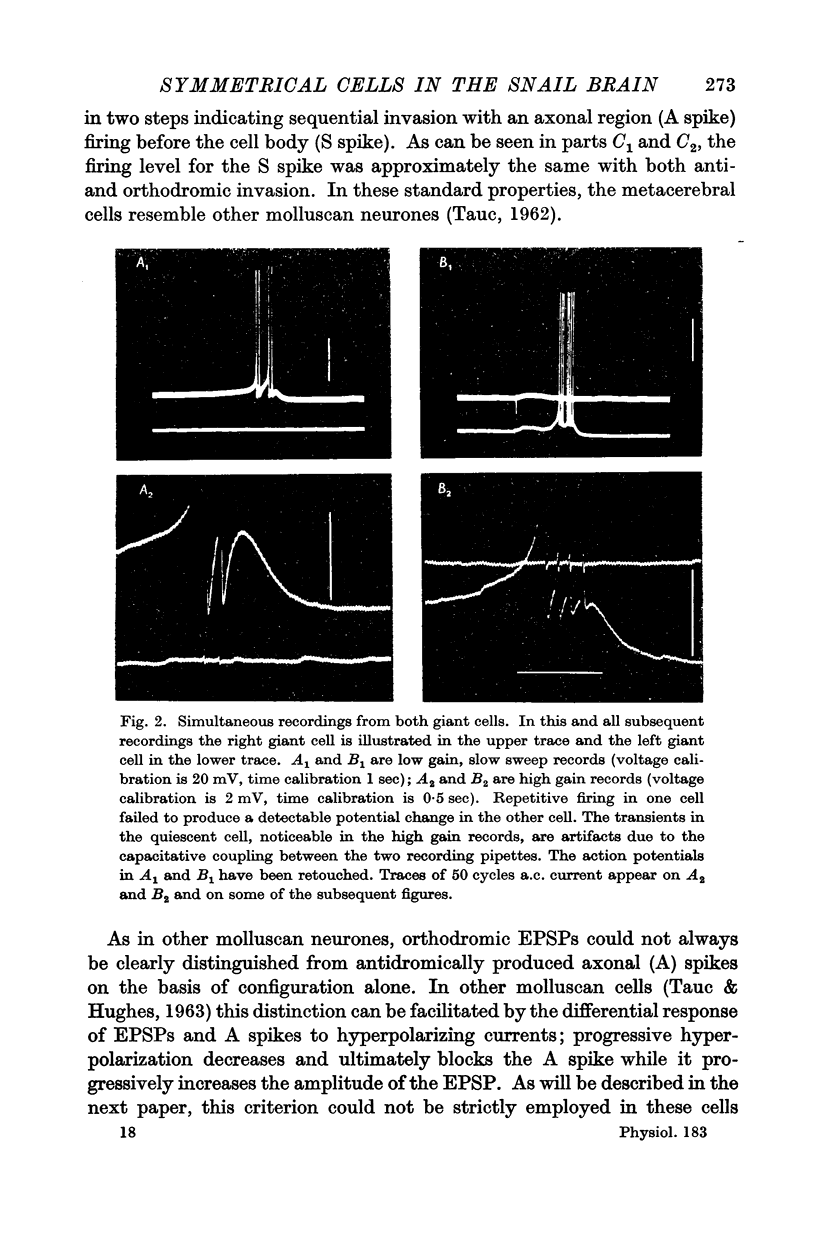
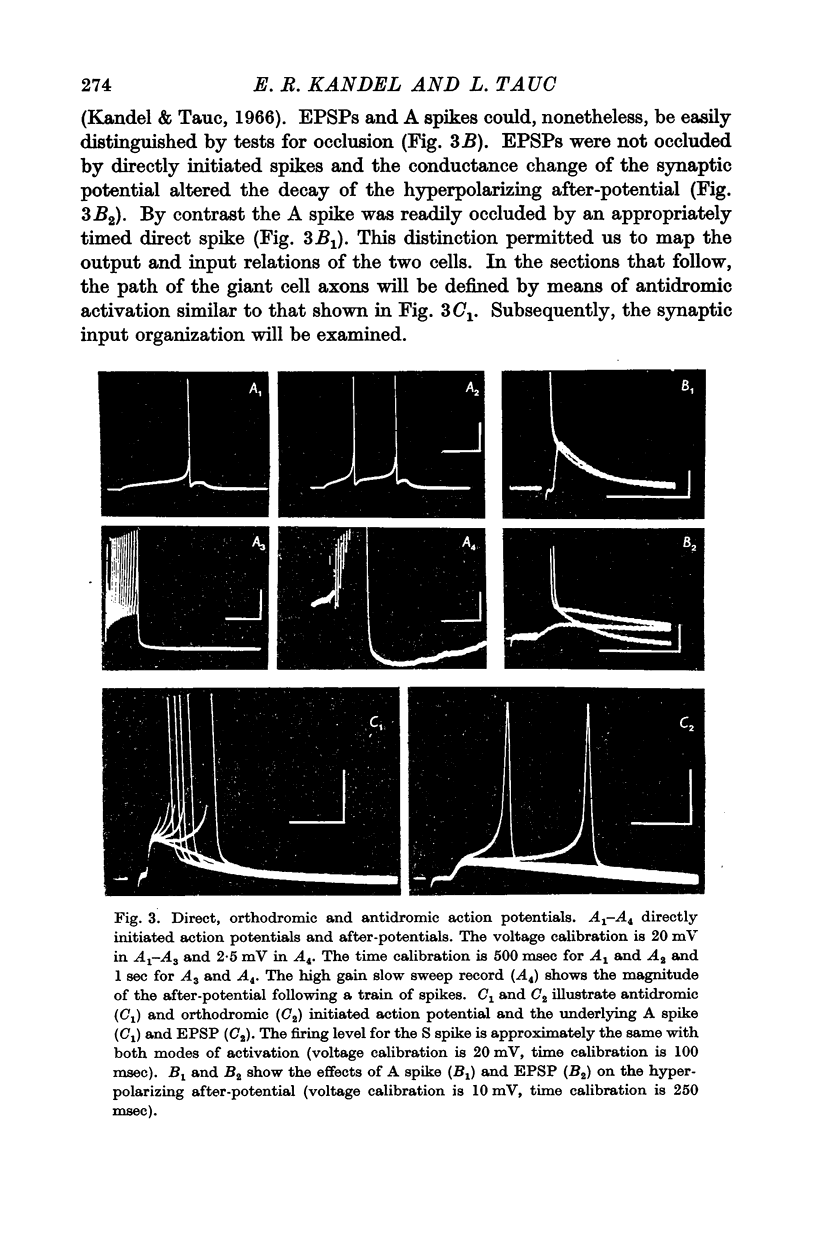
Abstract
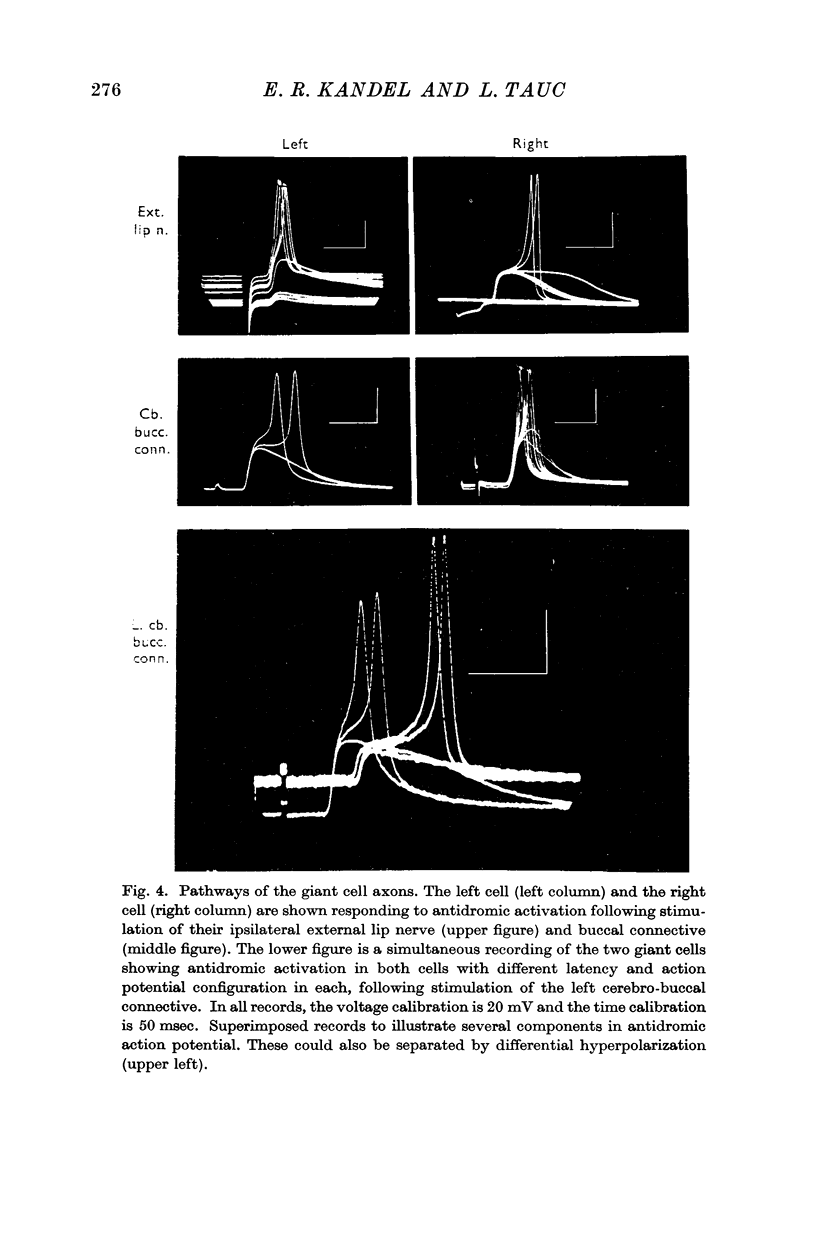
1. The ventral surface of the snail brain contains two symmetrical giant cells which are readily identifiable in each preparation. These cells lie in the left and right metacerebrum, a cerebral integrative structure which makes afferent and efferent connexions with the periphery and with the infraoesophageal ganglia on each side by means of four ipsi- and four contralateral peripheral nerves and three ipsi- and three contralateral connectives.
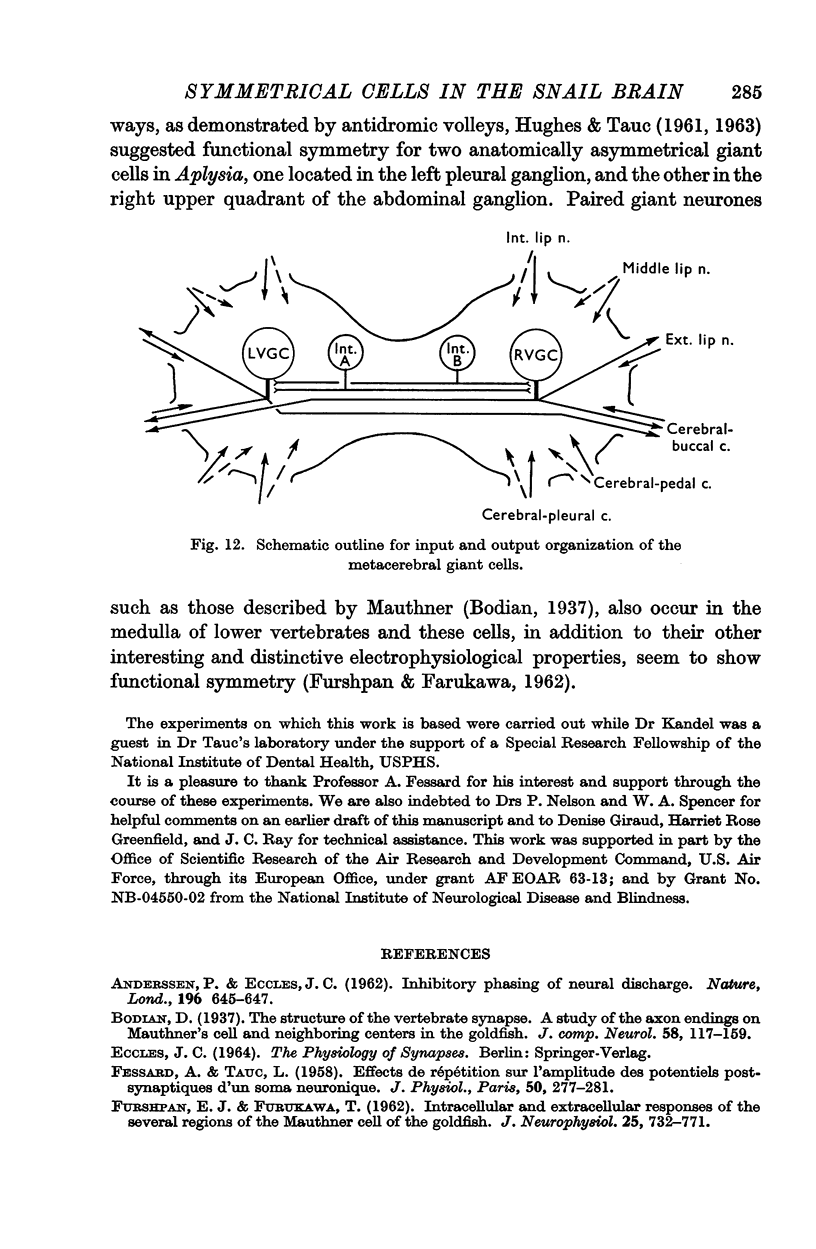
2. In order to examine the functional consequences of this anatomical symmetry single or double micro-electrodes, for intracellular recording and for direct stimulation, were placed in one or both of the ventral metacerebral giant cells.
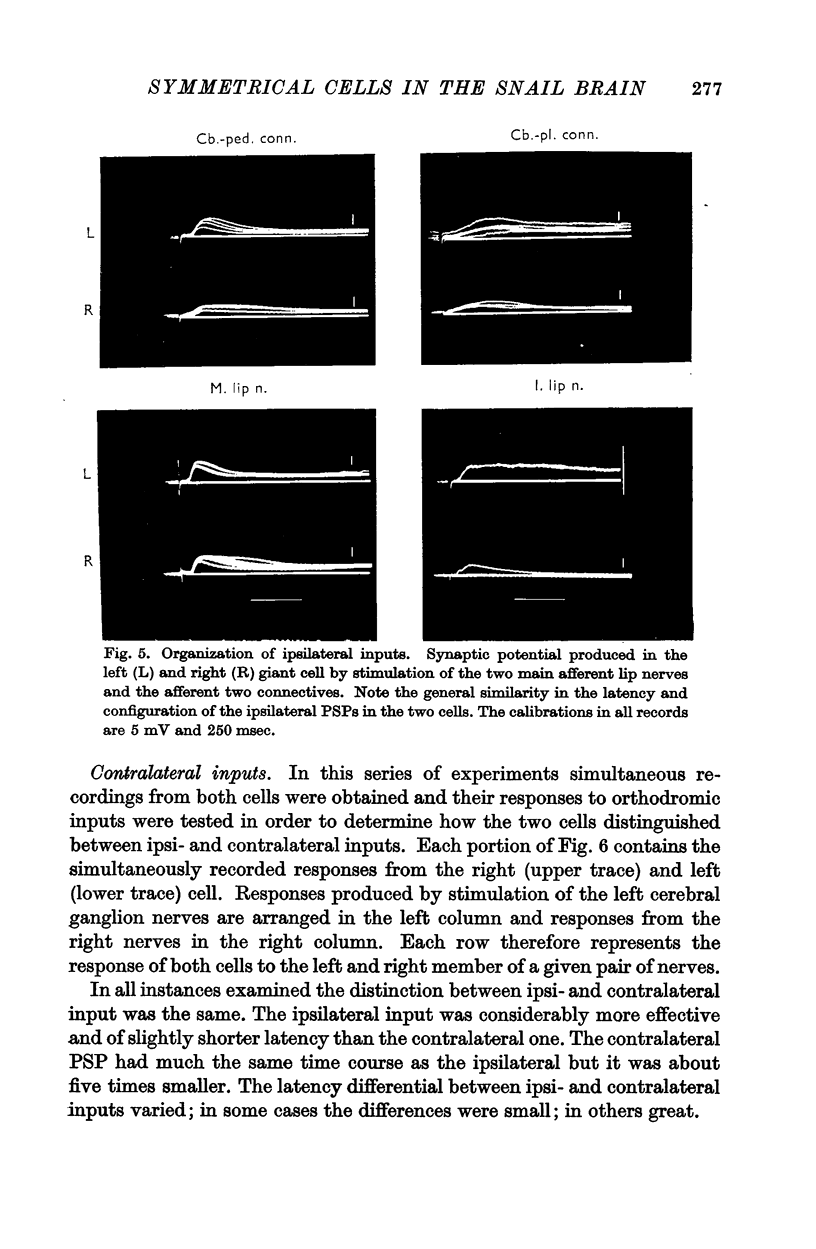
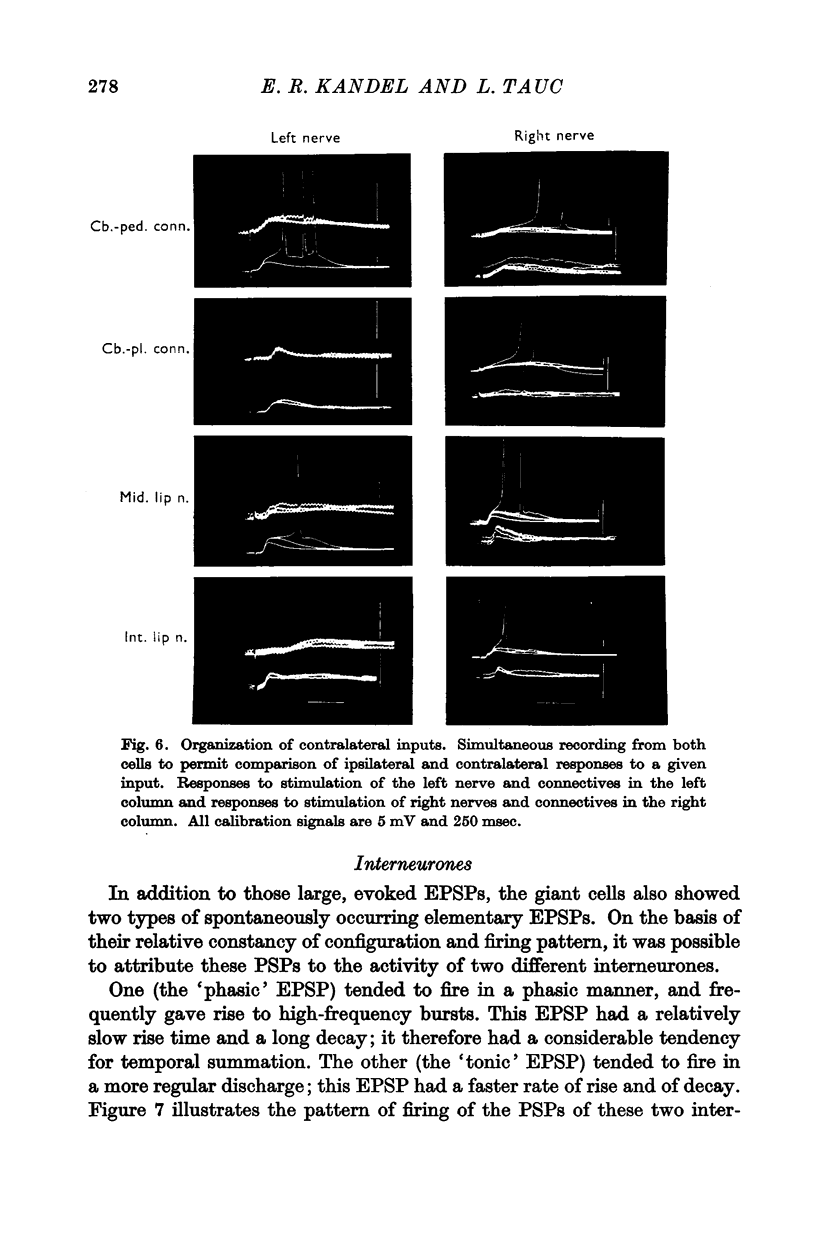
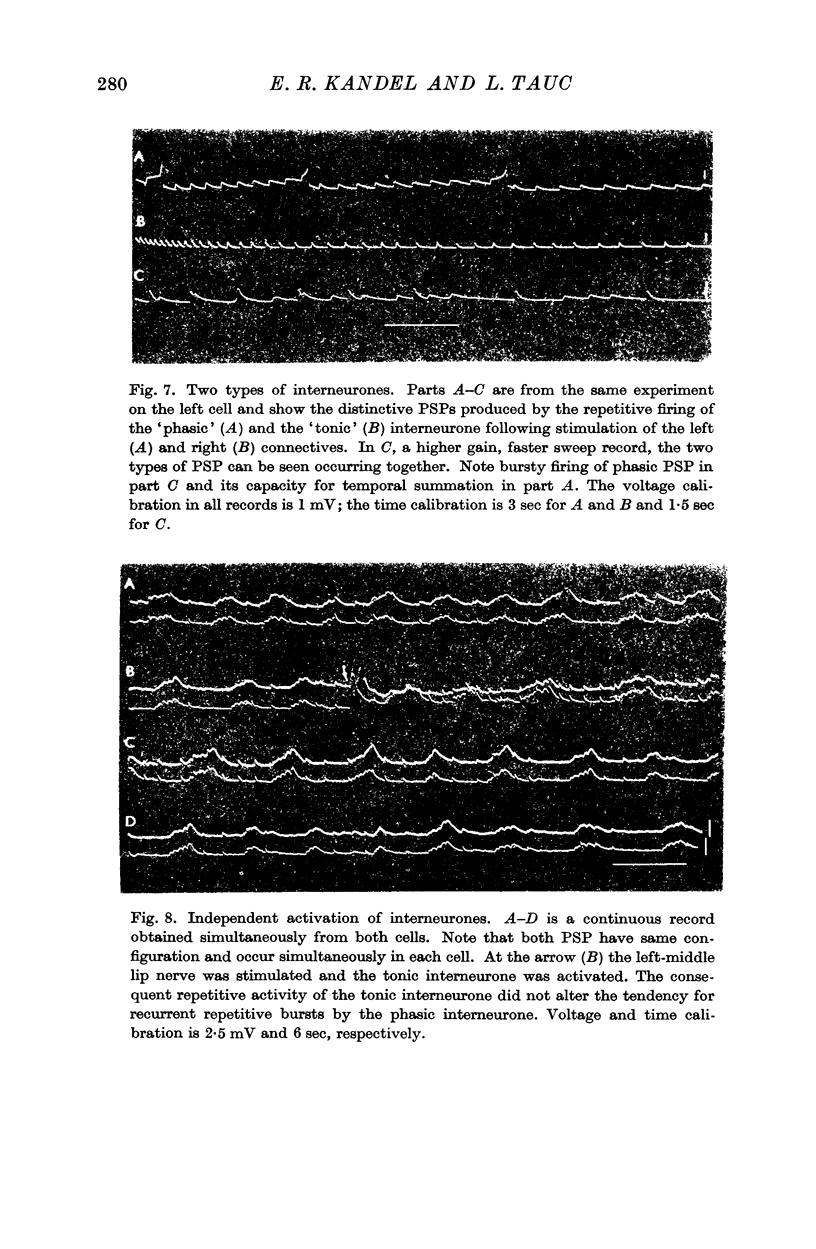
3. Responses from 6 ipsi- and 6 contralateral inputs were examined for orthodromic and antidromic components. The results of these experiments revealed that the output organization (the pathways of the three axonal branches of the giant cells) as well as the input organization were completely symmetrical in the two cells. The anatomical symmetry therefore seems to have been functionally preserved. Simultaneous recordings from both cells failed to reveal direct interconnexions, but did show that the cells do share in common the output of at least two interneurones.
4. By recording from both cells it was also possible to demonstrate, for each of the symmetrical nerves and connectives, that laterality of an input was signalled by latency and by synaptic efficacy; the ipsilateral input produced EPSPs that were consistently more effective and of shorter latency than the contralateral ones.
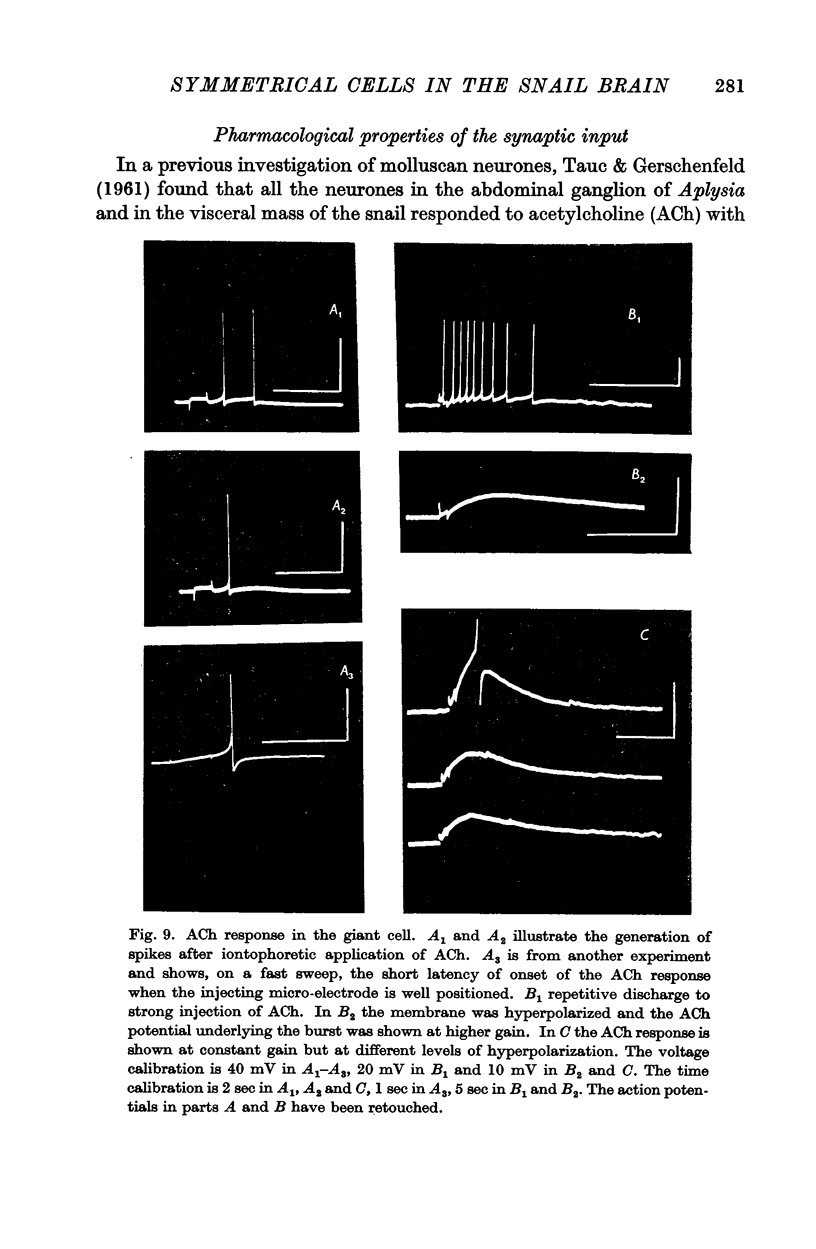
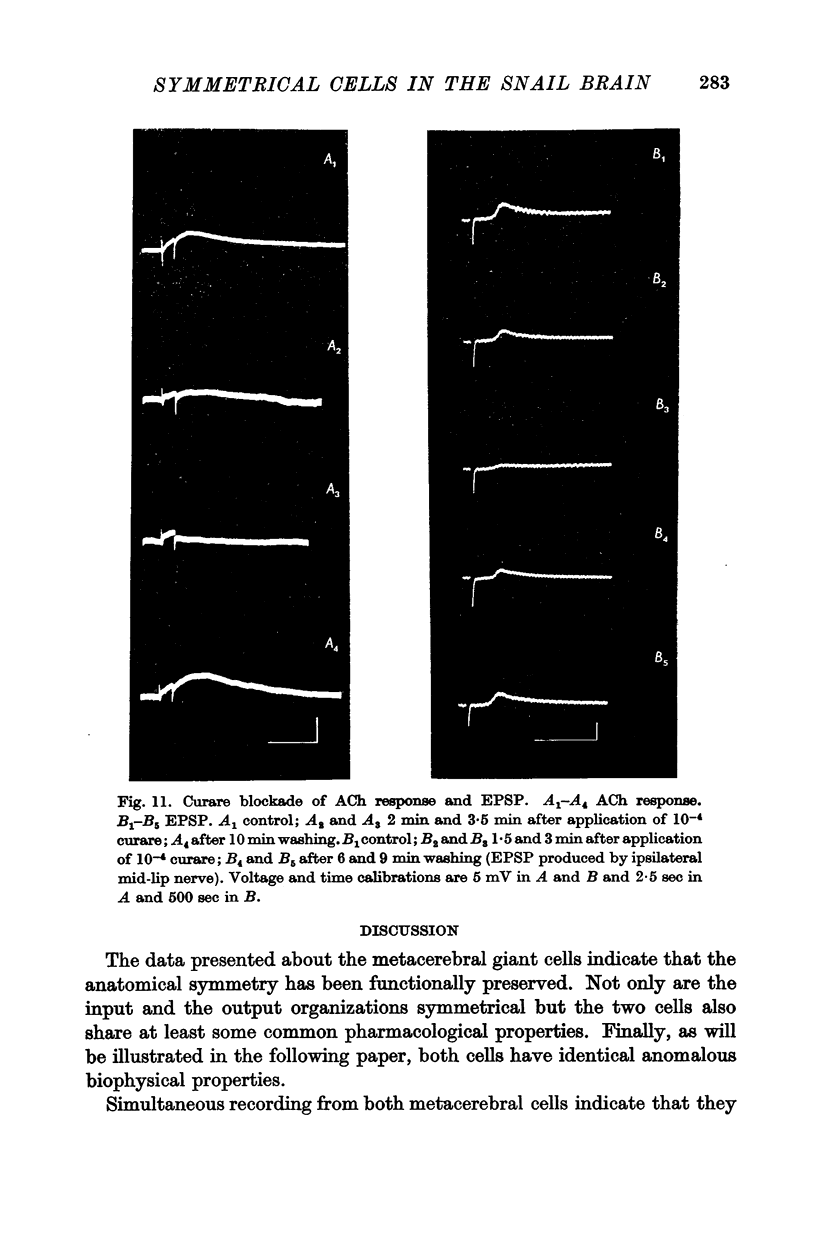
5. Pharmacological studies revealed that both cells fall into the category called D cells by Tauc & Gerschenfeld (1961); they responded with depolarization to iontophoretic injection of ACh and all excitatory synaptic inputs were blocked by d-tubocurarine.
6. With none of the twelve afferent inputs was synaptic inhibition observed. The results support the notion that D cells do not receive inhibitory input.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. Inhibitory phasing of neuronal discharge. Nature. 1962 Nov 17;196:645–647. doi: 10.1038/196645a0. [DOI] [PubMed] [Google Scholar]
- FESSARD A., TAUC L. Effets de répétition sur l'amplitude des potentiels postsynaptiques d'un soma neuronique. J Physiol (Paris) 1958 Mar;50(2):277–281. [PubMed] [Google Scholar]
- FURSHPAN E. J., FURUKAWA T. Intracellular and extracellular responses of the several regions of the Mauthner cell of the goldfish. J Neurophysiol. 1962 Nov;25:732–771. doi: 10.1152/jn.1962.25.6.732. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. AN ELECTROPHYSIOLOGICAL STUDY OF THE ANATOMICAL RELATIONS OF TWO GIANT NERVE CELLS IN APLYSIA DEPILANS. J Exp Biol. 1963 Sep;40:469–486. doi: 10.1242/jeb.40.3.469. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. The path of the giant cell axons in Aplysia depilans. Nature. 1961 Jul 22;191:404–405. doi: 10.1038/191404a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans. J Physiol. 1965 Nov;181(1):1–27. doi: 10.1113/jphysiol.1965.sp007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURPURA D. P., COHEN B. Intracellular recording from thalamic neurons during recruiting responses. J Neurophysiol. 1962 Sep;25:621–635. doi: 10.1152/jn.1962.25.5.621. [DOI] [PubMed] [Google Scholar]
- TAUC L., BRUNER J. "Desensitization" of cholinergic receptors by acetylcholine in molluscan central neurones. Nature. 1963 Apr 6;198:33–34. doi: 10.1038/198033a0. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- TAUC L., HUGHES G. M. Modes of initiation and propagation of spikes in the branching axons of molluscan central neurons. J Gen Physiol. 1963 Jan;46:533–549. doi: 10.1085/jgp.46.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L. Site of origin and propagation in spike in the giant neuron of Aplysia. J Gen Physiol. 1962 Jul;45:1077–1097. doi: 10.1085/jgp.45.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]


