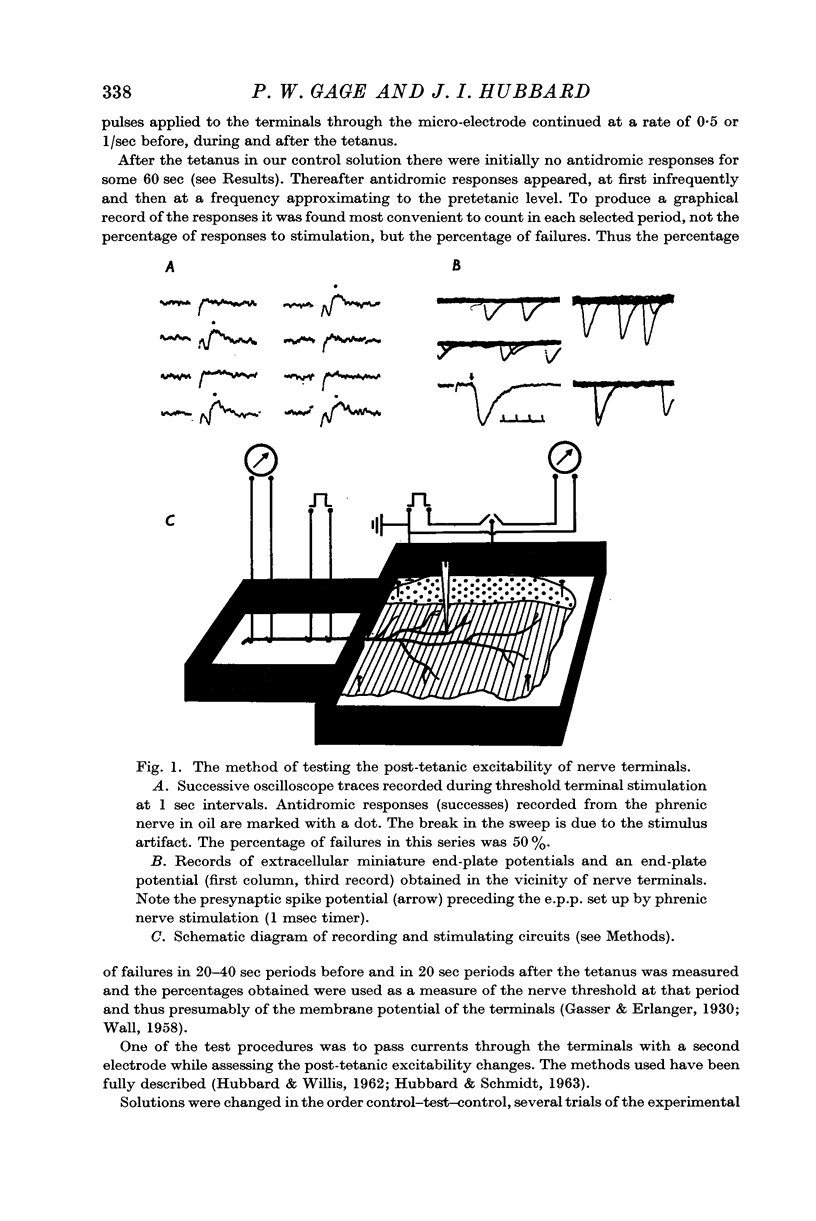
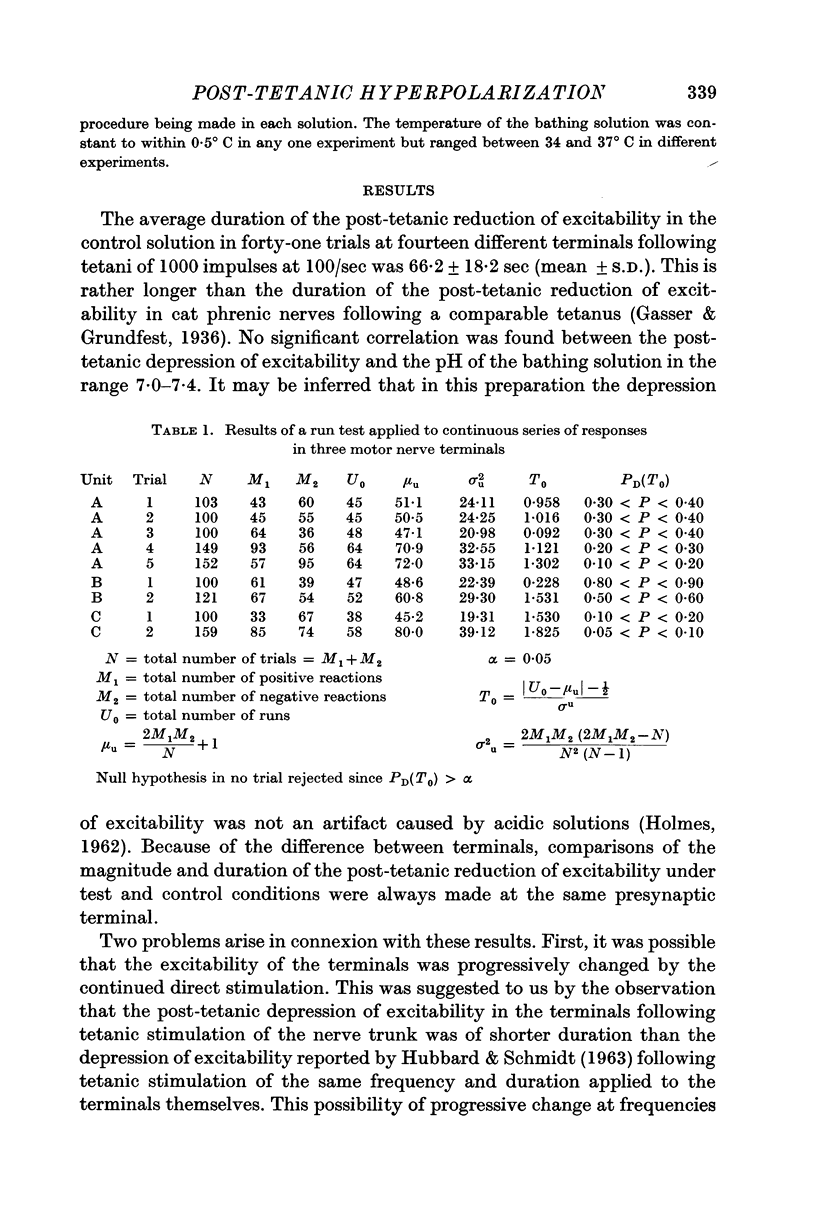
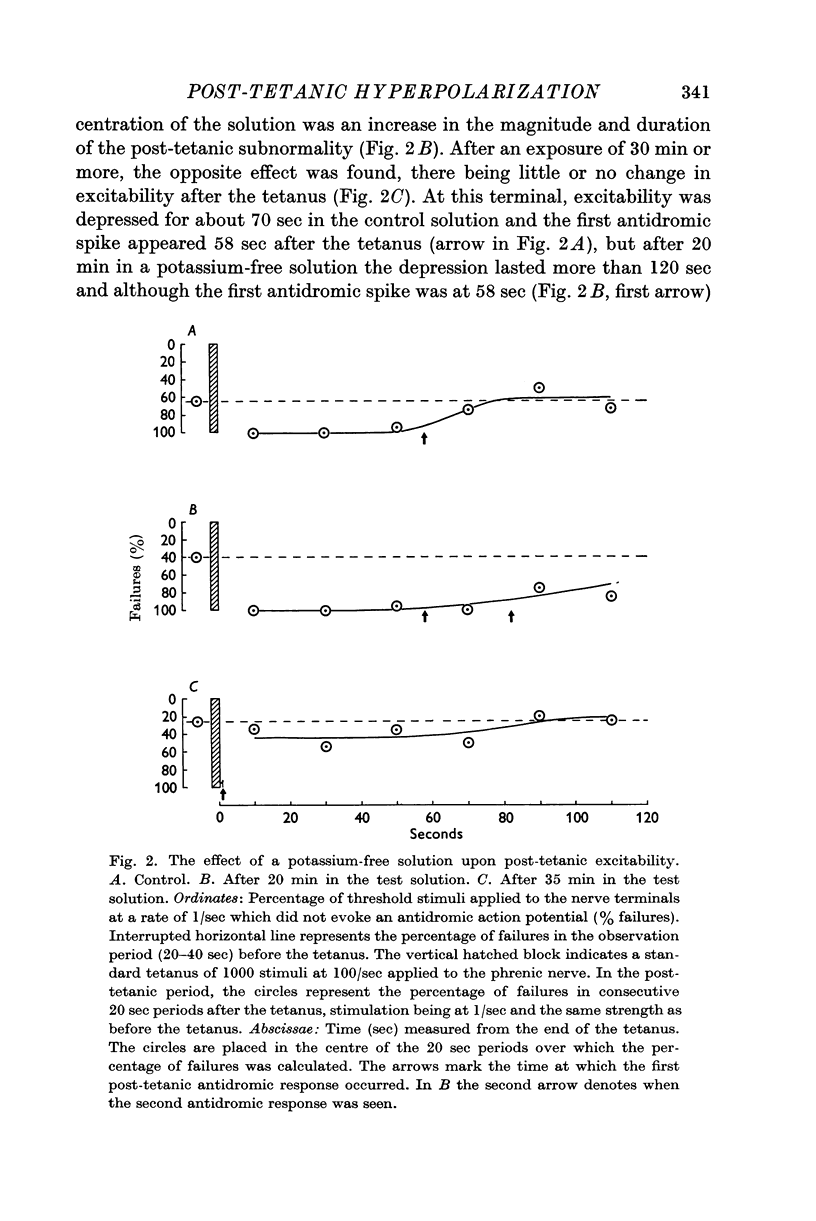
Abstract
1. Motor nerve terminals in magnesium-poisoned rat hemidiaphragm-phrenic nerve preparations in vitro were stimulated with short depolarizing pulses of approximately threshold strength and the evoked antidromic responses recorded from the phrenic nerve. The percentage of these 1/sec or 0·5/sec stimuli to which there was no antidromic response was used as a quantitative measure of the terminal excitability. After standard tetanic stimulation (1000 impulses at 100/sec) the excitability of the terminals was depressed for an average duration of 60-70 sec, during most of which time no antidromic responses to stimuli of pretetanic intensity were recorded. There was no significant interaction between stimuli to the terminals at rates of 1 or 0·5/sec.
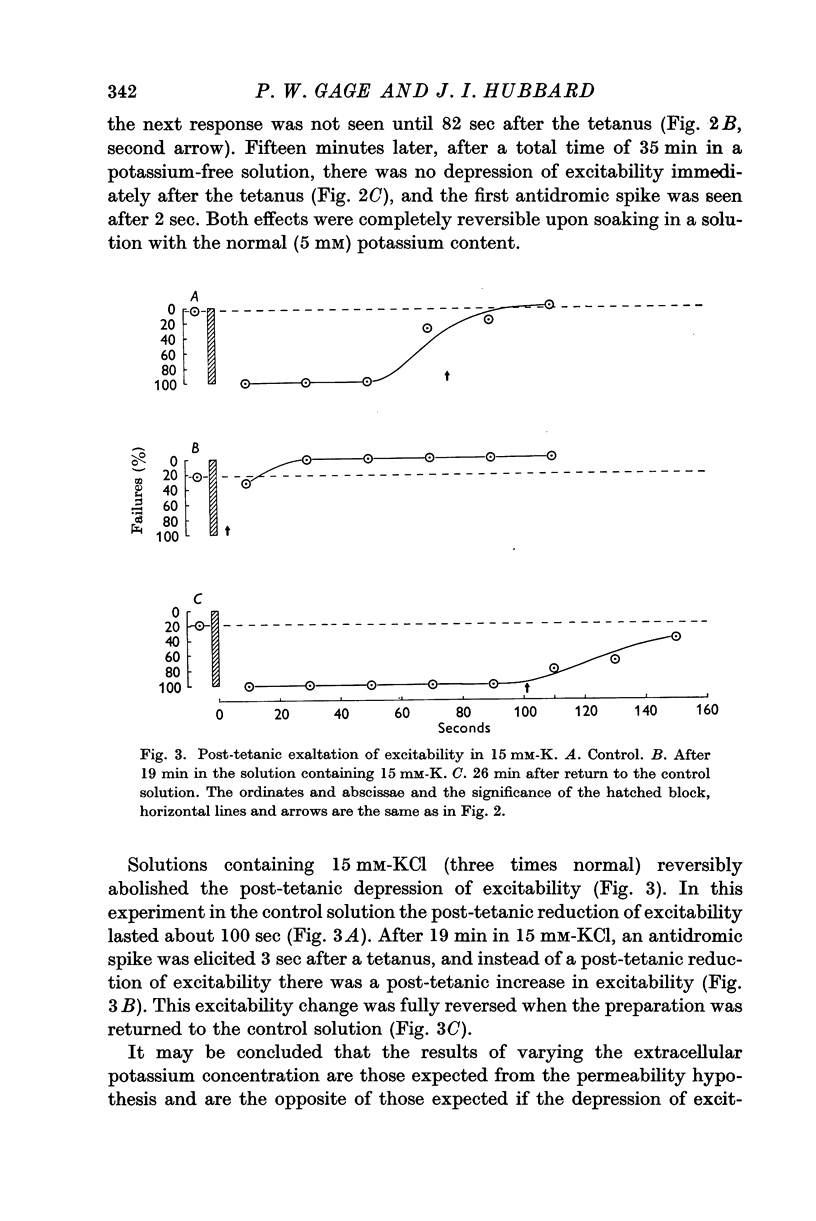
2. Potassium-free solutions at first increased, then decreased, the post-tetanic depression of excitability. Raising [K]o threefold (15 mM) abolished the post-tetanic depression and often converted it to an exaltation of excitability.
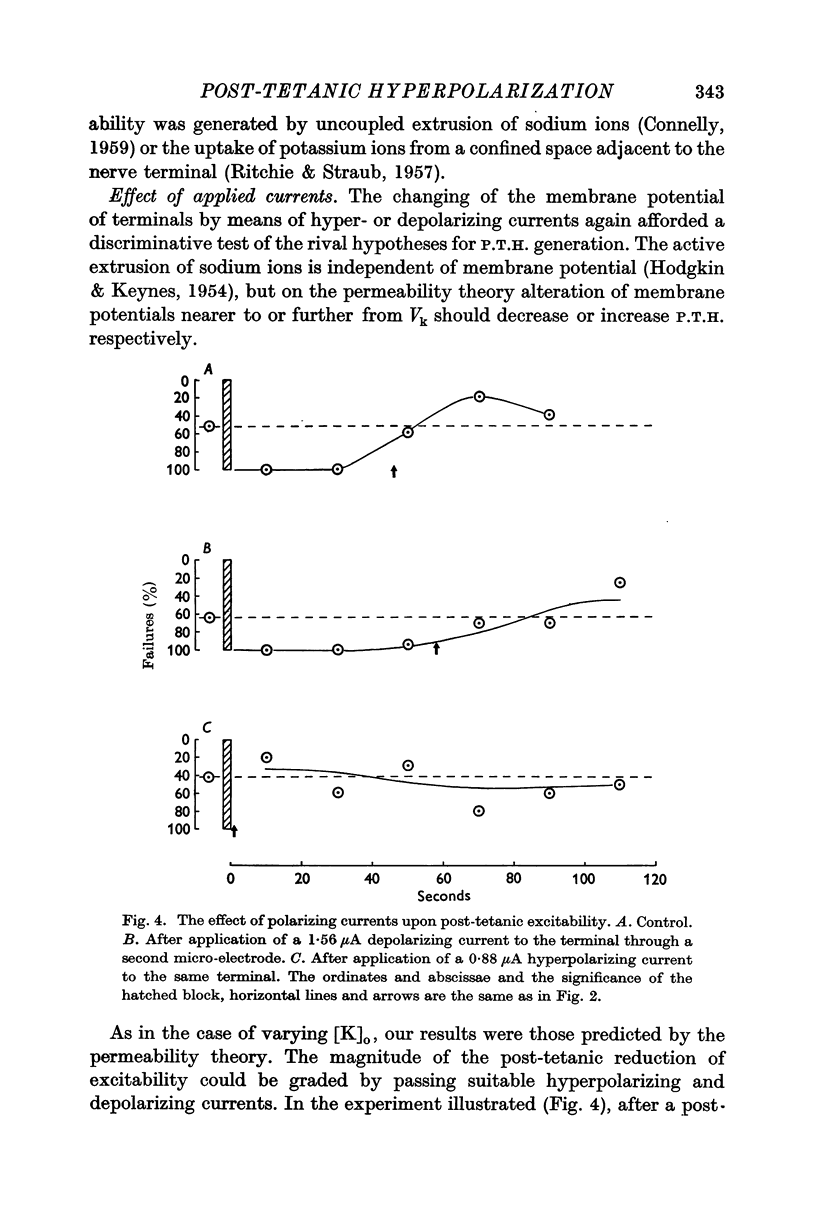
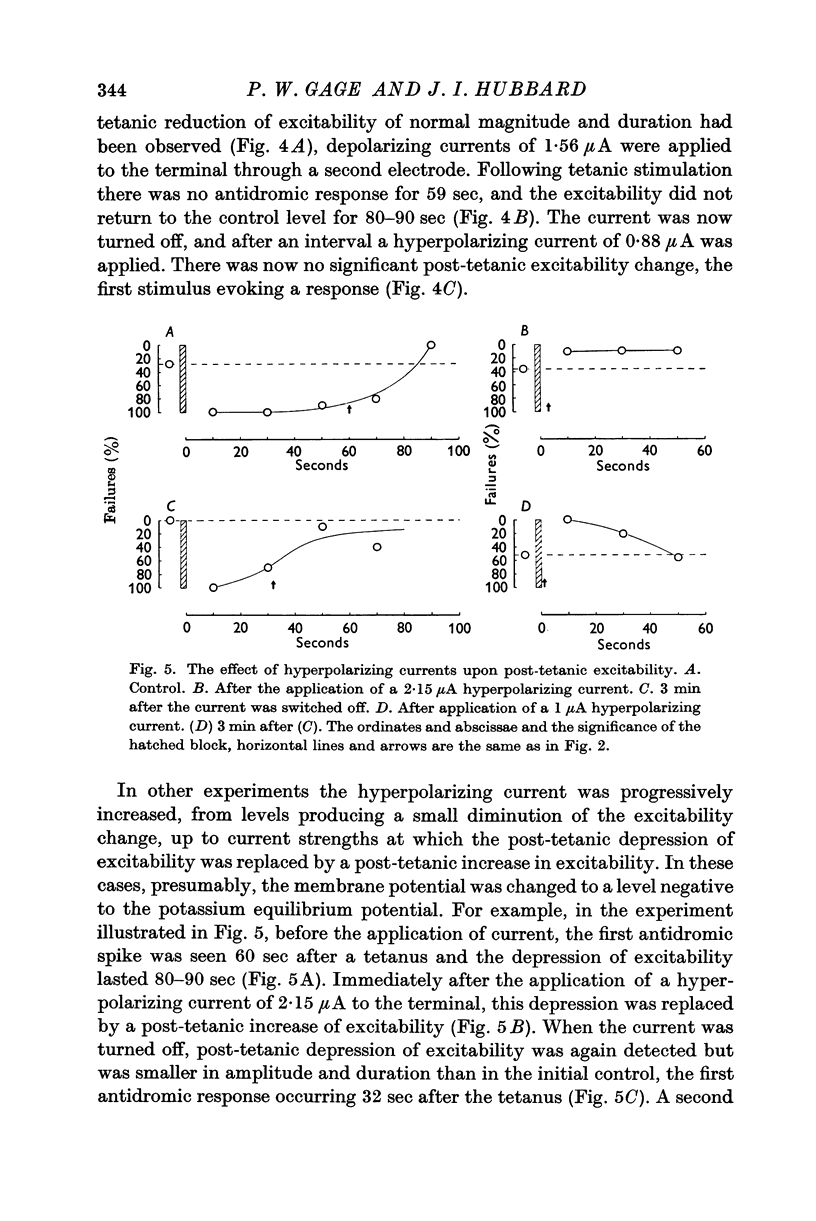
3. Polarizing currents were applied to the terminals with a second electrode. Depolarizing currents increased, while hyperpolarizing currents decreased, the post-tetanic depression of excitability.
4. In solutions with 70% of the normal NaCl content replaced by sucrose, the post-tetanic depression of excitability was reversibly prolonged.
5. In the presence of 7·7 × 10-6 M digoxin or 0·42 mM ouabain there was a small reversible reduction of post-tetanic excitability.
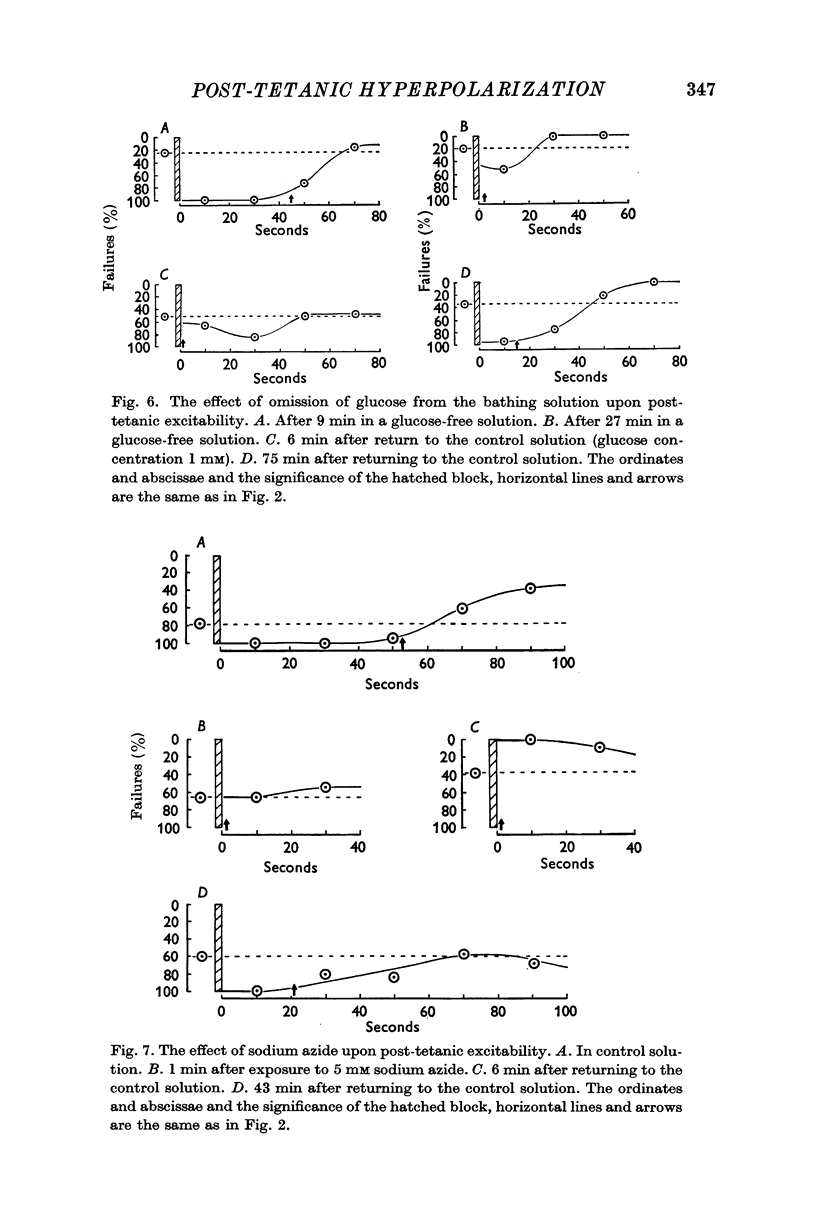
6. After exposure to solutions containing no glucose or to solutions containing 3-5 mM sodium azide the excitability of the terminals was not altered by the tetanus. After washing with the control solution, post-tetanic depression of excitability returned. Antimycin-A (1·8 × 10-6 M) had little or no effect upon post-tetanic excitability.
7. It was concluded that the post-tetanic depression of excitability reflected hyperpolarization of the terminals and that this hyperpolarization was caused by a shift of the membrane potential towards the potassium equilibrium potential because of an increase in potassium permeability.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. The recovery process of excitable tissues: Part I. J Physiol. 1920 Aug 19;54(1-2):1–31. doi: 10.1113/jphysiol.1920.sp001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE W. V. Structural variations of nerve endings in the striated muscles of the rat. J Comp Neurol. 1957 Dec;108(3):445–463. doi: 10.1002/cne.901080306. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K. Presynaptic changes associated with post-tetanic potentiation in the spinal cord. J Physiol. 1959 Dec;149:274–287. doi: 10.1113/jphysiol.1959.sp006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S. SODIUM PUMP: ITS ELECTRICAL EFFECTS IN SKELETAL MUSCLE. Science. 1965 Mar 19;147(3664):1442–1443. doi: 10.1126/science.147.3664.1442. [DOI] [PubMed] [Google Scholar]
- GAGE P. W., HUBBARD J. I. IONIC CHANGES RESPONSIBLE FOR POST-TETANIC HYPERPOLARIZATION. Nature. 1964 Aug 8;203:653–654. doi: 10.1038/203653a0. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEFF K., WESTERMANN E. Untersuchungen über die muskellähmende Wirkung des Strophanthins. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1955;226(2):103–113. [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Young J. Z. The re-innervation of muscle after various periods of atrophy. J Anat. 1944 Jan;78(Pt 1-2):15–43. [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES O. Effects of pH, changes in potassium concentration and metabolic inhibitors on the after-potentials of mammalian non-medullated nerve fibres. Arch Int Physiol Biochim. 1962 Mar;70:211–245. doi: 10.3109/13813456209092855. [DOI] [PubMed] [Google Scholar]
- HOROWICZ P., GERBER C. J. EFFECTS OF SODIUM AZIDE ON SODIUM FLUXES IN FROG STRIATED MUSCLE. J Gen Physiol. 1965 Jan;48:515–525. doi: 10.1085/jgp.48.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., GAGE P. W. ABOLITION OF POST-TETANIC POTENTIATION. Nature. 1964 Apr 18;202:299–300. doi: 10.1038/202299a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBUT W. P. Effects of azide and choretone on the sodium and potassium contents and the respiration of frog sciatic nerves. J Gen Physiol. 1958 May 20;41(5):959–988. doi: 10.1085/jgp.41.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBUT W. P. POTASSIUM FLUXES IN DESHEATHED FROG SCIATIC NERVE. J Gen Physiol. 1963 Jul;46:1223–1248. doi: 10.1085/jgp.46.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON J. A. Influence of ouabain, strophanthidin and dihydrostrophanthidin on sodium and potassium transport in frog sartorii. Am J Physiol. 1956 Nov;187(2):328–332. doi: 10.1152/ajplegacy.1956.187.2.328. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C. Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J Gen Physiol. 1949 Nov;33(2):147–170. doi: 10.1085/jgp.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEVES H. [The after-potentials of isolated medullated nerve fibers of the frog in tetanic stimulation]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;272:336–359. [PubMed] [Google Scholar]
- MULLINS L. J., AWAD M. Z. THE CONTROL OF THE MEMBRANE POTENTIAL OF MUSCLE FIBERS BY THE SODIUM PUMP. J Gen Physiol. 1965 May;48:761–775. doi: 10.1085/jgp.48.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDIN D. O., EISENMAN G. The action potential of spinal axons in vitro. J Gen Physiol. 1954 Mar;37(4):505–538. doi: 10.1085/jgp.37.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SLATER E. C. The constitution of the respiratory chain in animal tissues. Adv Enzymol Relat Subj Biochem. 1958;20:147–199. doi: 10.1002/9780470122655.ch6. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]