Abstract
Uracil DNA glycosylase (UDG), a highly conserved DNA repair enzyme, excises uracil from DNA. Crystal structures of several UDGs have identified residues important for their exquisite specificity in detection and removal of uracil. Of these, Y66 and N123 in Escherichia coli UDG have been proposed to restrict the entry of non-uracil residues into the active site pocket. In this study, we show that the uracil excision activity of the Y66F mutant was similar to that of the wild-type protein, whereas the activities of the other mutants (Y66C, Y66S, N123D, N123E and N123Q) were compromised ∼1000-fold. The latter class of mutants showed an increased dependence on the substrate chain length and suggested the existence of long-range interactions of the substrate with UDG. Investigation of the phosphate interactions by the ethylation interference assay reaffirmed the key importance of the –1, +1 and +2 phosphates (with respect to the scissile uracil) to the enzyme activity. Interestingly, this assay also revealed an additional interference at the –5 position phosphate, whose presence in the substrate had a positive effect on substrate utilisation by the mutants that do not possess a full complement of interactions in the active site pocket. Such long-range interactions may be crucial even for the wild-type enzyme under in vivo conditions. Further, our results suggest that the role of Y66 and N123 in UDG is not restricted merely to preventing the entry of non-uracil residues. We discuss their additional roles in conferring stability to the transition state enzyme–substrate complex and/or enhancing the leaving group quality of the uracilate anion during catalysis.
INTRODUCTION
Uracil arises in DNA by spontaneous deamination of an inherently unstable base, cytosine, and results in the appearance of G:U mismatches in the genome (1,2). In addition, during DNA replication, DNA polymerases may also erroneously incorporate dUMP opposite adenosine (as an A:U base pair) (2). The G:U mismatches are promutagenic and, if left unrepaired, lead to GC→AT mutations. On the other hand, the occurrence of an A:U base pair in DNA regulatory sequences can impede recognition by the cognate DNA-binding proteins. Therefore, to maintain genomic integrity and safeguard the physiological functions, the cells possess a highly efficient and ubiquitous base excision repair enzyme, uracil DNA glycosylase (UDG) (1). UDGs have pride of place amongst DNA repair enzymes in having a notably high turnover number and strict specificity in admitting only uracil in DNA into the active site pocket. The UDGs also interact with a number of proteins such as the Bacillus subtilis phage-encoded uracil DNA glycosylase inhibitor Ugi and cellular factors such as single-stranded DNA-binding proteins, involved in various vital processes such as DNA replication, repair and recombination (3–5). Subsequently, it was shown that UDGs interact with proliferating cell nuclear antigen (PCNA) and exist as part of a multiprotein complex involving PCNA, polymerase δ, FEN1 and DNA ligase I at the replication foci (6). These observations demonstrate the importance of uracil excision repair in the regions of the genome that become transiently single stranded. Thus, UDGs constitute a remarkably interesting model system to understand the basis of catalytic prowess and specificity associated with protein–DNA and protein– protein interactions.
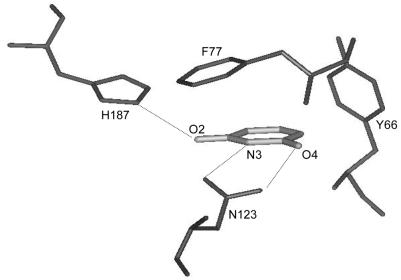
UDGs belong to a highly conserved class of proteins (7,8) and their crystal structures have shown an extraordinary conservation of the overall architecture and active site geometry. The uracil binds in the active site pocket by extensive shape and electrostatic complementarity (8,9). Several hydrogen bonds are established between the conserved UDG residues such as the histidine of the HPSPLS motif [H187 in Escherichia coli UDG (EcoUDG)] and the asparagine of the GVLLLN motif (N123 in EcoUDG) and the 2, 3 and 4 positions of uracil. N123 forms hydrogen bonds with O4 and N3 of uracil, thus effectively restricting cytosine from entering the pocket (10–15). Also, the side chain of the tyrosine of the GQDPYH motif (Y66 in EcoUDG), which is in Van der Waals contact with the C5 position of the uracil ring, excludes thymine, with a methyl group at this position, and the purines, with bulky rings (Fig. 1). In keeping with the structural determinations, the Y147A and N204D mutants (equivalent to Y66 and N123 of EcoUDG) conferred detectable thymine DNA glycosylase (TDG) and cytosine DNA glycosylase (CDG) activities, respectively, to the human UDG (11). As expected, these mutants caused cytotoxicity and mutator phenotype to the cell. Interestingly, the mutants still retained ∼0.1% of the original UDG activity, which was more prominent than the newly acquired CDG and TDG activities. Also, a recent study on Pyrobaculum aerophilum UDG (16) suggested that the elements that determine the strict substrate specificity for uracil may be more complex, and warrants mutational analyses of these residues in other UDGs.
Figure 1.
Uracil specificity pocket showing the complementarity of the interactions between the uracil residue and the side chains of Y66, N123 and H187 in the active site pocket of EcoUDG. The drawing was generated by the INSIGHT II package using coordinates from the EcoUDG crystal structure (1FLZ; 40).
In the present study we have carried out mutational analysis of the Y66 and N123 positions in EcoUDG by using a novel in vitro transcription–translation approach for their production. The mutants are highly compromised in their uracil excision activity, and suggest that the roles of these residues in uracil excision are more crucial and significant than just restricting entry of non-uracil bases to the active site pocket. Furthermore, the mutants show an increased dependence on the substrate chain length, suggesting the existence of long-range interactions between the substrate and the enzyme.
MATERIALS AND METHODS
DNA oligomers and 5′-end-labelling
DNA oligomers (SSU4, 5′-AGCUCATAGTTTACCTGAAGAATAT-3′; SSU5, 5′-GAGCUCTGAGGATCCTUTTGGATCCT-3′; SSU9, 5′-CTCAAGTGUAGGCATGCAAGA GCT-3′) were obtained from Ransom Hill Bioscience (USA), 5′-32P-end-labelled and purified on Sephadex G 50 minicolumns (17). For UDG reactions, DNA oligomers were mixed with the radiolabelled counterparts to obtain the desired specific activities.
Generation of the constructs used for in vitro transcription–translation of EcoUDG
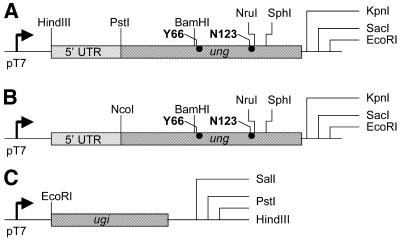
Two pTZ19R-derived laboratory vectors containing the 5′ untranslated region (5′-GGCTGTTGGGGTCCACCACGCCTTCCACCTGCCCCACTGCTTCTTCGCTTCTCTCTTGGAAAGTCCAGTCTCTCCTCGGCTTGCAGGGC-3′) of the human metallothionein-IF gene (18) ligated into the blunt ended SphI (pMTZ1) or HindIII (pMTZ2) site were used for further constructions. The pMTZ2 construct also contained a NcoI site and an ATG codon in a good Kozak context (GGCAACCATGGC). The E.coli ung gene ORF, whose ATG is naturally located within a good Kozak context was cloned as an AluI (position 501, from a limit digest)–EcoRI fragment from pTZUng2 (19) between the HincII and EcoRI sites of pMTZ1, and termed pMTZUng. This construct contains the complete UDG ORF downstream of a T7 RNA polymerase promoter (Fig. 2A).
Figure 2.
Diagrammatic sketches of constructs used for in vitro production of UDG and Ugi in RRLs or S30 lysates. (A) Wild-type, Y66C and N123D. (B) N123E, N123Q, Y66F and Y66S. Diagrammatic sketches of constructs used for in vitro production of Ugi (C). See Materials and Methods for details.
To introduce mutations at the Y66 and N123 positions, the HindIII–EcoRI fragment from pTZUng4S (19) harbouring the relevant portion of the ung gene was subcloned (20) into pTZ18R to yield pTZUng4S′. Single-stranded DNA was prepared from pTZUng4S′ and used for site-directed mutagenesis by a modified Kunkel protocol (21). The mutagenic oligomers containing limited randomisation at one or two positions, 5′-CAGGATCCTT(T/C/G)TCACGGACCG-3′ and 5′-CTGCTACTC(C/G)A(C/G)ACTGTGTTG-3′, were used to generate a limited spectrum of mutations at the Y66 and N123 positions, respectively. DNA sequence analysis identified the presence of the Y66S, Y66C, Y66F, N123E and N123Q mutants. The DNA segments (BamHI–SphI) from these plasmids were directly mobilised into the corresponding sites of pMTZUng, to generate pMTZUngY66C, or into pTrcUng (22), to obtain the corresponding pTrcUng constructs of the Y66S, Y66F, N123E and N123Q mutants, using standard methods (20). Subsequently, the mutant genes from the latter constructs were subcloned into pMTZ2 to yield pMTZUngY66S (or Y66F) and pMTZUngN123E (or N123Q). To obtain pMTZUngN123D, a mutagenic oligomer (5′-GCTACTCGATACTGTGTTG-3′) was used to introduce the mutation into pMTZUng by a megaprimer approach using Pfu DNA polymerase (23). All constructs were confirmed by DNA sequence analysis using Sanger’s dideoxy chain termination method (20).
In vitro transcription–translation by non-radioactive/radioactive methods
Plasmid DNAs were prepared by the cesium chloride density gradient centrifugation method (20), quantified and used for in vitro transcription–translation reactions in rabbit reticulocyte lysate, according to the supplier’s recommendations (Promega). A typical reaction (50 µl) comprised 25 µl of TNT rabbit reticulocyte lysate, 2 µl of reaction buffer, 1 µl of T7 RNA polymerase, 1 µl of amino acid mixture minus methionine (1 mM), 12.5 µl of [35S]methionine (1000 Ci/mmol, 10 mCi/ml), 40 U RNasin ribonuclease inhibitor and 1 µg DNA template (vector or the UDG gene-containing plasmids). In the non-radioactive translation reactions, 0.5 µl each of the amino acid mixes (–Leu) and (–Met) were used instead of the radiolabelled amino acid. The reaction contents were incubated at 37°C for 2 h. Subsequently, a 2.5 µl aliquot of the reaction was mixed with 10 µl of SDS sample loading dye, heated at 90°C and analysed by 15% SDS–PAGE (24). The gel was stained with Coomassie brilliant blue R-250, destained, dried and subjected to BioImage analysis (Fuji). Similarly, Ugi was in vitro translated in the presence of [35S]methionine using pTZUgi (25), wherein the expression of Ugi was under the control of the T7 RNA polymerase promoter (Fig. 2) in the TNT S30 lysates (Promega). The Ugi-containing translates were heated at 100°C for 20 min and centrifuged in a microfuge at 4°C for 10 min. As Ugi is thermostable (26), it could be partially purified and recovered from the supernatant while most other proteins in the lysates were denatured and partitioned in the pellet. The radioactive purity of the Ugi was ascertained by 18% SDS–PAGE and autoradiography.
Formation of UDG and Ugi complexes from the translates
UDG–Ugi complexes were formed by mixing 2.5 µl aliquots (corresponding to ∼2500 c.p.m. Ugi) from the supernatants of the heat-treated lysates with 1 µl of the translates programmed with the plasmids harbouring UDG genes in 20 mM Tris–HCl, pH 7.4, for 15 min at room temperature and 15 min on ice. Then, 5 µl of native dye (0.01% bromophenol blue, 10% v/v glycerol and 50 mM Tris–HCl pH 6.8) was added to the reaction mixture and resolved by 15% PAGE at 100 V for 1.5 h (25). The UDG–Ugi complex was detected by BioImage analysis.
UDG assays with translates
Oligomers (1 pmol, 20 000 c.p.m.) were incubated with the translation reactions (1 µl of the translates or dilutions made in the vector translate) for 10 min (unless indicated otherwise) at 37°C in 10 µl volumes (27). The reactions were terminated with 5 µl of 0.2 N NaOH, heated, dried in vacuo, taken up in 10 µl of loading dye (80% formamide, 0.1% xylene cyanol FF and bromophenol blue and 1 mM Na2EDTA) and half of the contents were electrophoresed on 15% polyacrylamide–8 M urea gels (20) and subjected to BioImage analysis.
Phosphate modification interference experiments
An aliquot of 1 pmol of high specific activity labelled oligomer SSU9 in 100 µl of 50 mM sodium cacodylate, pH 7.0, 1 mM Na2EDTA was mixed with 100 µl of freshly prepared saturated solution of N-ethyl N-nitrosourea in ethanol and incubated at 50°C for 1 h (28,29). The DNA was ethanol precipitated in the presence of 0.33 M sodium acetate, transferred to liquid nitrogen for 2 min, thawed and centrifuged for 15 min in a microfuge. The pellet was washed with ethanol, dried in vacuo, taken up in 10 µl of water and half of it was subjected to UDG reaction for 5 min at 37°C, with 1 µl of the undiluted or a 1:1000 dilution of the translate containing the N123D or the wild-type UDG, respectively. The reaction was quenched with 5 µl of 0.4 M NaOH at 37°C for 30 min to achieve selective cleavage at the abasic sites, resolved on a 15% polyacrylamide–8 M urea gel and autoradiographed. The bands corresponding to the leftover substrate and the product were cut out, eluted for 6 h in 400 µl of 50 mM Tris–HCl, pH 7.4, and 5 mM Na2EDTA and ethanol precipitated in the presence of 100 µg/ml yeast total RNA. The pellet was washed with 80% alcohol, dried, suspended in 100 µl of piperidine (1 M) and heated at 90°C for 30 min (30). The samples were lyophilised, taken up in 100 µl of water and re-lyophilised. Finally, the residue was taken up in 80% formamide dye and equal counts applied to an 18% polyacrylamide–8 M urea sequencing gel. The control sample was treated similarly except that no UDG was added.
RESULTS
In vitro synthesis of various UDGs
The mutations at Y147 and N204 in human UDG (the equivalents of Y66 and N123 in EcoUDG) have been reported to be cytotoxic (11). Hence, to study the effects of mutations at Y66 and N123 in EcoUDG, we resorted to their production in rabbit reticulocyte lysates (RRL) using an in vitro transcription–translation system (TNT) and the plasmid constructs shown in Figure 2. As will be seen below (see Fig. 4) and was reported earlier (31), the RRL does not contain any detectable endogenous DNA glycosylase activities.
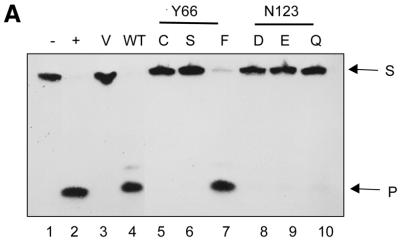
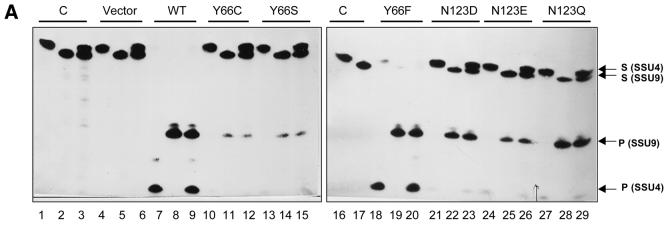
Figure 4.
Utilisation of SSU4 (A) or SSU9 (B) by the EcoUDG or mutants at positions Y66 and N123. The 5′-end-labelled oligomer (1 pmol, 20 000 c.p.m.) was either not treated (lane 1) or treated with the lysates (see Fig. 3A; 1 µl each) programmed with pTZ19R (lane 3) or the constructs containing wild-type (lane 4), Y66 mutant (lanes 5–7) or N123 mutant (lanes 8–10) UDG, under standard UDG assay conditions, and analysed on 15% polyacrylamide–8 M urea gels. Lane 2 is a control with native EcoUDG. The substrate (S) and the product (P) bands are shown by arrows.
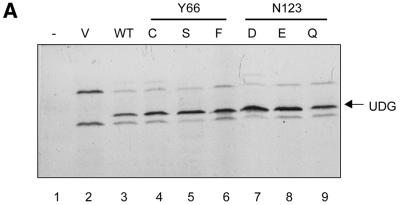
The autoradiogram in Figure 3A shows that the reaction containing vector DNA resulted in translation of two proteins (lane 2). The absence of these bands in the control translate containing no DNA (lane 1) suggested them to be vector specific. More importantly, when the translation reactions were programmed with the wild-type or the mutant ung gene plasmids (lanes 3–9), an additional band corresponding to UDG was observed.
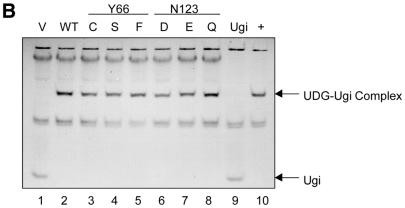
Figure 3.
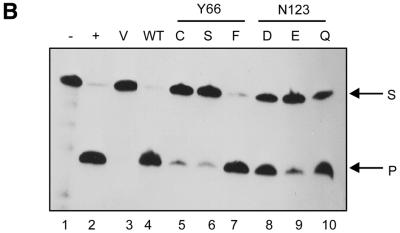
(A) Autoradiographic analysis of the in vitro translation reactions (2.5 µl each) fractionated by 15% SDS–PAGE. Lanes 1 and 2 are the lysate (no plasmid) and the vector (pTZ19R) controls, respectively. Lanes 3–9 represent the reactions programmed with the plasmids harbouring the wild-type or a mutant UDG gene. The band corresponding to UDG (lanes 3–9) is indicated by an arrow. (B) Autoradiographic analysis of UDG–Ugi complexes separated by 15% native PAGE (see Materials and Methods). The profile of partially purified radiolabelled Ugi (lane 9) and the results of incubation of this preparation with native UDG (lane 10), the vector alone translate (lane 1) or the UDG-containing translates (lanes 2–8) are as shown.
Interaction of the translated UDGs with Ugi
We used Ugi, a thermostable protein and an exceptional mimic of the DNA substrate which specifically interacts with the conserved class of UDGs (32–35), as a probe to ensure proper folding of the in vitro translated UDGs. However, the presence of an endogenous protein in the RRL which co-migrated with the UDG–Ugi complex on the native gels interfered with the analyses using native Ugi to form complexes with radiolabelled UDG in the in vitro translates (data not shown). Therefore, we radiolabelled Ugi and partially purified it to form complexes with the in vitro translates containing unlabelled proteins. Ugi was produced by in vitro transcription–translation in E.coli S30 lysates (TNT) in the presence of [35S]methionine and, as Ugi remains soluble upon heat treatment, it was enriched in the supernatants by thermal denaturation of most other proteins in the lysates. The [35S]methionine-labelled Ugi–UDG complexes thus formed were analysed by electrophoresis on native gels, wherein free Ugi (9.4 kDa), with a highly acidic pI of 4.2, migrates towards the bottom of the gel and the UDG–Ugi complex (35 kDa), with a pI of 4.9, migrates in the middle of the gel (25). As shown in Figure 3B, the partially purified preparation of (35S-labelled) Ugi showed two bands (lane 9), the lower one of which corresponded to Ugi. The specificity of the Ugi band was further verified by its mobility shift (but not of the other band) towards the middle of the gel upon its complexation with native EcoUDG (lane 10). As expected, no such shift of Ugi occurred with the vector alone RRL translate (lane 1). More importantly, incubation of Ugi with the translates primed with the wild-type or mutant UDG (Y66 and N123) templates produced UDG–Ugi complexes which co-migrated with the complex formed by native UDG (compare lanes 2–8 with 10). The ability of the UDG translates to form specific complexes with Ugi suggests that the translated UDGs folded correctly and were suitable for activity assays. It may be noted that incubation of the radiolabelled Ugi with RRL (lanes 1–8) but not pure UDG (lane 10) resulted in the presence of a diffuse band near the wells. As this band was absent from the Ugi preparation (lane 9), it most likely resulted from retention of the contaminating [35S]methionine or its derivatives (in the Ugi preparation) by the components of RRL on such native gels.
Uracil excision activity of the UDG mutants
Assays with the in vitro translates for UDG activity using SSU4, an oligomer harbouring uracil in the fourth position, are shown in Figure 4. The control translates programmed with the vector (pTZ19R) DNA showed no product bands corresponding to cleavage of the N-glycosidic bond at uracil or any other base, suggesting that RRL did not contain any detectable base excision activity (Fig. 4A, lane 3). However, uracil release by the in vitro translated wild-type UDG was highly proficient and resulted in a product band which co-migrated with the product of reaction with native UDG (Fig. 4A, compare lanes 2 and 4). On the other hand, excision of uracil from SSU4 by the mutants Y66C (lane 5), Y66S (lane 6), N123D (lane 8), N123E (lane 9) and N123Q (lane 10) was barely detectable (see also Fig. 5A). These data suggest that Y66 and N123 are critical for efficient UDG activity. Interestingly, the Y66F mutant, which contains a conservative change, was highly active in uracil excision (lane 7).
Figure 5.
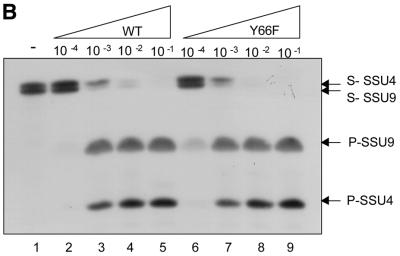
(A) Relative utilisation of SSU4 and SSU9. Control or UDG reactions were carried out using SSU4 (0.5 pmol, 20 000 c.p.m.; lanes 1, 4, 7, 10, 13, 16, 18, 21, 24 and 27) or SSU9 (0.5 pmol, 20 000 c.p.m.; lanes 2, 5, 8, 11, 14, 17, 19, 22, 25 and 28) or an equimolar mixture (0.5 pmol each) of the two (lanes 3, 6, 9, 12, 15, 20, 23, 26 and 29) using various translates (1 µl each) as indicated at the top of the lanes and analysed on 15% polyacrylamide–8 M urea gels. The substrate (S) and the product (P) bands are shown by arrows. (B) Relative utilisation of SSU4 and SSU9 (0.5 pmol each) by the wild-type or the Y66F mutant UDG. Lane 1 is the buffer control. Lanes 2–5 and 6–9 represent reactions with serially diluted translates of the wild-type or Y66F translates, respectively. S and P represent the substrate and product bands, respectively.
Subsequently, we used yet another substrate, SSU9, having uracil in the ninth position. Y66C, Y66S and N123E showed a detectable but weak uracil excision activity even with SSU9 (Fig. 4B, lanes 5, 6 and 9). Interestingly, the N123D and the N123Q UDGs displayed significant uracil excision from SSU9 (Fig. 4B, lanes 8 and 10). Furthermore, consistent with the ability of the mutants to form complexes with Ugi (Fig. 3B), the uracil excision activity of translates was abolished by Ugi, a specific inhibitor of UDGs (data not shown).
Uracil excision in mixed substrate reactions
The results in Figure 4 suggest that SSU4 and SSU9 were used differentially by the mutants (excepting Y66F). To confirm this observation, and to rule out the presence of any inhibitors in SSU4, we mixed the two substrates and assayed for their relative utilisation. As shown in Figure 5A, although the mutants excised uracil to different extents, they all (except for the Y66F mutant) showed preferential utilisation of SSU9 over SSU4 in both the single and the mixed substrate reactions. As expected, the buffer and the vector lysate control reactions did not show any UDG activity (Fig. 5A, lanes 1–3 and 4–6). The wild-type and Y66F mutant showed complete excision of uracil from both the substrates (lanes 7–9 and 18–20, respectively).
Wild-type and Y66F UDGs are indifferent to SSU4 and SSU9
To determine if the wild-type and Y66F proteins also preferentially utilised SSU9 over SSU4, the corresponding in vitro translation reactions were diluted up to 10 000-fold and used in the assay. These assays showed that the wild-type and Y66F translates did not discriminate between the two substrates (Fig. 5B, compare lanes 3–5 with 7–9). This experiment also demonstrated that the Y66F mutant was similar to the wild-type protein in its uracil excision activity (Fig. 5B, compare lanes 2–5 with 6–9). Furthermore, the observation that the translates utilised the substrates almost quantitatively even at a dilution of 1000-fold suggested that the RRL-based in vitro assay system is sensitive to detect activities as low as 0.1% of the wild-type activity.
Modification of the backbone phosphates and analysis of the interference in uracil excision
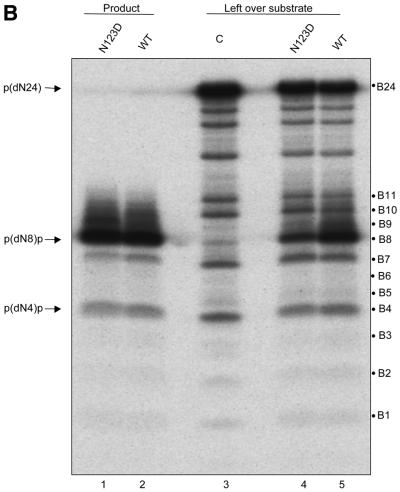
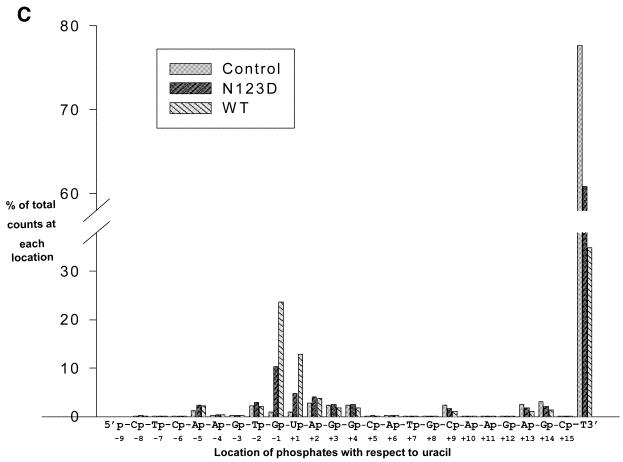
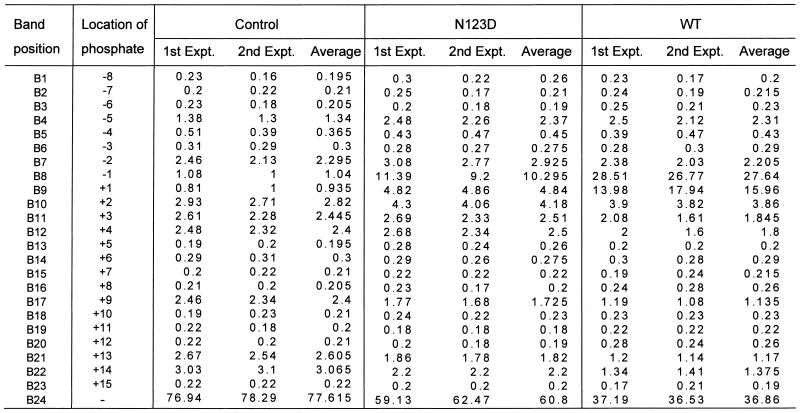
Phosphate group-mediated sequence-independent contacts between the nucleic acids and proteins contribute significantly to the stability of the complexes that arise by their specific interactions. Therefore, to assess the role of the backbone phosphates in substrate utilisation by UDG, we modified SSU9 with N-ethyl N-nitrosourea under the conditions which afforded low frequency phosphate ethylation (Fig. 6B, lane 3). We reasoned that if the flanking phosphates contributed to binding to the enzyme, their modification would interfere with substrate utilisation by UDG and, consequently, the unutilised substrate would be enriched for the population containing modifications at the phosphate groups crucial for interaction with UDG. To recover the unutilised substrate at the end of the reaction, we performed alkaline hydrolysis at 37°C. As shown in Figure 6A, the conditions used resulted in two major bands and afforded separation and recovery of the remaining substrate and the product. Subsequently, a rigorous alkaline hydrolysis at 90°C effected cleavage at the modified phosphates (Fig. 6B). Interestingly, quantification of the data (Fig. 6C and Table 1) showed that in the unutilised substrate there was a remarkable increase in the abundance of the bands corresponding to phosphate modification at the –1 (immediately 5′ to uracil), +1 and +2 (3′ to uracil) positions (compare the intensities of bands B8, B9 and B10 of the control with either the wild-type or N123 mutant). In addition, a detectable increase (∼2-fold) in the band intensity corresponding to the phosphate at the –5 position was also seen (Table 1, band B4). Since there were no changes in the abundance of most other bands, the observation suggests specific involvement of the phosphates at the –5, –1, +1 and +2 positions in interacting with EcoUDG. A higher abundance of these bands in the wild-type compared to the N123D mutant is most likely a consequence of the higher UDG activity of the wild-type protein (as is also evident from the lesser abundance of unutilised substrate in the reaction with the wild-type enzyme; Table 1, band B24). Further, in principle, for the positions 5′ to uracil the increase in the abundance of the bands representing the –5 and –1 positions should also be reflected in a decrease in the intensities of the corresponding bands in the product lanes. However, due to inaccuracies arising from the enormous background of unmodified substrate, analysis of the abundance of the modified phosphates in the product lanes was not pursued. Nevertheless, the product band in this analysis provided an exact marker for p(dN8)p.
Figure 6.
(A) N-ethyl N-nitrosourea-modified SSU9 was incubated with the buffer alone or supplemented with the translates (1 µl of a 1:1000 fold dilution, wild-type, lane 2; 1 µl of N123D, lane 3), incubated for 5 min at 37°C, treated with NaOH at 37°C, fractionated on 15% polyacrylamide–8 M urea gels, autoradiographed and the bands corresponding to the unutilised substrate (S) and product (P) were eluted. (B) Analysis of phosphate interference in substrate utilisation by UDGs. The eluted fragments were subjected to rigorous treatment with 1 M piperidine and the products were analysed on sequencing gels. All bands (B1–B24) were distinctly seen on a BioImage Analyser (FLA2000; Fuji) and the quantification of all the bands is as shown in Table 1. Some of the bands appear as doublets, most likely due to the slightly different mobilities of fragments with 3′ ends with an OH or ethyl phosphate group subsequent to cleavage with piperidine (28). (C) A histogram showing the changes in relative abundance (% of total) of the bands corresponding to cleavage at different phosphate positions (–8 to +15, B1–B23, respectively) and intact SSU9 (B24) from an average of two experiments. Quantification of the various bands was carried out on a BioImage analyzer (Fuji). Relative band intensities in per cent were estimated as [Bx/(B1 + B2… + B24) × 100], where Bx represents the counts (in pixels) in the band under consideration (either one of B1–B24). The denominator (B1 + B2… + B24) represents the total of counts in B1–B24.
Table 1. Per cent abundance of the bands corresponding to cleavage at different phosphate positions (B1–B23) or the intact SSU9 (B24).
Relative band intensities in per cent were estimated as [Bx/(B1 + B2… + B24) × 100], where Bx represents the counts (in pixels) in the band under consideration (either one of B1–B24). The denominator (B1 + B2… + B24) represents the total of counts in B1–B24 for a given lane.
Relative utilisation of SSU9, SSU5 and SSU4 by the wild-type and N123D UDGs
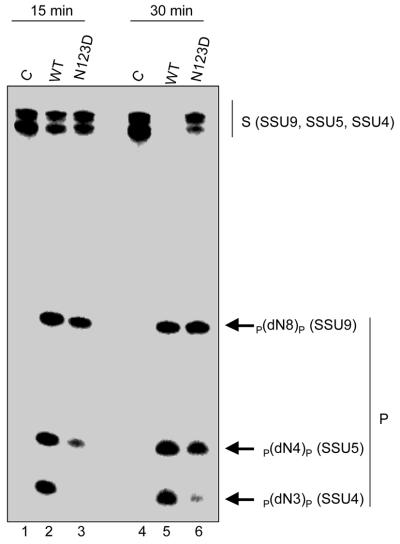
To further confirm our interpretation from the ethylation interference assays about the role of the phosphate occupying the –5 position in preferential utilisation of the substrates by UDG mutants, we carried out a triple substrate assay using DNAs with uracil at the fourth (SSU4), fifth (SSU5) or ninth (SSU9) position in equimolar amounts. Notably, 5′ phosphorylated SSU4 lacks the phosphate at the –5 position but the same is included in SSU5 due to the presence of an additional nucleotide upstream of the uracil. As can be seen from the equal intensities of the product bands, translate containing the wild-type UDG utilised all three substrates with similar efficiencies (Fig. 7, lane 2). On the other hand, the N123D mutant utilised them differentially and the results suggest that the longer 5′ flanks facilitated uracil release (Fig. 7, lanes 3 and 6). Interestingly, the relative intensities of the product bands corresponding to SSU4, SSU5 and SSU9 (lanes 3 or 6) show that when the 5′ flank size was increased from 3 nt (or four phosphates) in SSU4 to 4 nt (or five phosphates) in SSU5, there was a remarkable increase in uracil excision (as opposed to when the flank size was extended from 4 nt in SSU5 to 8 nt in SSU9). This observation further highlights the positive effect of the –5 phosphate in uracil excision by the N123D mutant. A likely reason for the apparent inconsequential nature of this interaction for the wild-type UDG (lane 2) could be that, at least in vitro, the contribution of this contact is only subtle for the wild-type enzyme (unlike the negative effect seen upon its ethylation in Fig. 6C). However, for the mutants (e.g. N123D) whose binding to the substrate is compromised owing to the loss of a specific contact(s), such as those in the active site pocket, even the minor contributions become crucial.
Figure 7.
Relative utilisation of SSU4, SSU5 and SSU9 by the translates programmed with the wild-type or N123 mutant UDG constructs. Equimolar amounts of oligomers (0.3 pmol each, 20 000 c.p.m.) were either incubated in buffer alone (lanes 1 and 4), with 1 µl of a 1:1000-fold dilution of the translate containing wild-type (lanes 2 and 5) or with 1 µl of the translate containing the N123D (lanes 3 and 6) UDG for either 15 (lanes 1–3) or 30 min (lanes 4–6) and analysed on 15% polyacrylamide–8 M urea gels. The substrate (S) and the product (P) bands corresponding to SSU4, SSU5 and SSU9 are as indicated.
DISCUSSION
Several high resolution DNA–UDG co-crystal structures have provided an in-depth understanding of the mechanisms that regulate extreme substrate specificity and catalysis by the conserved class of UDGs (10–15). According to a recent study, glycosidic bond cleavage occurs by an autocatalytic step facilitated by interactions between the Ser–Pro loops of the enzyme with the backbone phosphates in the substrate, then a water molecule activated by the highly conserved D64 reacts with the C1′ of the ribose sugar (36). The crystal structures and mutational analyses have also emphasised the role of two other highly conserved residues, Y66 and N123, in conferring strict substrate selectivity for uracil in DNA and in avoiding admission of other bases into the active site pocket (see Introduction). The role of Y66 in restricting entry of thymine or purines was further supported by the discovery of EcoMUG (mismatch-specific glycosylase), which removes U or T from G:U/T and where the topological equivalent of Y66 of EcoUDG is a G residue, which would allow entry of thymine (12,37).
In this study, we have carried out mutational analysis of Y66 and N123 in EcoUDG. Similar mutations in human UDGs have been shown to be cytotoxic on account of acquisition of weak thymine/cytosine DNA glycosylase activity (TDG or CDG) (11). At least two of the mutants (Y66C and Y66S) that we attempted to produce in E.coli could also not be expressed (data not shown). Therefore, we resorted to in vitro production of all the UDG mutants in a RRL-based transcription–translation system. Also, while our assays were not specifically designed to detect low CDG/TDG activities, the oligonucleotide-based assay was sensitive in detecting as low as 0.1% of the wild-type activity (Fig. 5B). However, in our assays, as we only see bands corresponding to excision of uracils in the oligomer sequence, suggest that the acquired CDG or TDG activity of the mutants is lower than this detection limit.
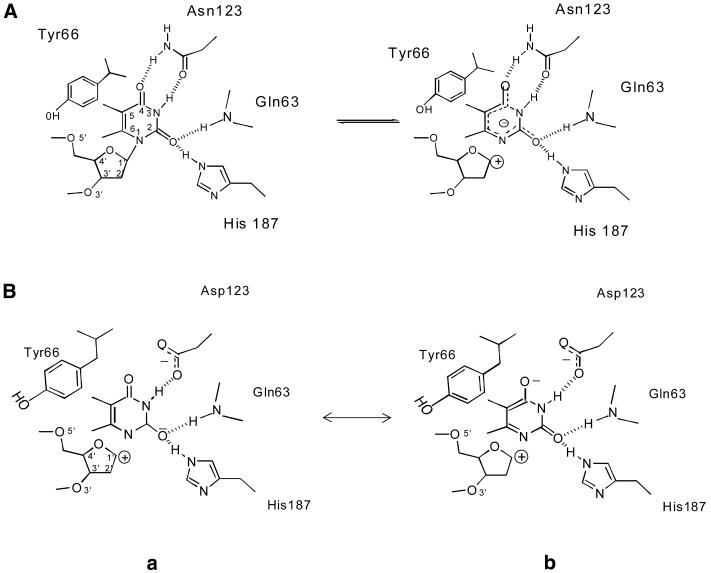
More importantly, is the role of Y66 and N123 limited only to conferring substrate selectivity? The observation that the Y66S and Y66C mutants showed drastically reduced activity in excision of uracil is inconsistent with the proposed role of Y66 in merely providing a steric block to prevent admittance of thymine or purines into the active site pocket. Had it been just this role, why should there have been a drastic drop in the uracil excision activity of these mutants? The observation that Y66F is similar to the wild-type enzyme in its activity suggests that from a structural view point mutations per se at this position can be tolerated. Thus, we propose that in addition to its role in blocking the entry of non-uracil residues, the Van der Waals interaction (or a possible C-H…π type interaction) that the Y66 (or F66 in the mutant) establishes with the C5 position of the uracil may also facilitate stabilisation of the transition state enzyme–substrate [ES] complex. Such a possibility also provides an explanation for the observation that the utilisation of SSU4 by the Y66S or Y66C mutant was insignificant but increased upon increasing the substrate chain length (SSU9). Most likely the loss of a contribution by Y66 towards stabilisation of the enzyme–substrate complex was partially compensated for by long-range interactions between the substrate and the enzyme (discussed below). Further, as the N123 position donates a hydrogen bond to the O4 of the uracil ring, N123 may also be similarly important in stabilising the transition state enzyme–substrate complex. From another viewpoint, the Asp in the N123D mutant should be in perfect geometry to form a hydrogen bond with the exocyclic 4-NH2 of cytosine. The finding that the CDG activity of the N123D mutant is, at best, less than 0.1% of the UDG activity of the wild-type protein signifies another possible role for N123. It has been proposed that the leaving group quality of the uracilate anion is enhanced by a hydrogen bond from H187 to the O2 group. However, in the native enzyme withdrawal of the electron density towards the amide of N123 will also lead to enhancement of the leaving group quality of the uracilate anion (Scheme 1A). Hence, even though cytosine- or uracil-containing DNA may well be accommodated in the pocket of the N123D mutant, the Asp at this position will not enhance the leaving group quality of the cleaved base. Worse even, the negative charge from the carboxyl of Asp will actually reduce its leaving group quality (Scheme 1B). Thus, taken together, it appears that Y66 and N123 participate in uracil excision by at least three mutually non-exclusive mechanisms. In addition to their role in selectivity for uracil, they may contribute to the stability of the transition state enzyme–substrate complex and/or enhance the leaving group quality of the uracilate anion.
Scheme 1. (A) Role of Q63, Y66, N123 and H187 in cleavage of the N-glycosidic bond between uracil and the deoxyribose sugar (adapted from 36). (B) Possible effects of mutations at N123 residue. Two resonance forms (a and b) of uracilate anion are shown. Note that the mutation of N123 to D123 results in loss of one hydrogen bond. Additionally, the repulsive force between the partially negatively charged O4 of the leaving group and the carboxylate anion (form b) would adversely affect stabilisation of the leaving group (for details see Discussion).
Further, to gain insights into the mechanism of differential utilisation of SSU4 and SSU9 by the UDG mutants, we carried out phosphate ethylation interference assays to study utilisation of SSU9 by the wild-type and the N123D UDG mutant (deficient in hydrogen bonding with the O4 of uracil; Scheme 1B). As shown in Figure 6C, modification of SSU9 at the –1, +1 and +2 phosphates (with respect to the scissile uracil) hampered its use as a substrate. This observation is consistent with our earlier studies providing the first evidence for the crucial role of the flanking phosphates (at the –1, +1 and +2 positions) in uracil excision, in solution, by UDG (38). The precise role of these phosphates became apparent from UDG–DNA co-crystal structures wherein the three Ser–Pro-rich loops accomplish kinking of the target DNA and extrahelical localisation of the uridine from the base stack by backbone compression upon establishing contacts with these phosphates (39). Our present observation that modification at an upstream phosphate (–5 position) interfered with substrate utilisation suggests an additional interaction with the enzyme. The significance of this interaction with the substrate phosphate in UDG activity was further emphasised by the fact that the N123D mutant showed a remarkable enhancement of uracil release when the size of SSU4 was increased (in SSU5) just to include this phosphate (Fig. 7). Although such long-range interactions between UDG and the substrate may be redundant or contribute only subtly to the wild-type protein, they do make an important contribution to the activity of the mutants (e.g. N123D) which fail to establish a full complement of interactions in the active site pocket. The long-range interactions between the substrate and the enzyme that we have been able to detect in the context of the mutant enzymes may be crucial even for the wild-type enzyme under in vivo conditions. Finally, the RRL in vitro translation system exploited herein provides a good method for expressing lethal/toxic proteins to investigate their biochemical properties.
Acknowledgments
ACKNOWLEDGEMENTS
We thank K. Saikrishnan for his help in generating Figure 1 and Dr Mini Thomas and other laboratory colleagues for their suggestions on the manuscript. This work was supported in part by research grants from the Council of Scientific and Industrial Research (CSIR), the Department of Biotechnology and the Department of Science and Technology, New Delhi, India. Use of the phosphorimaging central facility supported by the Department of Biotechnology is acknowledged. P.H. and N.A. are supported by a senior research fellowship of the CSIR.
REFERENCES
- 1.Lindahl T., (1982) DNA repair enzymes. Annu. Rev. Biochem., 51, 61–87. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- 3.Mosbaugh D.W., and Bennett,S.E. (1994) Uracil excision repair. Prog. Nucleic Acid Res. Mol. Biol., 48, 315–370. [DOI] [PubMed] [Google Scholar]
- 4.Nagelhus T.A., Haug,T., Singh,K.K., Keshav,K.F., Skorpen,F., Otterlei,M., Bharati,S., Lindmo,T., Benichou,S., Benarous,R. and Krokan,H.E. (1997) A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J. Biol. Chem., 272, 6561–6566. [DOI] [PubMed] [Google Scholar]
- 5.Handa P., Acharya,N. and Varshney,U. (2001) Chimeras between single stranded DNA binding proteins from Escherichia coli and Mycobacterium tuberculosis reveal that their C-terminal domains interact with uracil-DNA glycosylases. J. Biol. Chem., 276, 16992–16997. [DOI] [PubMed] [Google Scholar]
- 6.Otterlei M., Warbrick,E., Nagelhus,T.A., Haug,T., Slupphaug,G., Akbari,M., Aas,P.A., Steinsbekk,K., Bakke,O. and Krokan,H.E. (1999) Post replicative base excision repair in the replication foci. EMBO J., 18, 3834–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aravind L., and Koonin,E.V. (2000) The α/β fold uracil DNA glyosylases: a common origin with diverse fates. Genome Biol., 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krokan H.E., Standal,R. and Slupphaug,G. (1997) DNA glycosylases in the base excision repair of DNA. Biochem. J., 325, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearl L.H., (2000) Structure and function in the uracil-DNA glycosylase superfamily. Mutat. Res., 460, 165–181. [DOI] [PubMed] [Google Scholar]
- 10.Mol C.D., Arvai,A.S., Slupphaug,G., Kavli,B., Alseth,I., Krokan,H.E. and Tainer,J.A. (1995) Crystal structure and mutational analysis of human uracil-DNA glycosylase: structural basis for specificity and catalysis. Cell, 80, 869–878. [DOI] [PubMed] [Google Scholar]
- 11.Kavli B., Slupphaugh,G., Mol,C.D., Arvai,S.A., Petersen,S.B., Tainer,J.A. and Krokan,H.E. (1996) Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO J., 15, 3442–3447. [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett T.E., Savva,R., Panayotou,G., Barlow,T., Brown,T., Jiricny,J. and Pearl,L.H. (1998) Crystal structure of a G:T/U mismatch-specific DNA glycosylase: mismatch recognition by complementary-strand interactions. Cell, 92, 117–129. [DOI] [PubMed] [Google Scholar]
- 13.Savva R., McAuley-Hecht,K., Brown,T. and Pearl,L. (1995) The structural basis of specific base excision repair by uracil-DNA glycosylase. Nature, 373, 487–493. [DOI] [PubMed] [Google Scholar]
- 14.Parikh S.S., Walcher,G., Jones,G.D., Slupphaug,G., Krokan,H.E., Blackburn,G.M. and Tainer,J.A. (2000) Uracil-DNA glycosylase-DNA substrate and product structures: conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc. Natl Acad. Sci. USA, 97, 5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh S.S., Mol,C.D., Slupphaug,G., Bharati,S., Krokan,H.E. and Tainer,J.A. (1998) Base excision repair initiating revealed by crystal structures and binding kinetics of human uracil-DNA glycosylase with DNA. EMBO J., 17, 5214–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartori A.A., Schar,P., Gibbson,S., Miller,J.H. and Jiricny,J. (2001) Biochemical characterization of uracil processing activities in the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Biol. Chem., 276, 29979–29986. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N.V., and Varshney,U. (1994) Inefficient excision of uracil from loop regions of DNA oligomers by E.coli uracil-DNA glycosylase. Nucleic Acids Res., 18, 3737–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varshney U., Jahroudi,N., Foster,R. and Gedamu,L. (1986) Structure, organization and regulation of human metallothionein IF gene: differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol. Cell. Biol., 6, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varshney U., Hutcheon,T. and van de Sande,J.H. (1988) Sequence analysis, expression and conservation of Escherichia coli uracil-DNA glycosylase and its gene (ung). J. Biol. Chem., 263, 7776–7784. [PubMed] [Google Scholar]
- 20.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Handa P., and Varshney,U. (1998) Rapid and reliable site directed mutagenesis using Kunkel’s approach. Indian J. Biochem. Biophys., 35, 63–66. [PubMed] [Google Scholar]
- 22.Handa P., Roy,S. and Varshney,U. (2001) The role of leucine 191 of Escherichia coli uracil DNA glycosylase in forming a stable complex with a substrate mimic, Ugi and in uracil excision from synthetic substrates. J. Biol. Chem., 276, 17324–17331. [DOI] [PubMed] [Google Scholar]
- 23.Picard V., Ersdal-Badju,E., Lu,A. and Bock,S.C. (1994) A rapid and efficient one-tube PCR-based mutagenesis technique using Pfu DNA polymerase. Nucleic Acids Res., 22, 2587–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U.K., (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 25.Roy S., Purnapatre,K., Handa,P., Boyanapalli,M. and Varshney,U. (1998) Use of coupled transcriptional system for consistent overexpression and purification of UDG-Ugi complex and Ugi from E. coli. Protein Expr. Purif., 13, 155–162. [DOI] [PubMed] [Google Scholar]
- 26.Cone R., Bonura,T. and Friedberg,E.C. (1980) Inhibitor of uracil-DNA glycosylase induced by bacteriophage PBS2. Purification and preliminary characterization. J. Biol. Chem., 255, 10354–10358. [PubMed] [Google Scholar]
- 27.Kumar N.V., and Varshney,U. (1997) Contrasting effects of single stranded DNA binding protein on the activity of uracil-DNA glycosylase from Escherichia coli towards different DNA substrates. Nucleic Acids Res., 25, 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seibenlist U., and Gilbert,W. (1980) Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc. Natl Acad. Sci. USA, 77, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrickson W., and Schleif,R. (1985) A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc. Natl Acad. Sci. USA, 82, 3129–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxam A.M., and Gilbert,W. (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol., 65, 499–560. [DOI] [PubMed] [Google Scholar]
- 31.Caradonna S., Worrad,D. and Lirette,R. (1987) Isolation of a herpes simplex virus cDNA encoding the DNA repair enzyme, uracil DNA glycosylase. J. Virol., 61, 3040–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mol C.D., Arvai,A.S., Sanderson,R.J., Slupphaug,G., Kavli,B., Krokan,H.E., Mosbaugh,D.W. and Tainer,J.A. (1995) Crystal structure of the human uracil-DNA glycosylase in complex with a protein inhibitor: protein mimicry of DNA. Cell, 82, 701–708. [DOI] [PubMed] [Google Scholar]
- 33.Savva R., and Pearl,L.H. (1995) Nucleotide mimicry in the crystal structure of the uracil-DNA glycosylase-uracil glycosylase inhibitor protein complex. Nature Struct. Biol., 2, 752–755. [DOI] [PubMed] [Google Scholar]
- 34.Ravishankar R., Bidya Sagar,M., Roy,S., Purnapatre,K., Handa,P., Varshney,U. and Vijayan,M. (1998) X-ray analysis of a complex of E.coli uracil-DNA glycosylase (EcUDG) with its proteinaceous inhibitor. The structure elucidation of a prokaryotic UDG. Nucleic Acids Res., 26, 4880–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putnam C.D., Shroyer,M.J.N., Lundquist,A.J., Mol,C.D., Arvai,A.S. and Mosbaugh,D.W. (1999) Protein mimicry of DNA from crystal structures of the uracil-DNA glycosylase inhibitor protein and its complex with Escherichia coli uracil-DNA glycosylase. J. Mol. Biol., 287, 331–346. [DOI] [PubMed] [Google Scholar]
- 36.Dinner A.R., Blackburn,G.M. and Karplus,M. (2001) Uracil-DNA glycosylase acts by substrate autocatalysis. Nature, 413, 752–755. [DOI] [PubMed] [Google Scholar]
- 37.Gallinari P., and Jiricny,J.A. (1996) A new class of uracil-DNA glycosylase related to human thymine-DNA glycosylase. Nature, 383, 735–738. [DOI] [PubMed] [Google Scholar]
- 38.Varshney U., and van de Sande,J.H. (1991) Specificities and kinetics of uracil excision from uracil containing DNA oligomers by Escherichia coli uracil-DNA glycosylase. Biochemistry, 30, 4055–4061. [DOI] [PubMed] [Google Scholar]
- 39.Slupphaug G., Mol,C.D., Kavli,B., Arvai,A.S., Krokan,H.E. and Tainer,J.A. (1996) A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA. Nature, 384, 87–92. [DOI] [PubMed] [Google Scholar]
- 40.Werner R.M., Jiang,Y.L., Gordley,R.G., Jagadeesh,G.J., Ladner,J.E., Xiao,G., Tordova,M., Gilliland,G.L. and Stivers,J.T. (2000) Stressing out DNA? The contribution of serine-phosphodiester interactions in catalysis by uracil-DNA glycosylase. Biochemistry, 39, 12585–12594. [DOI] [PubMed] [Google Scholar]