Abstract
1. Membrane potential changes of the smooth muscle cells of the taenia coli were recorded during stimulation of the perivascular inhibitory nerves.
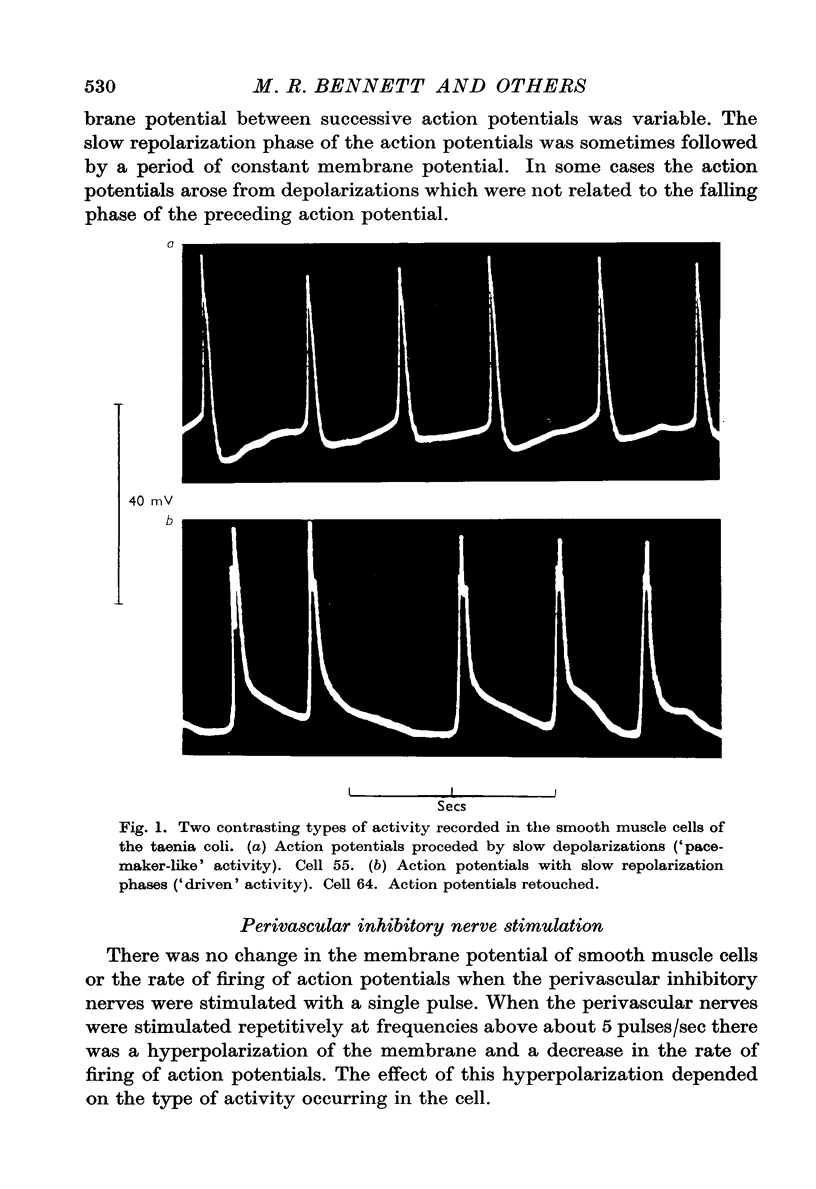
2. Some spontaneous action potentials were preceded by a slow pacemaker-like potential. Others began at or near the maximum level of the membrane potential and were not preceded by pacemaker-like potentials.
3. There were no changes in the membrane potential of smooth muscle cells when the inhibitory nerves were stimulated with a single pulse. Stimulation at frequencies greater than 5 pulses/sec caused a hyperpolarization of the smooth muscle membrane. This resulted in a decrease in spike frequency and relaxation.
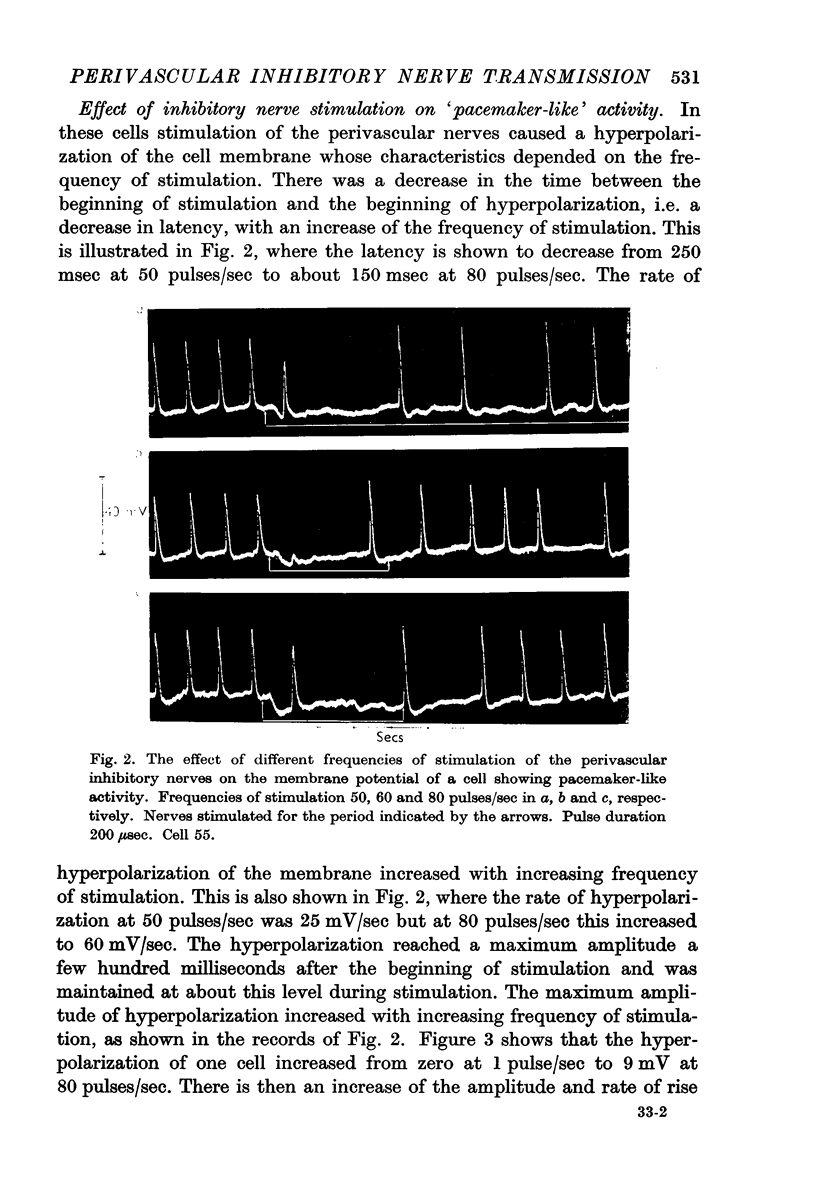
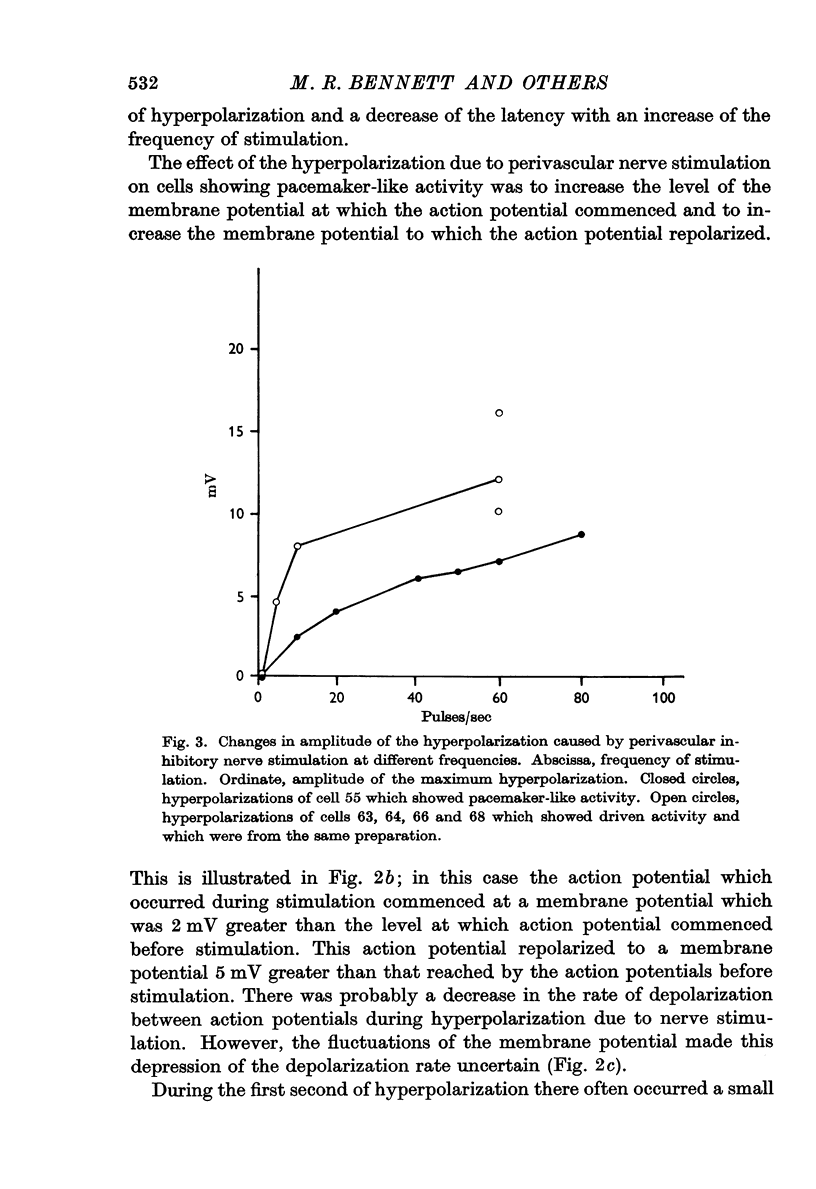
4. When the frequency of stimulation of the inhibitory nerves was increased there was an increase in the amplitude and rate of rise of the hyperpolarization and a decrease of the latency. The latency varied from 150 to 300 msec, and the largest hyperpolarization recorded was 16 mV.
5. The effect of the hyperpolarization due to nerve stimulation in cells showing pacemaker-like activity was to increase the level of the membrane potential at which the action potentials began and to increase the membrane potential to which the action potentials repolarized. Action potentials which occurred during hyperpolarizations of the membrane had greater rates of rise and fall and larger amplitudes than did the action potentials which occurred before hyperpolarization.
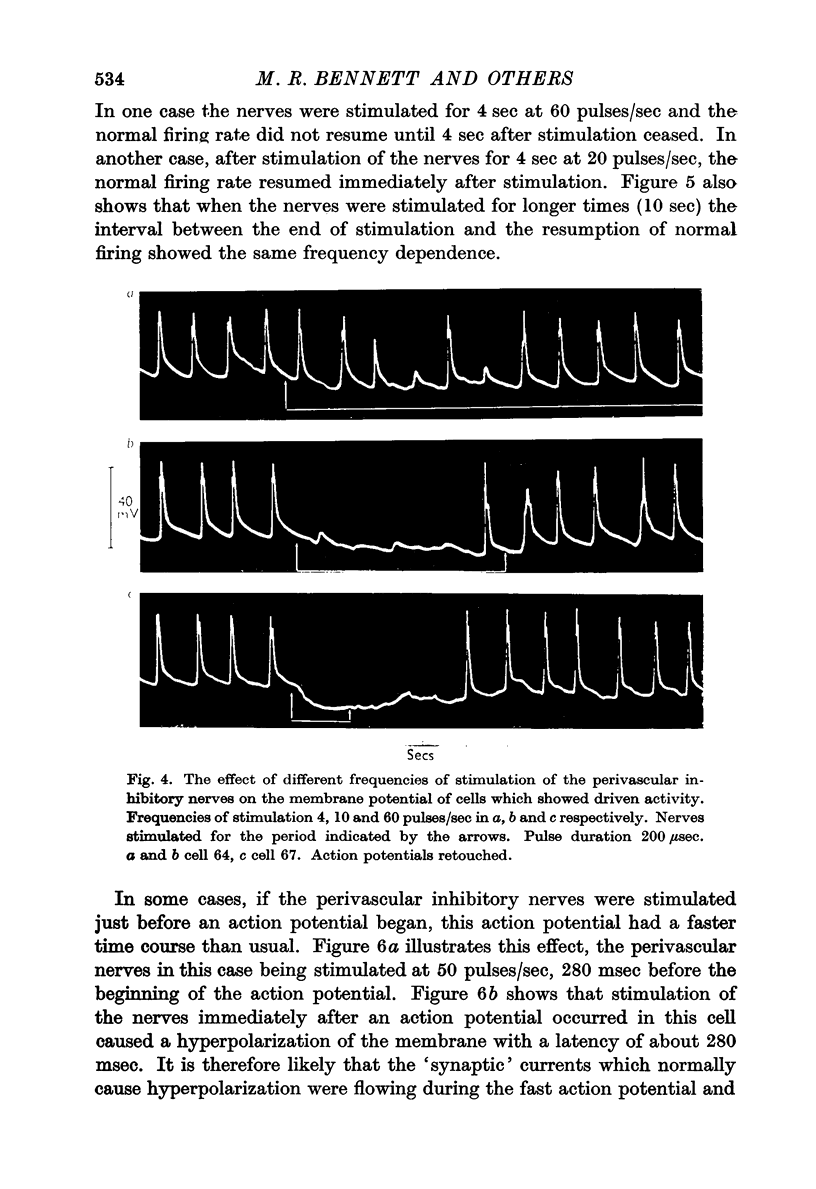
6. The effect of the hyperpolarization due to nerve stimulation in cells which did not show pacemaker-like activity depended on the amplitude of the hyperpolarization. Small hyperpolarizations exposed small depolarizations of the membrane which occurred when an action potential would normally have been expected. Large hyperpolarizations blocked the action potentials entirely.
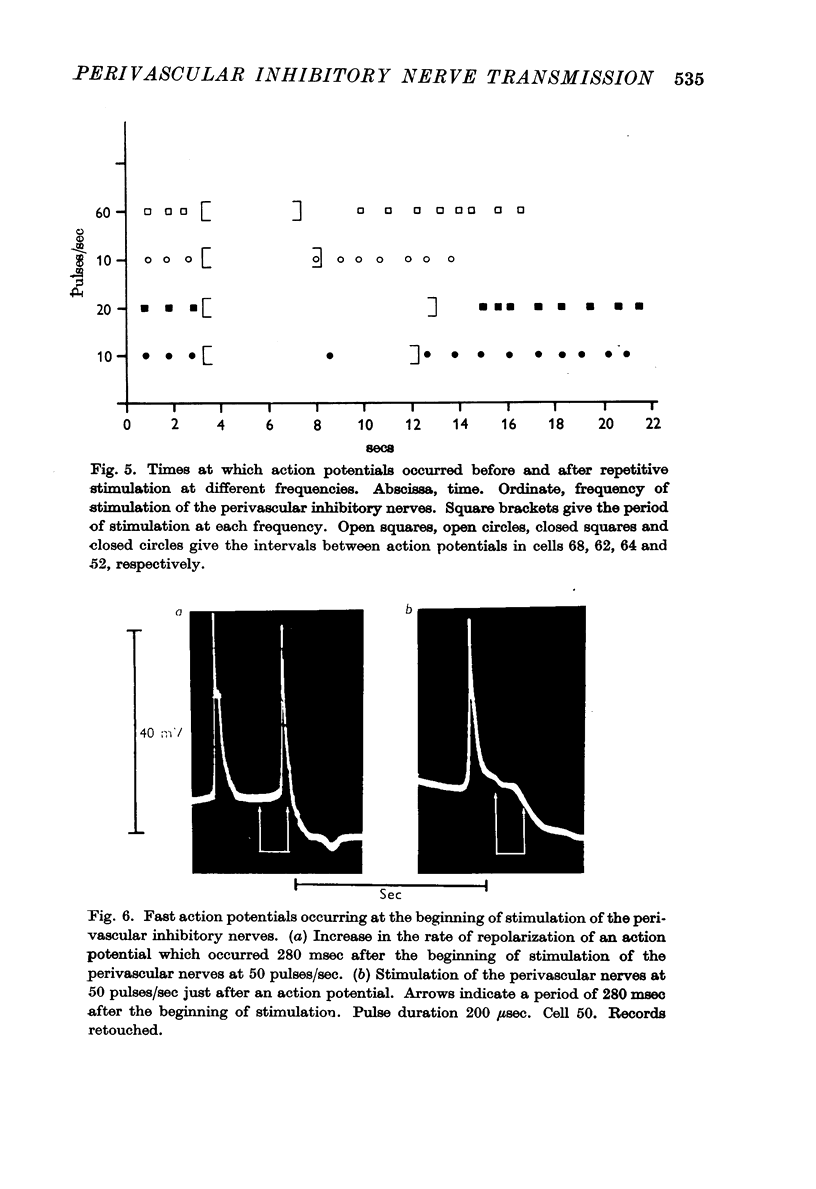
7. Action potentials did not begin firing again at the normal rate immediately after stimulation ceased. The time taken for the rate of firing of action potentials to return to normal increased with increasing frequency of stimulation.
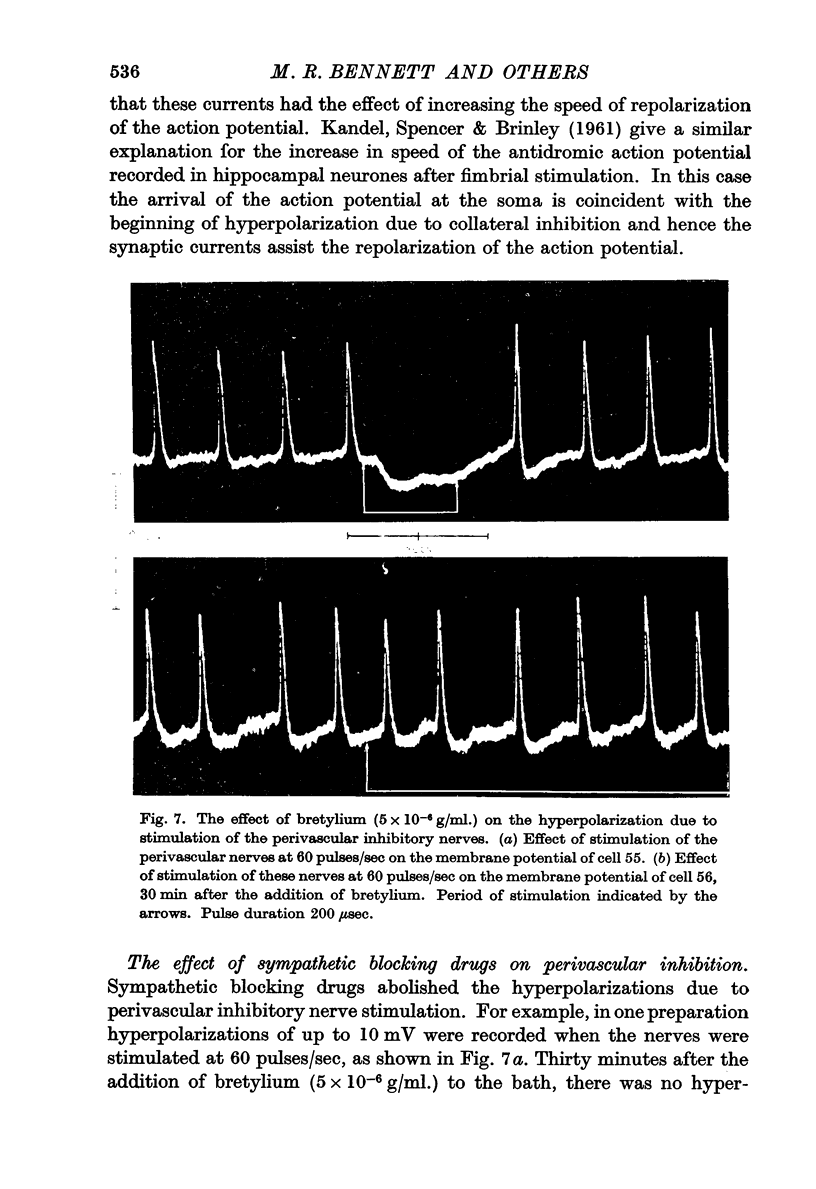
8. The hyperpolarization in response to perivascular inhibitory nerve stimulation was blocked by guanethidine and bretylium.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUELBRING E., KURIYAMA H. THE EFFECT OF ADRENALINE ON THE SMOOTH MUSCLE OF GUINEA-PIG TAENIA COLI IN RELATION TO THE DEGREE OF STRETCH. J Physiol. 1963 Nov;169:198–212. doi: 10.1113/jphysiol.1963.sp007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Changes in configuration of spontaneously discharged spike potentials from smooth muscle of the guinea-pig's taenia coli; the effect of electrotonic currents and of adrenaline, acetylcholine and histamine. J Physiol. 1957 Feb 15;135(2):412–425. doi: 10.1113/jphysiol.1957.sp005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The action of adrenaline on excitability and membrane potential in the taenia coli of the guinea-pig and the effect of DNP on this action and on the action of acetylcholine. J Physiol. 1958 Aug 29;143(1):183–194. doi: 10.1113/jphysiol.1958.sp006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. M., Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962 Aug 31;137(3531):670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J. "ELECTRICAL TRANSMISSION" AT AN EXCITATORY SYNAPSE IN A VERTEBRATE BRAIN. Science. 1964 May 15;144(3620):878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J. S. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J Physiol. 1962 Jun;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. ELECTROPHYSIOLOGICAL OBSERVATIONS ON THE MOTOR INNERVATION OF THE SMOOTH MUSCLE CELLS IN THE GUINEA-PIG VAS DEFERENS. J Physiol. 1963 Nov;169:213–228. doi: 10.1113/jphysiol.1963.sp007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OOSAKI T., ISHII S. JUNCTIONAL STRUCTURE OF SMOOTH MUSCLE CELLS. THE ULTRASTRUCTURE OF THE REGIONS OF JUNCTION BETWEEN SMOOTH MUSCLE CELLS IN THE RAT SMALL INTESTINE. J Ultrastruct Res. 1964 Jun;10:567–577. doi: 10.1016/s0022-5320(64)80030-7. [DOI] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- URSILLO R. C. Electrical activity of the isolated nerve-urinary bladder strip preparation of the rabbit. Am J Physiol. 1961 Sep;201:408–412. doi: 10.1152/ajplegacy.1961.201.3.408. [DOI] [PubMed] [Google Scholar]
- WOODBURY J. W., BRADY A. J. Intracellular recording from moving tissues with a flexibly mounted ultramicroelectrode. Science. 1956 Jan 20;123(3186):100–101. doi: 10.1126/science.123.3186.100-a. [DOI] [PubMed] [Google Scholar]


