Abstract
1. Studies on the rate of efflux from the isolated perfused rat heart of plasma albumin conjugated with Evans Blue showed the conjugate to have penetrated extensively the extravascular compartment of the organ during a period of 2 min. This was confirmed by direct analysis of hearts for Evans Blue after perfusion.
2. Exposure of the hearts to Evans Blue-albumin conjugate for 8 min in vivo showed no significant penetration of the interstitial space.
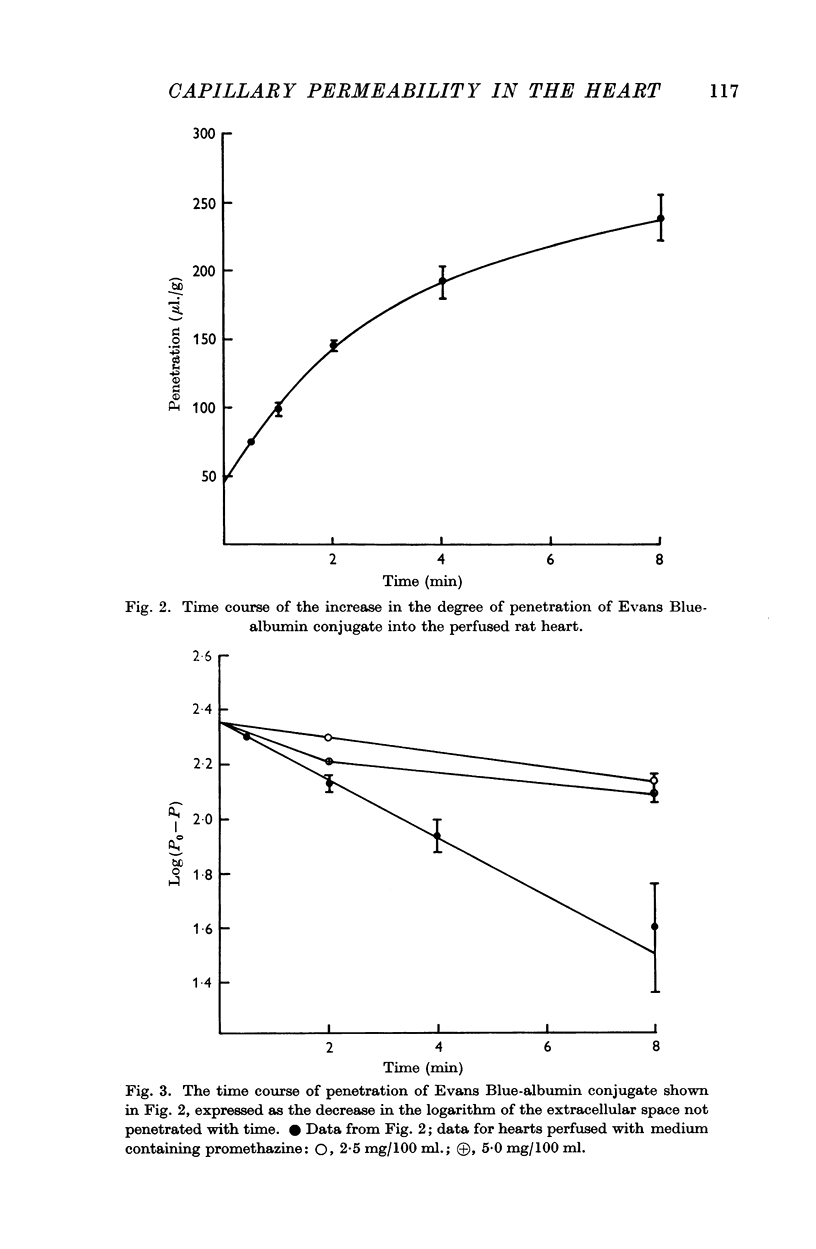
3. With the isolated preparation inclusion of promethazine in the perfusing medium significantly diminished the rate of penetration of the extravascular compartment by the conjugate as did injection of the animals with either reserpine 2 days before, or bretylium immediately before the experiment.
4. Penetration of the interstitial compartment in vivo could be induced by repeated asphyxiation. This penetration could also be diminished by promethazine but was not influenced by mepyramine maleate.
The increased permeability of capillaries to plasma proteins can be readily demonstrated in the whole animal by detecting the leakage from the vasculature of the plasma proteins conjugated with a dye (Menkin & Menkin, 1930; Miles & Miles, 1952). Evans Blue, which has been widely used as a vascular marker because of the stability of its conjugate with plasma albumin, is the most suitable dye for this purpose. In the present work this method has been applied to the isolated rat heart, to determine if changes in capillary permeability occur in the perfused tissue.
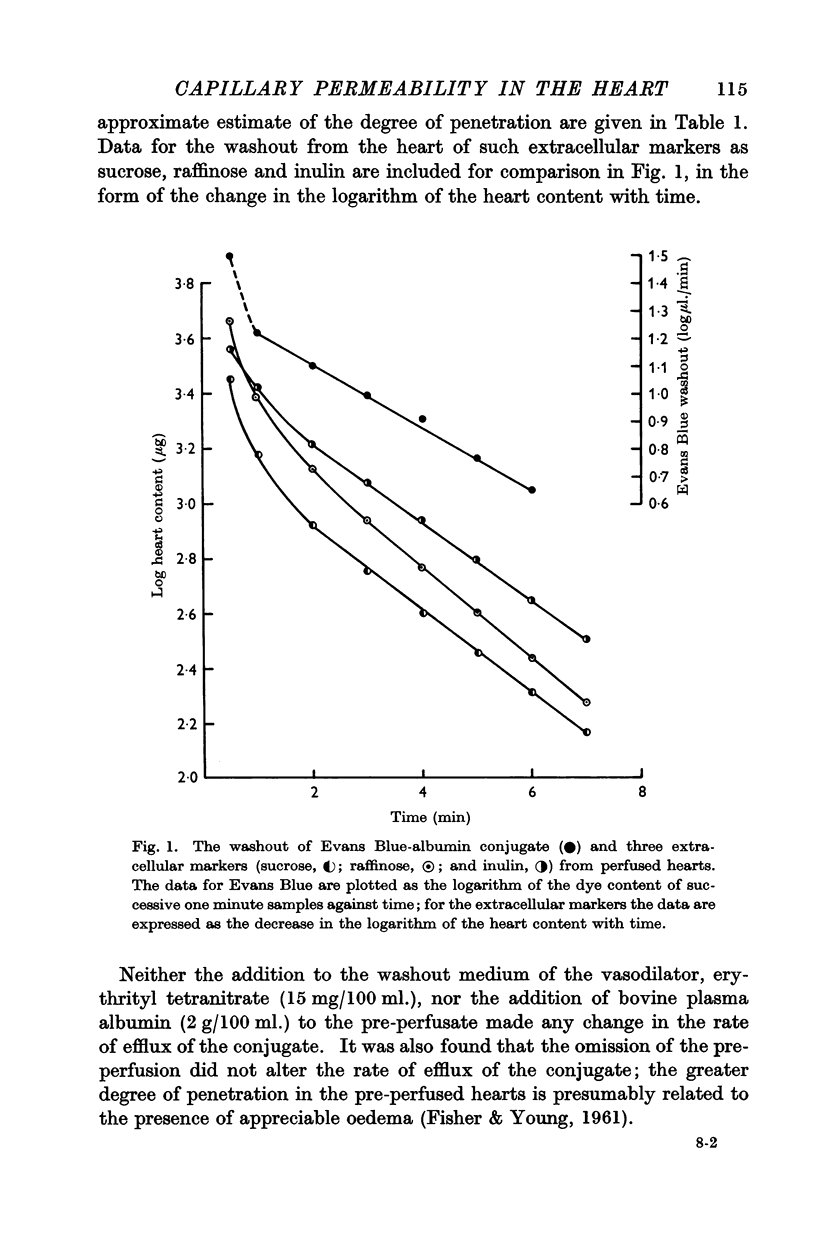
When plasma albumin conjugated with Evans Blue was used as a vascular marker in this preparation, its rate of clearance was very much less than that of erythrocytes, and the amount contained in the heart corresponded to a space approaching that occupied by extracellular markers such as raffinose and inulin. It was concluded, therefore, that there was a failure of a considerable number of the cardiac capillaries to retain the plasma albumin, and subsequent work was directed towards the identification of the condition that gives rise to these changes and the possible involvement of a permeability factor.
A preliminary account of part of this work was given to the Physiologcal Society (Sutherland & Young, 1961).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEEHEN N. M., FISHER R. B. The action of insulin in the isolated rat heart. J Physiol. 1954 Feb 26;123(2):260–276. doi: 10.1113/jphysiol.1954.sp005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURA A. L., GREEN A. F. The actions of bretylium: adrenergic neurone blocking and other effects. Br J Pharmacol Chemother. 1959 Dec;14:536–548. doi: 10.1111/j.1476-5381.1959.tb00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOLEY G. Some observations on impurities present in samples of Evan's blue (T 1824) and their influence on blood-volume determinations effected by the dye method. J Physiol. 1954 Jan;123(1):16–21. doi: 10.1113/jphysiol.1954.sp005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., YOUNG D. A. Direct determination of extracellular fluid in the rat heart. J Physiol. 1961 Sep;158:50–58. doi: 10.1113/jphysiol.1961.sp006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILMORE J. P., SIEGEL J. H. Mechanism of the myocardial effects of bretylium. Circ Res. 1962 Mar;10:347–353. doi: 10.1161/01.res.10.3.347. [DOI] [PubMed] [Google Scholar]
- LEE W. C., SHIDEMAN F. E. Role of myocardial catecholamines in cardiac contractility. Science. 1959 Apr 10;129(3354):967–968. doi: 10.1126/science.129.3354.967. [DOI] [PubMed] [Google Scholar]
- MILES A. A., MILES E. M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952 Oct;118(2):228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARRATT J. R., WEST G. B. Release of 5-hydroxytryptamine and histamine from tissues of the rat. J Physiol. 1957 Jul 11;137(2):179–192. doi: 10.1113/jphysiol.1957.sp005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M. Compound 48/80: a potent histamine liberator. Br J Pharmacol Chemother. 1951 Sep;6(3):499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPARROW E. M., WILHELM D. L. Species differences in susceptibility to capillary permeability factors: histamine, 5-hydroxytrytamine and compound 48/80. J Physiol. 1957 Jun 18;137(1):51–65. doi: 10.1113/jphysiol.1957.sp005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILHELM D. L. The mediation of increased vascular permeability in inflammation. Pharmacol Rev. 1962 Jun;14:251–280. [PubMed] [Google Scholar]
- YOUNG A. B. A METHOD FOR EXTRACTION OF EVANS BLUE FROM PLASMA AND TISSUES. Proc Soc Exp Biol Med. 1964 May;116:220–222. doi: 10.3181/00379727-116-29207. [DOI] [PubMed] [Google Scholar]


