Abstract
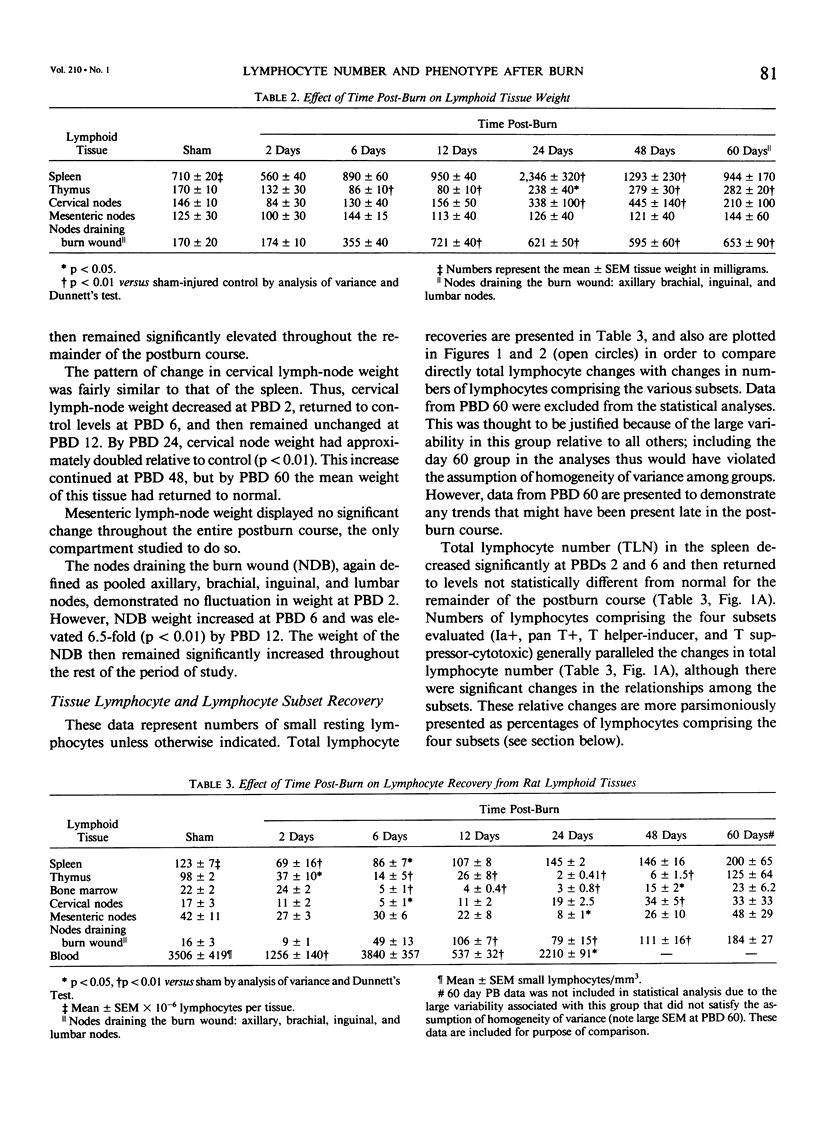
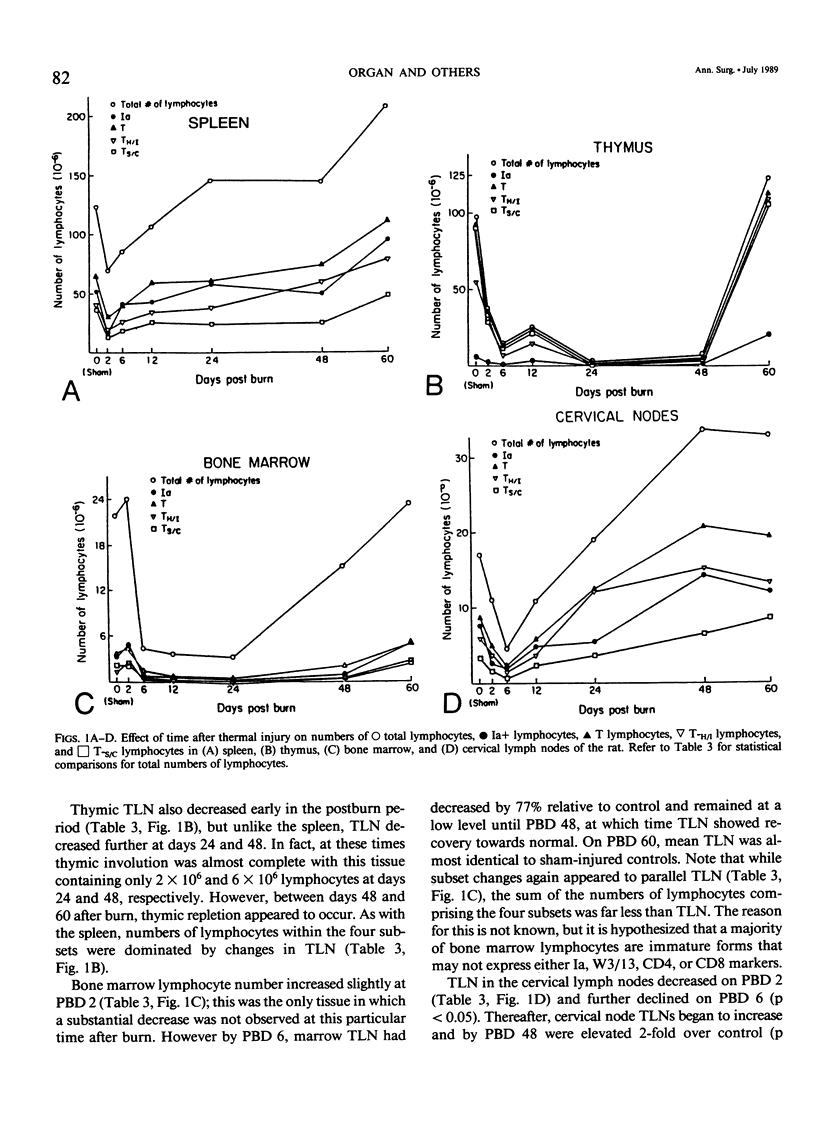
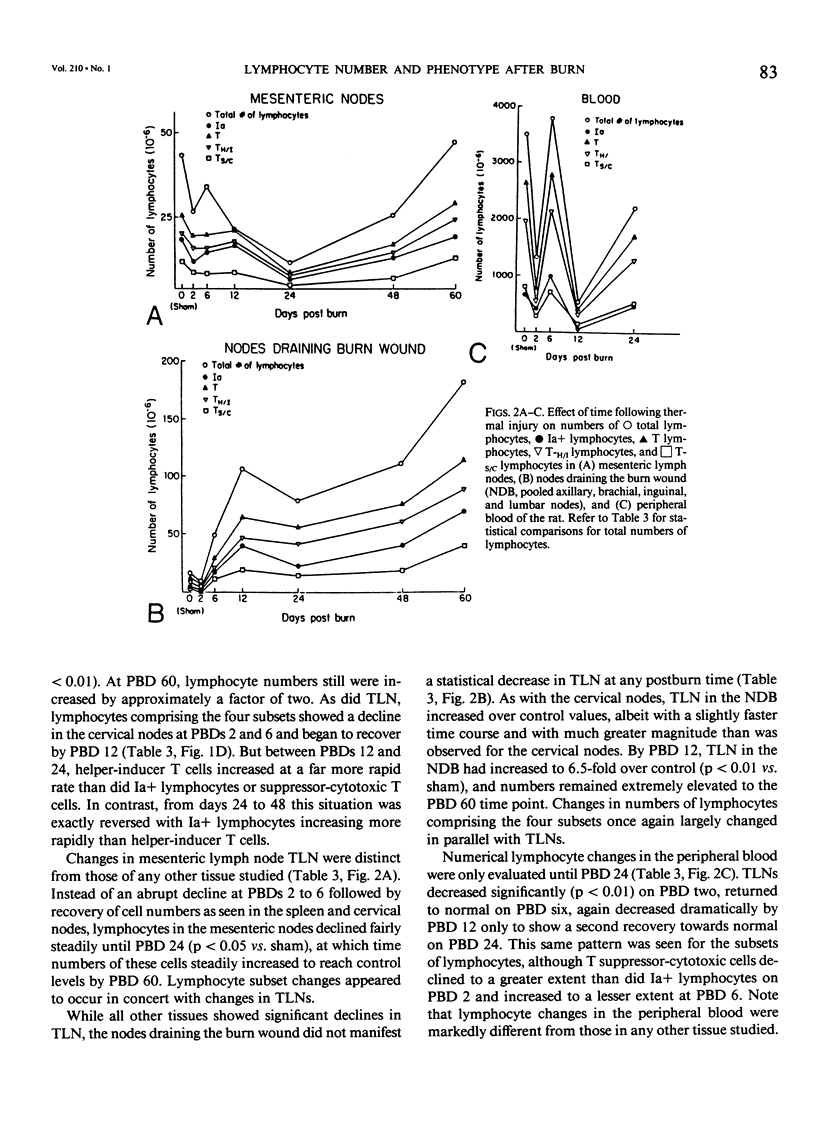
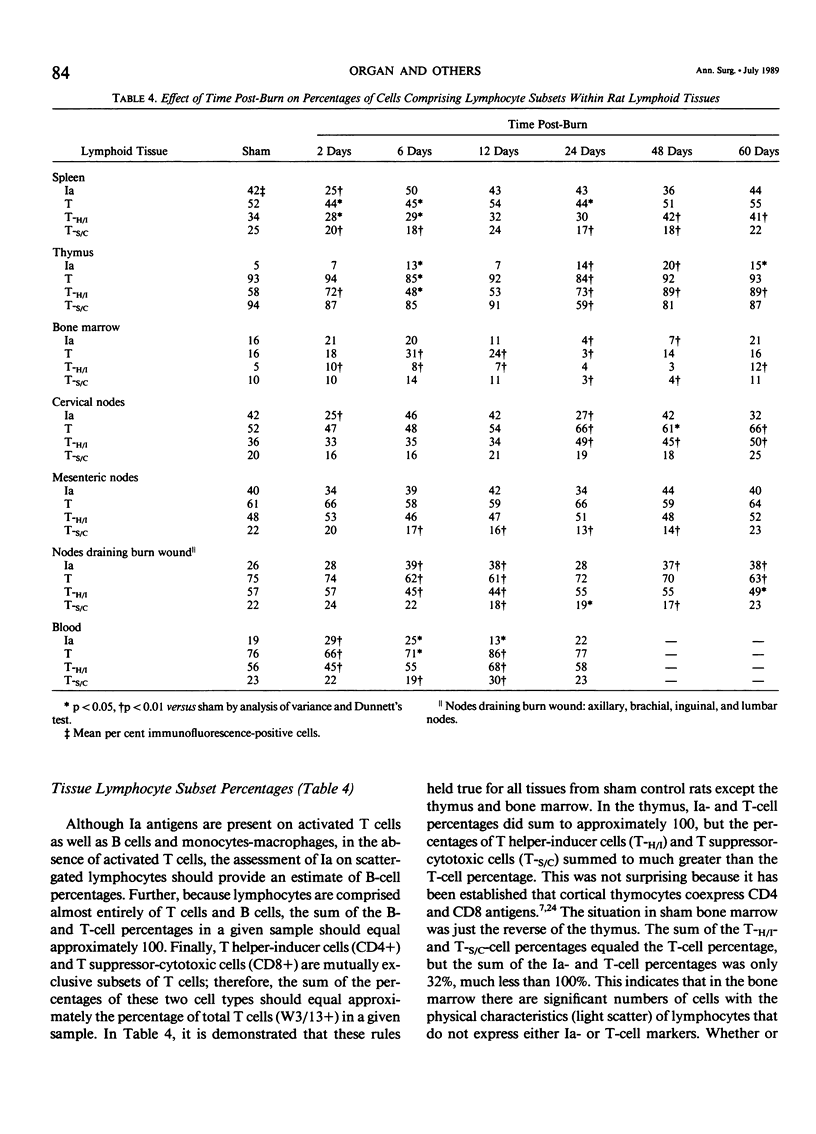
Thermal injury is associated with dysfunction of host defense systems. The present study used flow cytometric immunofluorescence analyses to investigate changes in number and phenotype of lymphocytes in seven different lymphoid compartments at 2, 6, 12, 24, 48, and 60 days after 50% total body-surface area thermal injury in the rat. Relative to sham-injured control rats, at postburn day 2, significant lymphopenia was observed in the peripheral blood along with depletion of lymphocytes from the spleen and thymus. By day 6 after injury, lymphocytes in the bone marrow and cervical lymph nodes decreased significantly while numbers in the spleen and thymus remained depressed. Splenic and cervical node lymphocyte numbers normalized by day 12, the bone marrow and thymus numbers still were significantly lower than control, and a 6.5-fold increase in number of lymphocytes was observed in the nodes draining the burn wound, pooled axillary, brachial, inguinal, and lumbar lymph nodes. At day 24 after injury, the thymus and bone marrow virtually were depleted of lymphocytes, the mesenteric lymph nodes manifested a significant decrease, and lymphocytes in the nodes draining the burn wound continued to increase in number. This same pattern was maintained on day 48, but numbers of lymphocytes in the mesenteric nodes normalized. At day 60 after injury, lymphocyte numbers in all tissues were normalized, but the spleen and nodes draining the burn wound where increased numbers compared to control persisted. Cell-surface phenotyping was performed on all lymphoid tissues at all time intervals to determine the percentages of lymphocytes comprising the following subsets: Ia+ cells (B cells and activated T cells), T cells, T-Helper/Inducer cells (T-H/I), and T-Suppressor/Cytotoxic (T-S/C) cells. Although changes in lymphocyte subset percentages were complex, they could be divided grossly into two phases. First, all compartments showed significant phenotypic changes in the first six days after burn. With the exception of the nodes draining the burn wound and the blood, this was followed by a return towards normal on day 12. The second phase then ensued with significant phenotypic changes again occurring in most tissues from days 24 to 60 after injury. These studies demonstrate that burn injury results in dramatic alterations in lymphocyte numbers and subset percentages in different lymphoid compartments. Immune alterations observed following thermal injury may be due, in part, to a redistribution of the cellular elements responsible for generation of the immune response.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonacci A. C., Calvano S. E., Reaves L. E., Prajapati A., Bockman R., Welte K., Mertelsmann R., Gupta S., Good R. A., Shires G. T. Autologous and allogeneic mixed-lymphocyte responses following thermal injury in man: the immunomodulatory effects of interleukin 1, interleukin 2, and a prostaglandin inhibitor, WY-18251. Clin Immunol Immunopathol. 1984 Feb;30(2):304–320. doi: 10.1016/0090-1229(84)90064-3. [DOI] [PubMed] [Google Scholar]
- Antonacci A. C., Gupta S., Good R. A., Shires G. T., Fernandes G. Natural killer and antibody-dependent cytotoxicity following thermal injury in humans. Curr Surg. 1983 Jan-Feb;40(1):20–23. [PubMed] [Google Scholar]
- Arturson G., Högman C. F., Johansson S. G., Killander J. Changes in immunoglobulin levels in severely burned patients. Lancet. 1969 Mar 15;1(7594):546–548. doi: 10.1016/s0140-6736(69)91957-6. [DOI] [PubMed] [Google Scholar]
- Barbul A., Wasserkrug H. L., Seifter E., Rettura G., Levenson S. M., Efron G. Immunostimulatory effects of arginine in normal and injured rats. J Surg Res. 1980 Sep;29(3):228–235. doi: 10.1016/0022-4804(80)90165-1. [DOI] [PubMed] [Google Scholar]
- Burleson D. G., Mason A. D., Jr, Pruitt B. A., Jr Lymphoid subpopulation changes after thermal injury and thermal injury with infection in an experimental model. Ann Surg. 1988 Feb;207(2):208–212. doi: 10.1097/00000658-198802000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson D. G., Vaughn G. K., Mason A. D., Jr, Pruitt B. A., Jr Flow cytometric measurement of rat lymphocyte subpopulations after burn injury and burn injury with infection. Arch Surg. 1987 Feb;122(2):216–220. doi: 10.1001/archsurg.1987.01400140098013. [DOI] [PubMed] [Google Scholar]
- Calvano S. E., Mark D. A., Good R. A., Fernandes G. In vitro assessment of immune function in adrenalectomized rats. Immunopharmacology. 1982 Aug;4(4):291–302. doi: 10.1016/0162-3109(82)90050-9. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Dialynas D. P., Fitch F. W., MacDonald H. R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983 Nov 1;158(5):1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- Cox J. H., Ford W. L. The migration of lymphocytes across specialized vascular endothelium. IV. Prednisolone acts at several points on the recirculation pathways of lymphocytes. Cell Immunol. 1982 Jan 15;66(2):407–422. doi: 10.1016/0008-8749(82)90190-3. [DOI] [PubMed] [Google Scholar]
- Echinard C. E., Sajdel-Sulkowska E., Burke P. A., Burke J. F. The beneficial effect of early excision on clinical response and thymic activity after burn injury. J Trauma. 1982 Jul;22(7):560–565. doi: 10.1097/00005373-198207000-00006. [DOI] [PubMed] [Google Scholar]
- Ford W. L. Lymphocyte migration and immune responses. Prog Allergy. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Morris B. The immediate effect of antigens on the cell output of a lymph node. Br J Exp Pathol. 1965 Aug;46(4):450–454. [PMC free article] [PubMed] [Google Scholar]
- Jung L. K., Hara T., Fu S. M. Detection and functional studies of p60-65 (Tac antigen) on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1597–1602. doi: 10.1084/jem.160.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Claudy B. J. Suppressor T-cell activity induced as a result of thermal injury. Cell Immunol. 1979 Apr;44(1):201–208. doi: 10.1016/0008-8749(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Eurenius K. Enhanced hemolytic antibody response following thermal injury. Int Arch Allergy Appl Immunol. 1972;43(3):321–326. doi: 10.1159/000230847. [DOI] [PubMed] [Google Scholar]
- Munster A. M., Eurenius K., Katz R. M., Canales L., Foley F. D., Mortensen R. F. Cell-mediated immunity after thermal injury. Ann Surg. 1973 Feb;177(2):139–143. doi: 10.1097/00000658-197302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M., Hoagland H. C., Pruitt B. A., Jr The effect of thermal injury on serum immunoglobulins. Ann Surg. 1970 Dec;172(6):965–969. doi: 10.1097/00000658-197012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Frank H. A. Prolonged survival of human skin allografts following thermal injury. Transplantation. 1978 Feb;25(2):69–72. doi: 10.1097/00007890-197802000-00006. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. The contribution of bone marrow to the economy of the lymphoid system. Monogr Allergy. 1980;16:157–172. [PubMed] [Google Scholar]
- Rapaport F. T., Milgrom F., Kano K., Gesner B., Solowey A. C., Casson P., Silverman H. I., Converse J. M. Immunologic sequelae of thermal injury. Ann N Y Acad Sci. 1968 Aug 14;150(3):1004–1008. doi: 10.1111/j.1749-6632.1968.tb14753.x. [DOI] [PubMed] [Google Scholar]
- Renk C. M., Long C. L., Blakemore W. S. Comparison between in vitro lymphocyte activity and metabolic changes in trauma patients. J Trauma. 1982 Feb;22(2):134–140. doi: 10.1097/00005373-198202000-00010. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Gowans J. L., Atkins R. C., Ford W. L., Smith M. E. The specific selection of recirculating lymphocytes by antigen in normal and preimmunized rats. J Exp Med. 1972 Sep 1;136(3):499–513. doi: 10.1084/jem.136.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Daniels J. C., Beathard G. A., Lewis S. R., Lynch J. B., Ritzmann S. E. Mixed lymphocyte culture reaction in patients with acute thermal burns. J Trauma. 1974 Jan;14(1):53–57. doi: 10.1097/00005373-197401000-00007. [DOI] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Contribution of thymus lymphocytes to the peripheral lymphoid tissues and the effect of antigen on the rate of cell exit from the thymus. Am J Anat. 1984 Jul;170(3):331–338. doi: 10.1002/aja.1001700308. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Functions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo. Immunol Rev. 1986 Jun;91:195–218. doi: 10.1111/j.1600-065x.1986.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Walker H. L., Mason A. D., Jr A standard animal burn. J Trauma. 1968 Nov;8(6):1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Zatz M. M., Lance E. M. The distribution of 51Cr-labeled lymphocytes into antigen-stimulated mice. Lymphocyte trapping. J Exp Med. 1971 Jul 1;134(1):224–241. doi: 10.1084/jem.134.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


