Abstract
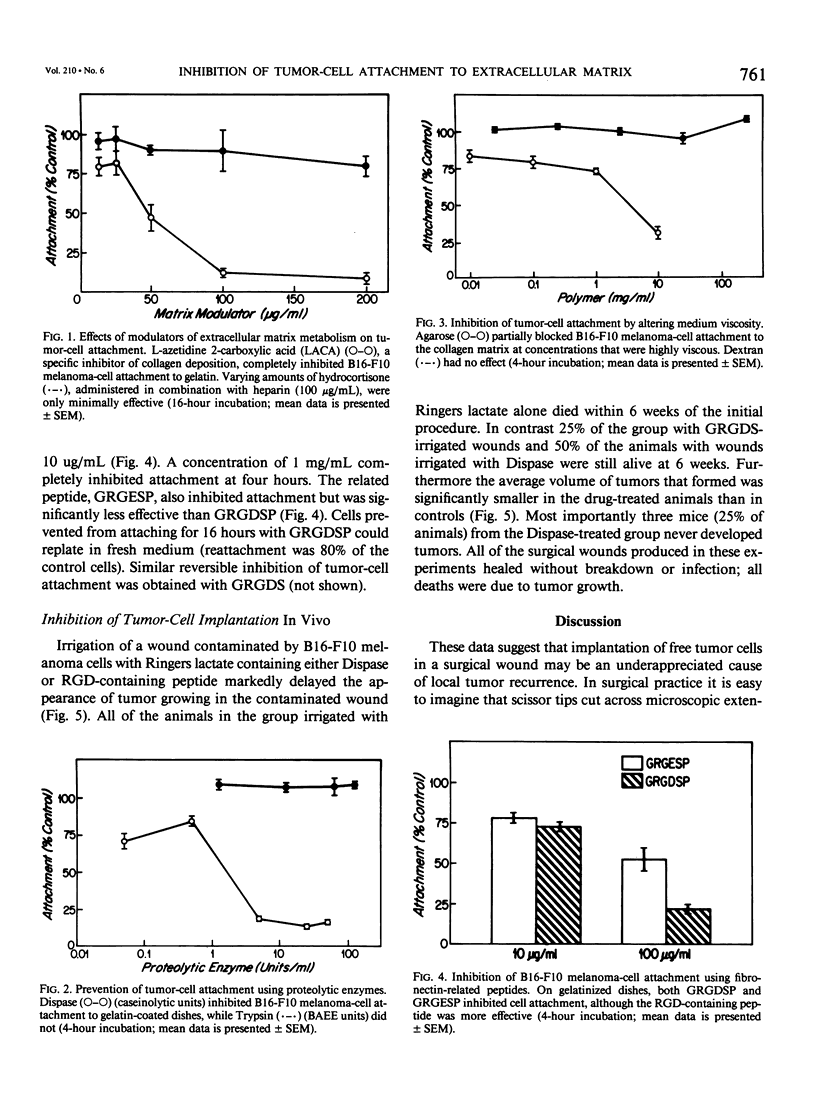
Studies with four different transplantable murine tumors demonstrated that surgical instruments contaminated by contact with a tumor mass could produce tumors in a surgical wound. Eighty-seven per cent of mice with wounds made by invisibly contaminated scissors developed tumors. Irrigation with water did not prevent tumor growth. Before spilled tumor cells can invade and grow into a recurrence in the wound site, they must first attach to underlying extracellular matrix. We have devised a simple in vitro assay to identify inhibitors of tumor-cell attachment to develop therapeutic compounds that can prevent tumor-cell reimplantation. Various test compounds, including proteases (trypsin and Dispase), known modulators of matrix metabolism (proline analogues, cycloheximide, heparin, cortisone, cortexolone, and heparin-steroid combinations), large molecular weight polymers (agarose, dextran, polyethylene oxide), and synthetic fibronectin peptides were tested for their ability to inhibit mouse melanoma (B16-F10) cell attachment to gelatinized dishes. Most of these compounds had little or no effect on tumor-cell adhesion when cells were plated in serum-containing medium. However we identified three compounds that inhibited tumor-cell attachment in a reversible fashion: (1) a specific inhibitor of collagen deposition (L-azetidine-2-carboxylic acid); (2) a bacterial neutral protease (Dispase); and (3) synthetic fibronectin peptides that contained the arginine-glycine-asparate (RGD) sequence that is responsible for cell binding. Dispase and the RGD-containing peptides also inhibited cell implantation and prevented tumor formation in a surgical wound. We propose that inhibitors of attachment might be used either alone or with other biologic modifiers to prohibit implantation of free tumor cells at the time of surgery and thus, to prevent local tumor recurrence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONS M. S., SMITH R. R., MYERS M. H. Significance of cancer cells in operative wounds. Cancer. 1961 Sep-Oct;14:1041–1044. doi: 10.1002/1097-0142(196109/10)14:5<1041::aid-cncr2820140519>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Connolly D. T., Knight M. B., Harakas N. K., Wittwer A. J., Feder J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal Biochem. 1986 Jan;152(1):136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- DerHagopian R. P., Sugarbaker E. V., Ketcham A. Inflammatory oncotaxis. JAMA. 1978 Jul 28;240(4):374–375. [PubMed] [Google Scholar]
- Fidler I. J., Sone S., Fogler W. E., Barnes Z. L. Eradication of spontaneous metastases and activation of alveolar macrophages by intravenous injection of liposomes containing muramyl dipeptide. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1680–1684. doi: 10.1073/pnas.78.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Greenspan H. P. Influence of geometry on control of cell growth. Biochim Biophys Acta. 1975 Dec 31;417(3-4):211–236. doi: 10.1016/0304-419x(75)90011-6. [DOI] [PubMed] [Google Scholar]
- HAVERBACK C. Z., SMITH R. R. Transplantation of tumor by suture thread and its prevention. An experimental study. Cancer. 1959 Sep-Oct;12:1029–1042. doi: 10.1002/1097-0142(195909/10)12:5<1029::aid-cncr2820120524>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986 Dec;103(6 Pt 2):2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986 Oct;119(4):1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987 May;23(5):387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Ingber D., Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988 Jul;59(1):44–51. [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Skubitz A. P., Palm S. L., Furcht L. T. Metastasis inhibition of different tumor types by purified laminin fragments and a heparin-binding fragment of fibronectin. J Natl Cancer Inst. 1988 Mar 16;80(2):108–116. doi: 10.1093/jnci/80.2.108. [DOI] [PubMed] [Google Scholar]
- Nagata M., Matsumura T. Action of the bacterial neutral protease, dispase, on cultured cells and its application to fluid suspension culture with a review on biomedical application of this protease. Jpn J Exp Med. 1986 Dec;56(6):297–307. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Soloway M. S., Masters S. Urothelial susceptibility to tumor cell implantation: influence of cauterization. Cancer. 1980 Sep 1;46(5):1158–1163. doi: 10.1002/1097-0142(19800901)46:5<1158::aid-cncr2820460514>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Neckers L. M., Johnson D. W., Liotta L. A. Protein synthesis but not DNA synthesis is required for tumor cell invasion in vitro. Invasion Metastasis. 1984;4(2):73–83. [PubMed] [Google Scholar]
- Vembu D., Liotta L. A., Paranjpe M., Boone C. W. Correlation of tumorigenicity with resistance to growth inhibition by cis-hydroxypyroline. Exp Cell Res. 1979 Dec;124(2):247–252. doi: 10.1016/0014-4827(79)90200-3. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wittelsberger S. C., Kleene K., Penman S. Progressive loss of shape-responsive metabolic controls in cells with increasingly transformed phenotype. Cell. 1981 Jun;24(3):859–866. doi: 10.1016/0092-8674(81)90111-2. [DOI] [PubMed] [Google Scholar]


