkonzentration. Helv Physiol Pharmacol Acta. 1958;16(2):127–145. [PubMed] [Google Scholar]
- TASAKI I. Demonstration of two stable states of the nerve membrane in potassium-rich media. J Physiol. 1959 Oct;148:306–331. doi: 10.1113/jphysiol.1959.sp006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA T., SAIMI T., TOIDA N. Repetitive hyperpolarizing response of the nerve fibre of crayfish. Nature. 1961 Apr 15;190:271–272. doi: 10.1038/190271a0. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G. On the mechanism of spontaneous impulse generation in the pacemaker of the heart. J Gen Physiol. 1961 Nov;45:317–330. doi: 10.1085/jgp.45.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT E. B., TOMITA T. Separation of sodium and potassium ion carrier systems in crustacean motor axon. Am J Physiol. 1962 May;202:856–864. doi: 10.1152/ajplegacy.1962.202.5.856. [DOI] [PubMed] [Google Scholar]


