Abstract
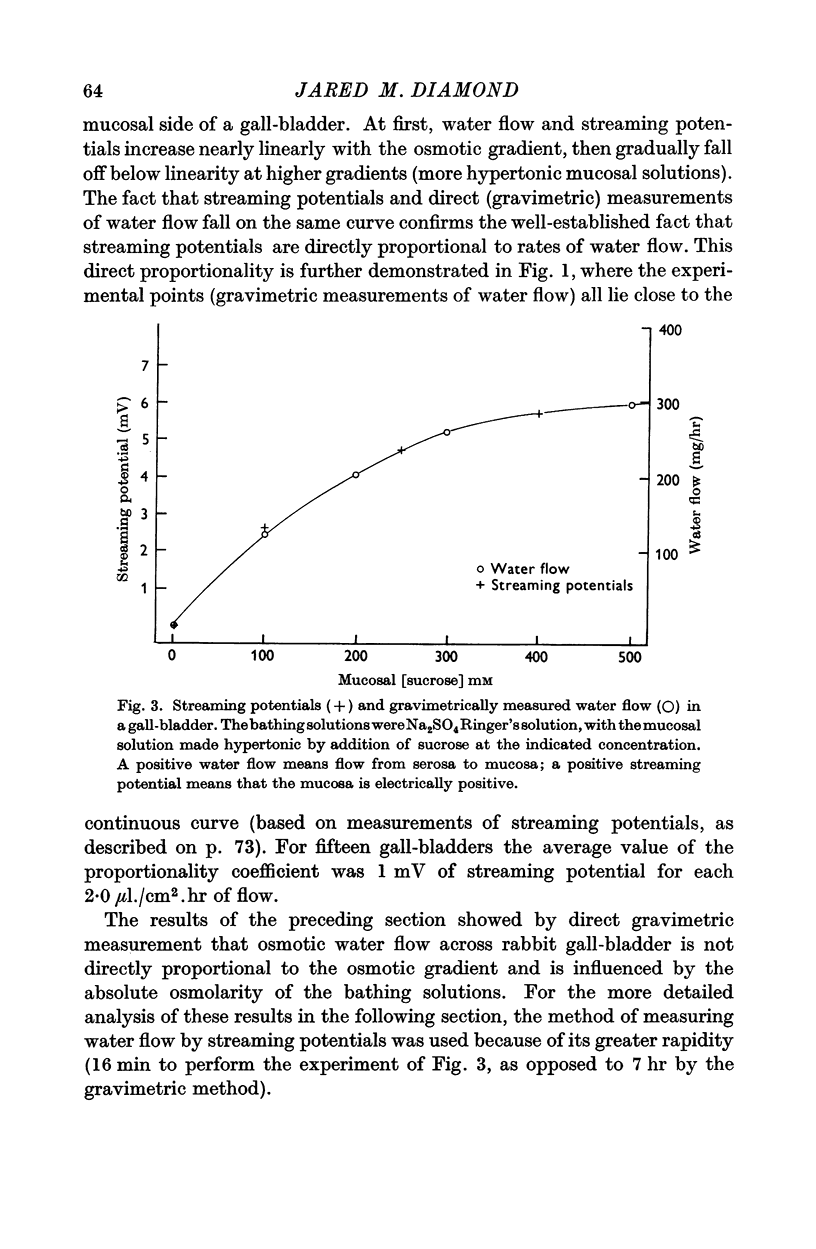
1. The relation between osmotic gradient and rate of osmotic water flow has been measured in rabbit gall-bladder by a gravimetric procedure and by a rapid method based on streaming potentials. Streaming potentials were directly proportional to gravimetrically measured water fluxes.
2. As in many other tissues, water flow was found to vary with gradient in a markedly non-linear fashion. There was no consistent relation between the water permeability and either the direction or the rate of water flow.
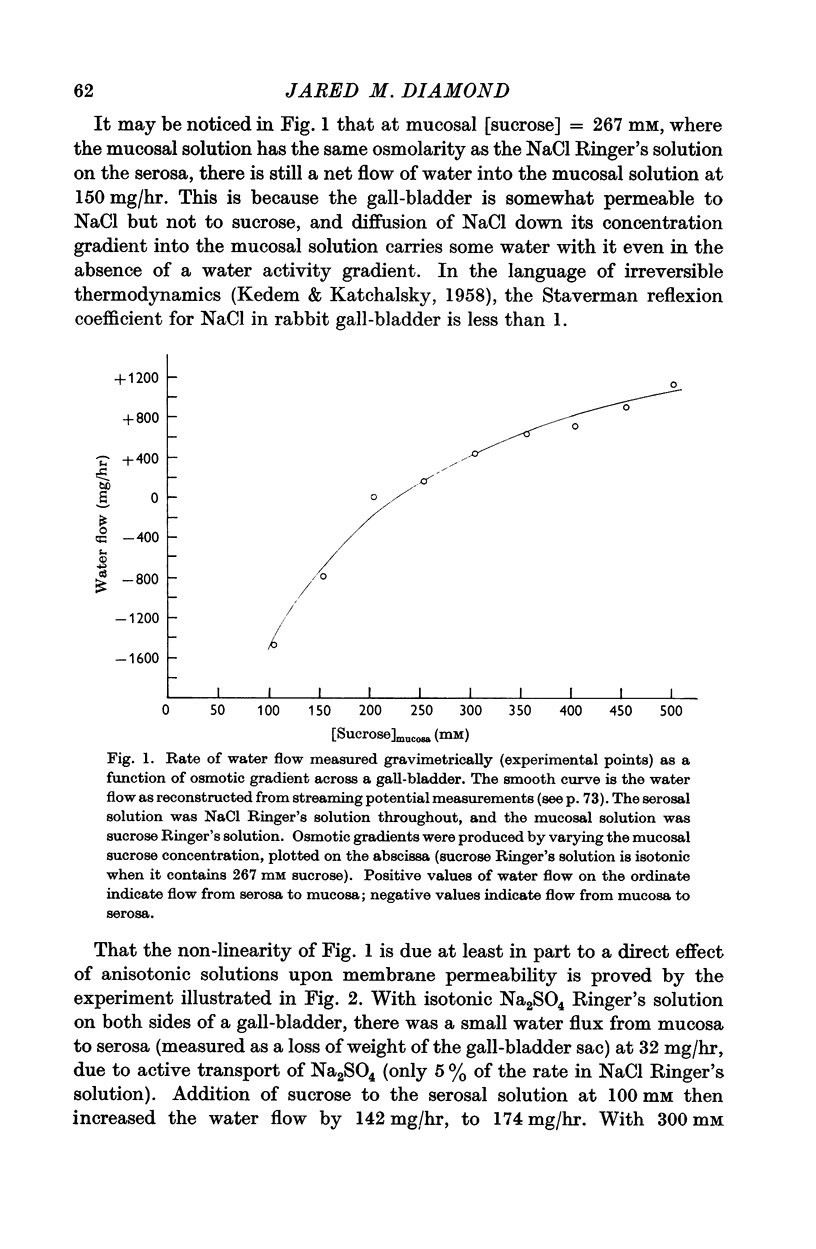
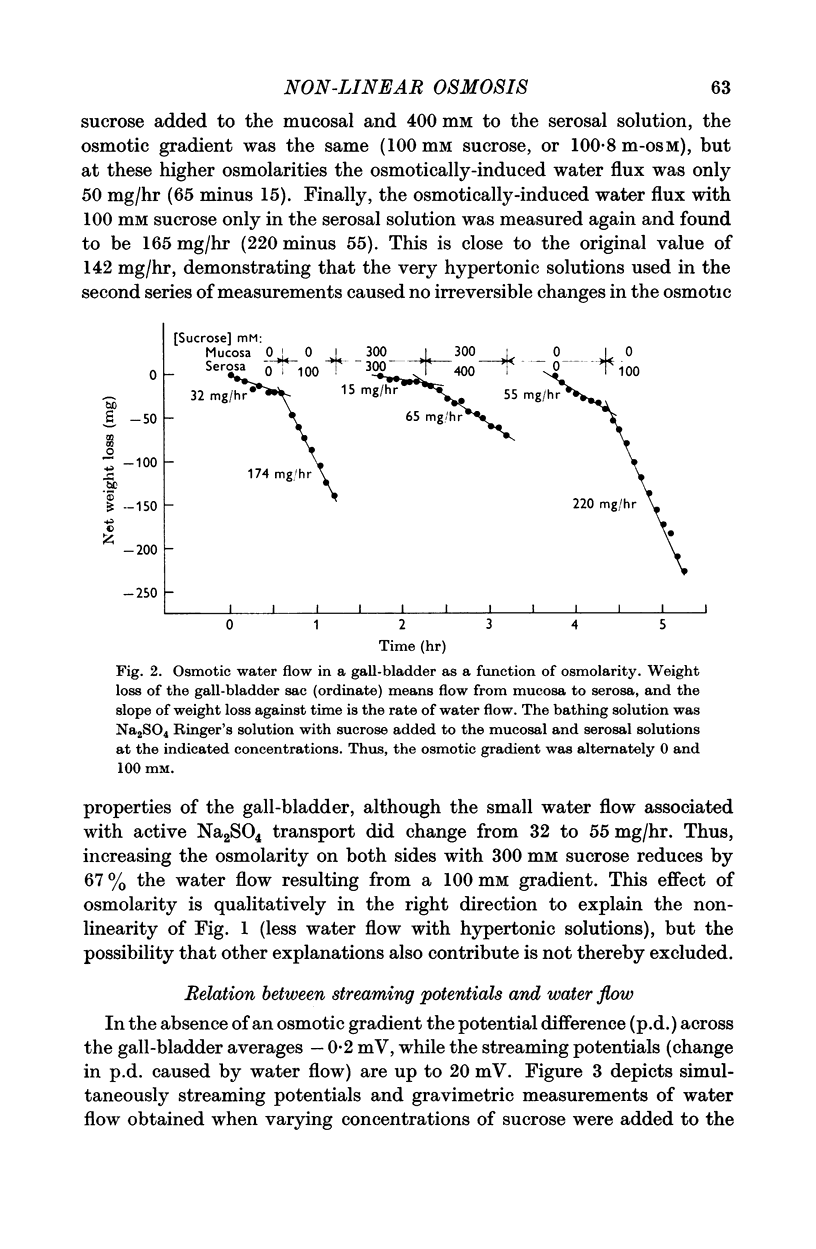
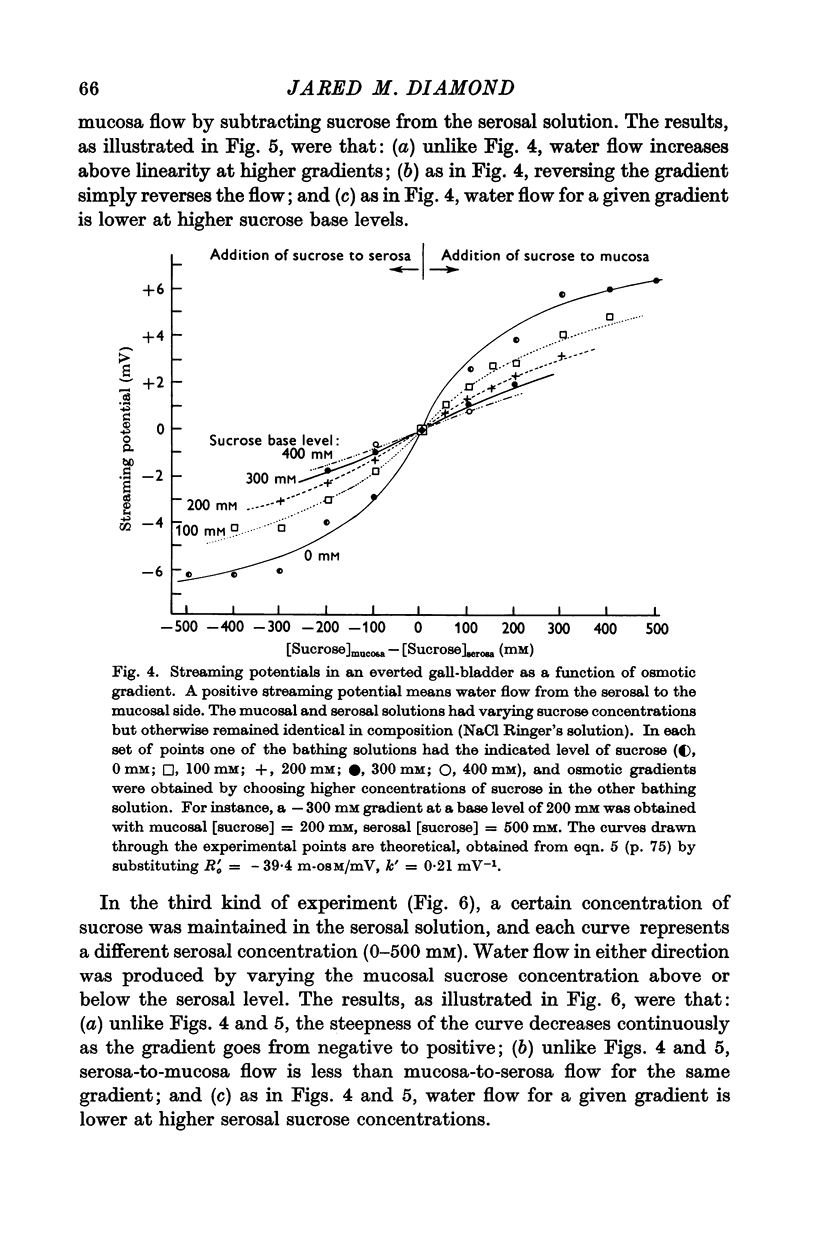
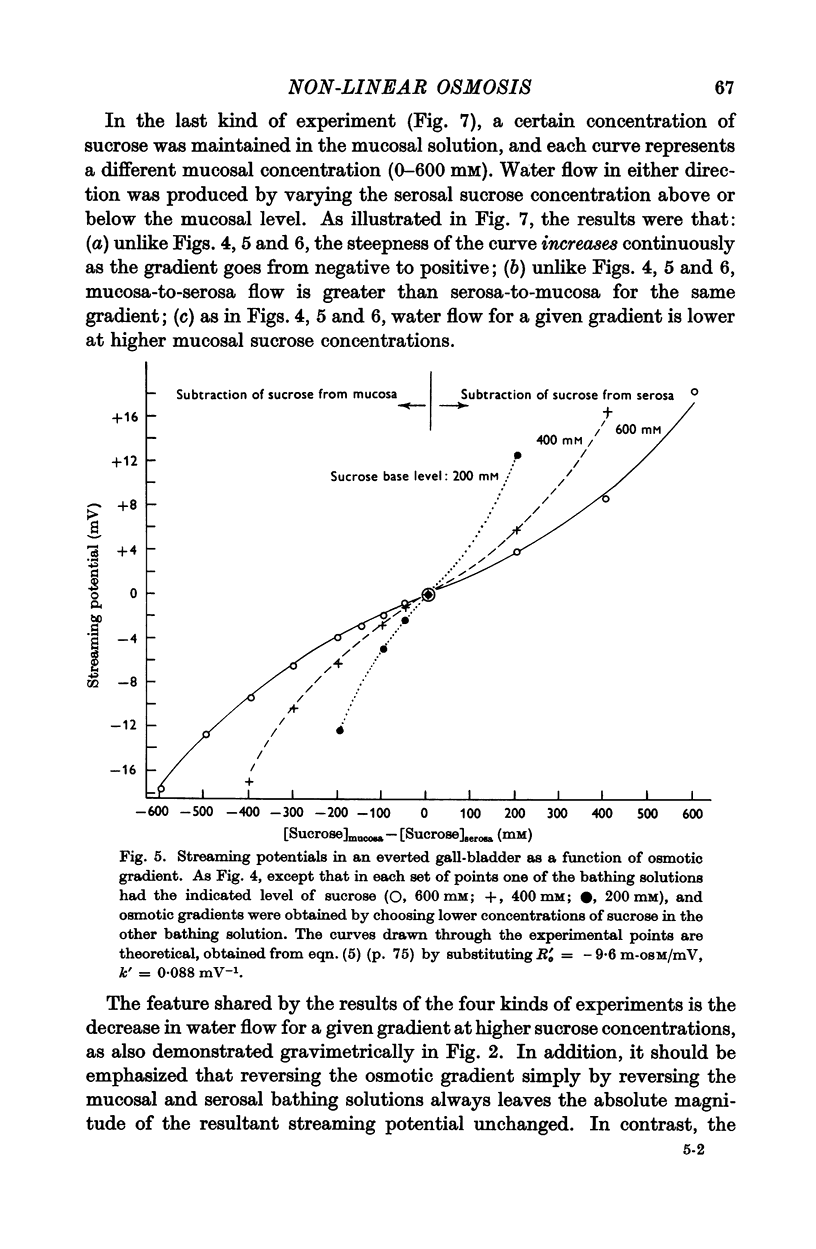
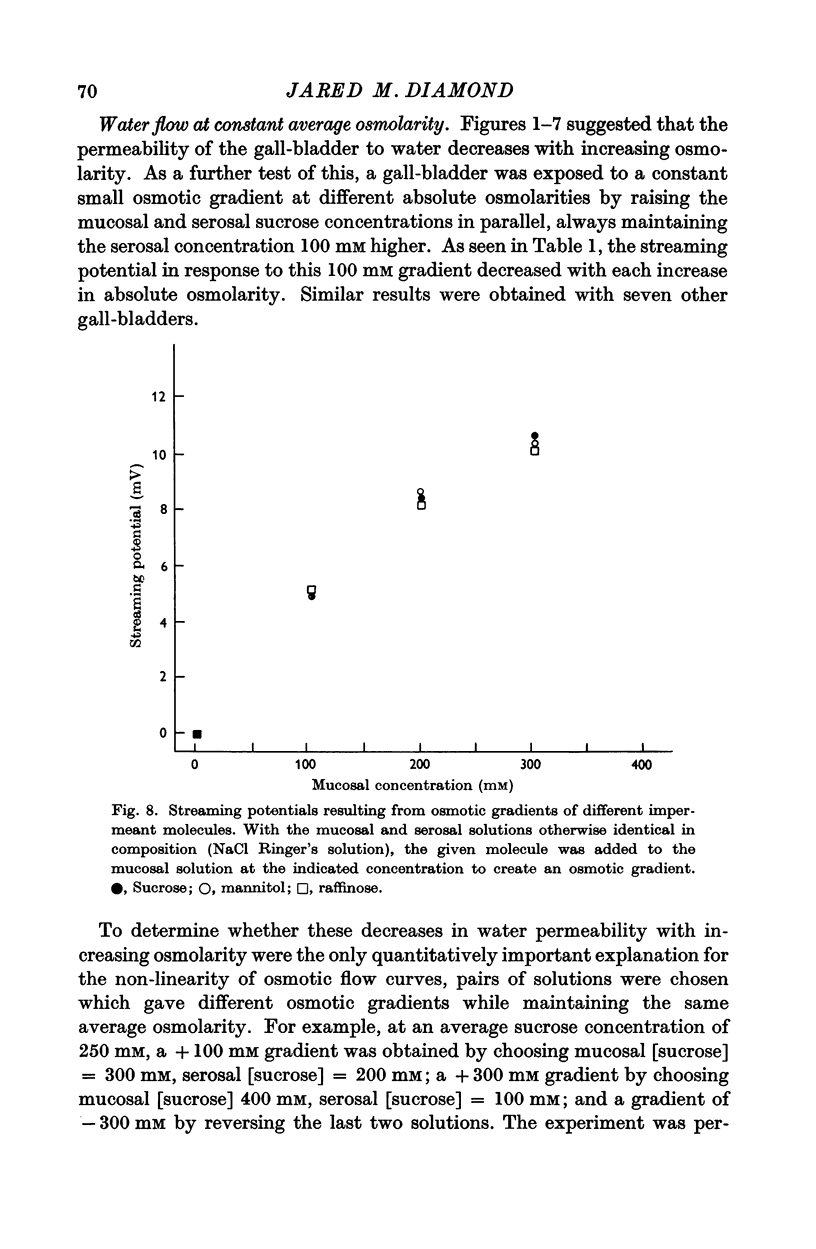
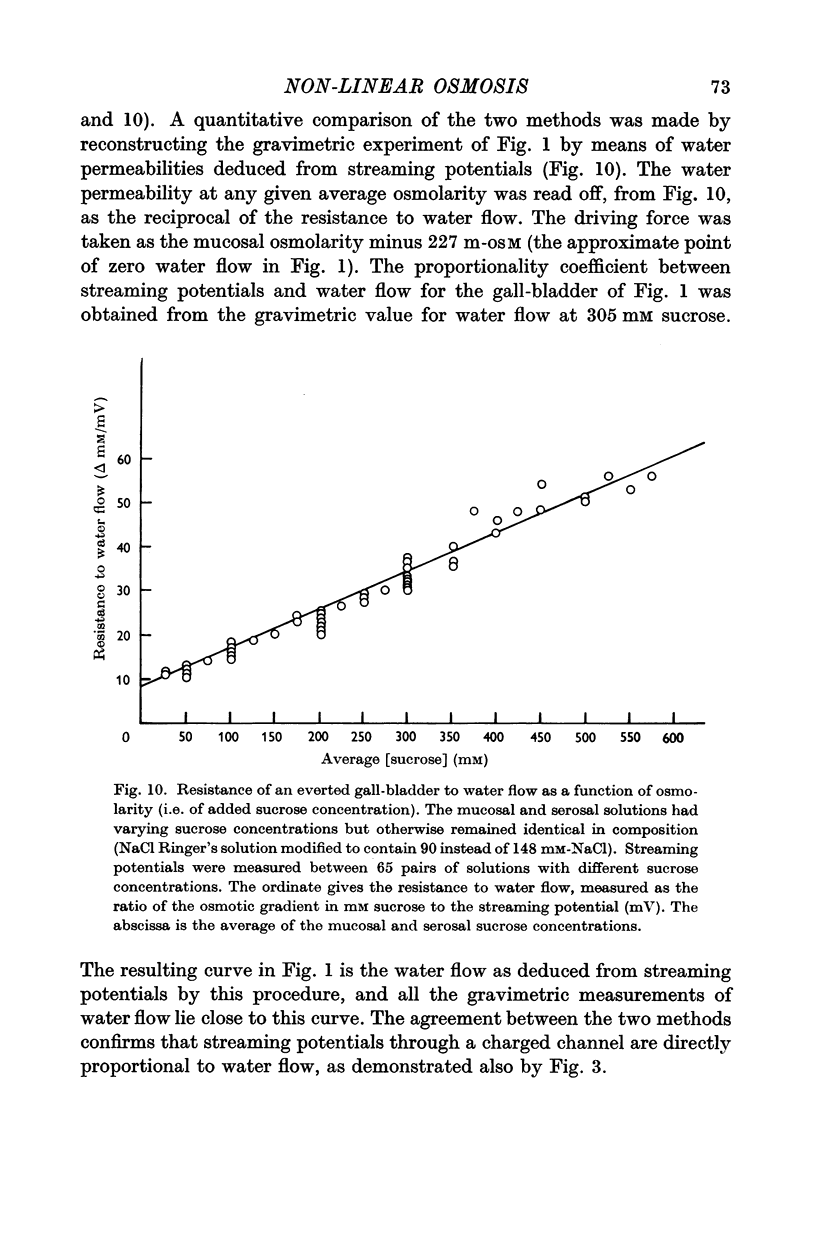
3. Water flow in response to a given gradient decreased at higher osmolarities. The resistance to water flow increased linearly with osmolarity over the range 186-825 m-osM.
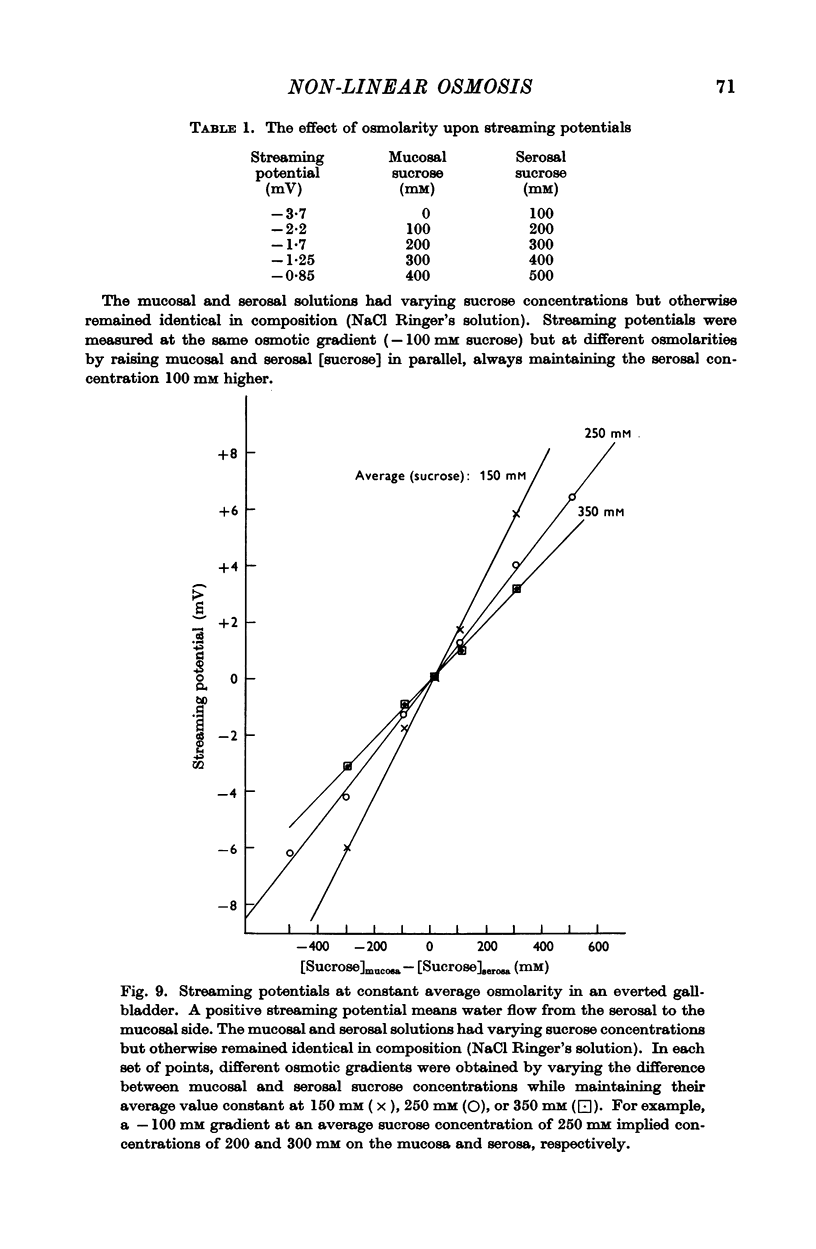
4. The resistance to water flow was the same when the gall-bladder separated any two bathing solutions with the same average osmolarity, regardless of the magnitude of the gradient. In other words, the rate of water flow is given by the expression (Om — Os)/[Ro′ + ½k′ (Om + Os)], where Ro′ and k′ are constants and Om and Os are the bathing solution osmolarities.
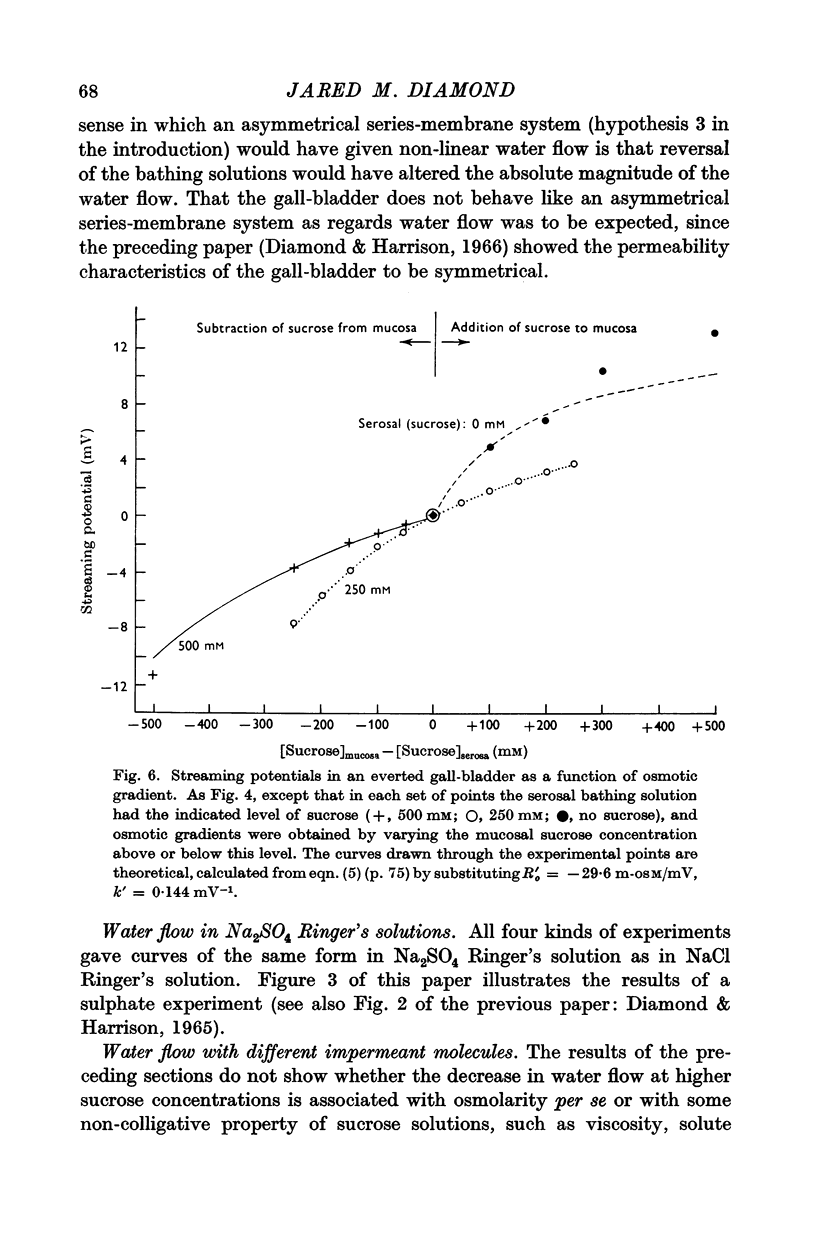
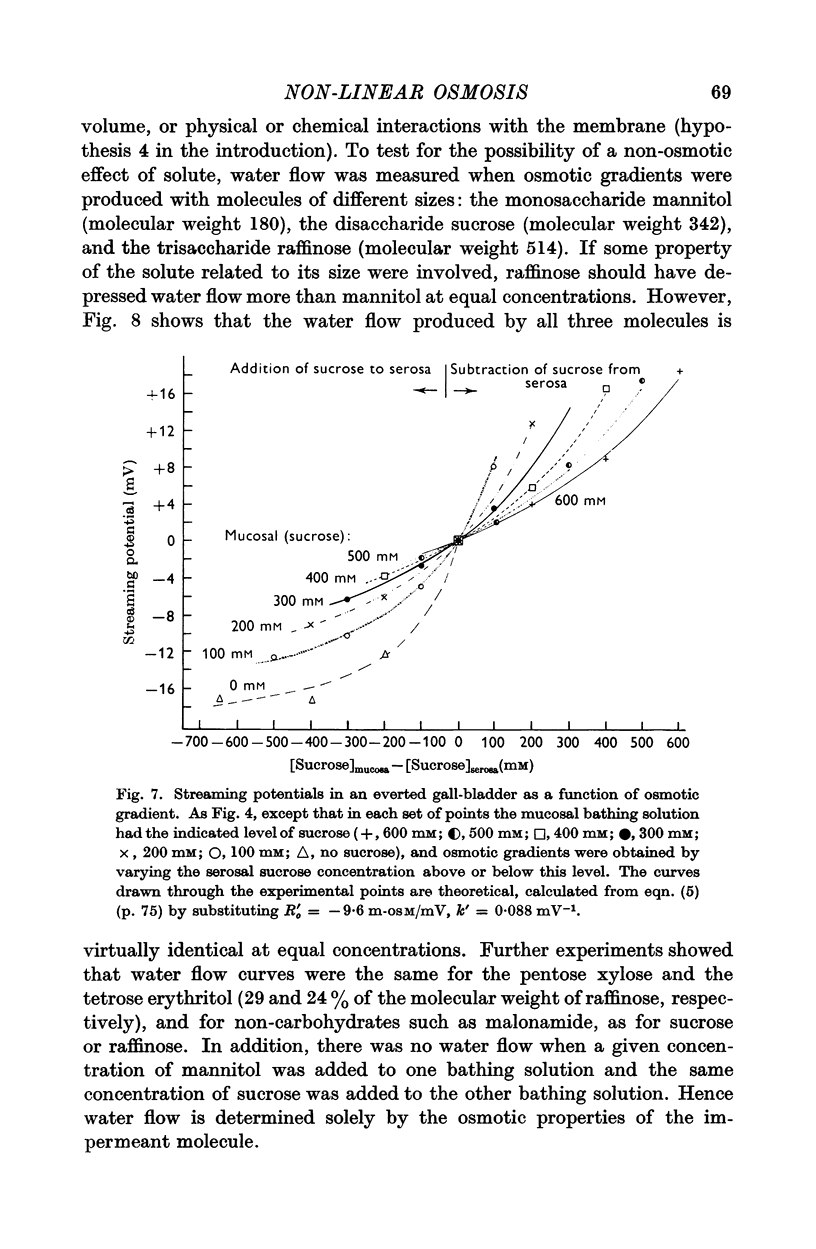
5. Of the theories advanced to explain non-linear osmosis in other tissues, flow-induced membrane deformations, unstirred layers, asymmetrical series-membrane effects, and non-osmotic effects of solutes could not explain the results. However, experimental measurements of water permeability as a function of osmolarity permitted quantitative reconstruction of the observed water flow—osmotic gradient curves. Hence non-linear osmosis in rabbit gall-bladder is due to a decrease in water permeability with increasing osmolarity.
6. The results suggest that aqueous channels in the cell membrane behave as osmometers, shrinking in concentrated solutions of impermeant molecules and thereby increasing membrane resistance to water flow. A mathematical formulation of such a membrane structure is offered.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODSKY W. A., SCHILB T. P. OSMOTIC PROPERTIES OF ISOLATED TURTLE BLADDER. Am J Physiol. 1965 Jan;208:46–57. doi: 10.1152/ajplegacy.1965.208.1.46. [DOI] [PubMed] [Google Scholar]
- DAINTY J., GINZBURG B. Z. THE MEASUREMENT OF HYDRAULIC CONDUCTIVITY (OSMOTIC PERMEABILITY TO WATER) OF INTERNODAL CHARACEAN CELLS BY MEANS OF TRANSCELLULAR OSMOSIS. Biochim Biophys Acta. 1964 Jan 27;79:102–111. doi: 10.1016/0926-6577(64)90043-9. [DOI] [PubMed] [Google Scholar]
- DAINTY J., GINZBURG B. Z. THE PERMEABILITY OF THE CELL MEMBRANES OF NITELLA TRANSLUCENS TO UREA, AND THE EFFECT OF HIGH CONCENTRATIONS OF SUCROSE ON THIS PERMEABILITY. Biochim Biophys Acta. 1964 Jan 27;79:112–121. doi: 10.1016/0926-6577(64)90044-0. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of water transport by the gall-bladder. J Physiol. 1962 May;161:503–527. doi: 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Harrison S. C. The effect of membrane fixed charges on diffusion potentials and streaming potentials. J Physiol. 1966 Mar;183(1):37–57. doi: 10.1113/jphysiol.1966.sp007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- OGILVIE J. T., McINTOSH J. R., CURRAN P. F. Volume flow in a series-membrane system. Biochim Biophys Acta. 1963 May 21;66:441–444. [PubMed] [Google Scholar]
- PIDOT A. L., DIAMOND J. M. STREAMING POTENTIALS IN A BIOLOGICAL MEMBRANE. Nature. 1964 Feb 15;201:701–702. doi: 10.1038/201701a0. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Goldstein D. A., Hoffman J. F. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963 Nov;5(3):426–442. doi: 10.1016/0022-5193(63)90088-2. [DOI] [PubMed] [Google Scholar]


