Abstract
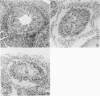
Tumor necrosis factor (TNF) is a cytokine that mediates many of the metabolic responses after endotoxemia, septicemia, and tissue injury. The effect of TNF on testicular function was determined in a series of studies in which rhTNF (0, 2, and 4 X 10(5) units/kg/24 hours) was administered by continuous infusion to male Wistar rats maintained on total parenteral nutrition adequate for growing rats. Testicular weight and histology, and plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone levels were determined at 1, 3, and 6 days. Testicular weight decreased within 24 hours and this was associated with a fall in plasma testosterone and increased LH and FSH levels. These changes persisted for 6 days, indicating a loss of testosterone-mediated negative feedback on gonadotropin release. Histologic examination demonstrated significant damage to the germ cells in the adluminal compartment of the seminiferous epithelium; extensive exfoliation of spermatocytes and spermatids occurred at day six. However the primary spermatogonia in the basal compartment were relatively spared. Damage to the seminiferous epithelium at earlier times was noted in some tubules. The decrease in testosterone concentration and increase in gonadotropin levels suggest that TNF interferes with Leydig cell function. Germ cell damage may be a direct effect of TNF on these cells or may occur through secondary mechanisms involving Leydig or Sertoli cell dysfunction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Shiomi H., Matthews J. Induction of the intermediate pituitary by stress: synthesis and release of a nonopioid form of beta-endorphin. Science. 1985 Jan 25;227(4685):424–426. doi: 10.1126/science.3155575. [DOI] [PubMed] [Google Scholar]
- Bambino T. H., Hsueh A. J. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981 Jun;108(6):2142–2148. doi: 10.1210/endo-108-6-2142. [DOI] [PubMed] [Google Scholar]
- Blackwell R. E., Amoss M. S., Jr A sex difference in the rate of rise of plasma LH in rats following gonadectomy. Proc Soc Exp Biol Med. 1971 Jan;136(1):11–14. doi: 10.3181/00379727-136-35181. [DOI] [PubMed] [Google Scholar]
- CLERMONT Y., MORGENTALER H. Quantitative study of spermatogenesis in the hypophysectomized rat. Endocrinology. 1955 Sep;57(3):369–382. doi: 10.1210/endo-57-3-369. [DOI] [PubMed] [Google Scholar]
- Challis J. R., Davies I. J., Benirschke K., Hendrickx A. G., Ryan K. J. The effects of dexamethasone on the peripheral plasma concentrations of androstenedione, testosterone and cortisol in the pregnant rhesus monkey. Endocrinology. 1975 Jan;96(1):185–192. doi: 10.1210/endo-96-1-185. [DOI] [PubMed] [Google Scholar]
- Charpenet G., Tache Y., Forest M. G., Haour F., Saez J. M., Bernier M., Ducharme J. R., Collu R. Effects of chronic intermittent immobilization stress on rat testicular androgenic function. Endocrinology. 1981 Oct;109(4):1254–1258. doi: 10.1210/endo-109-4-1254. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeff N., Auclair M. C., Benassayag C., Carli A., Nunez E. A. Endotoxin-induced changes in sex steroid hormone levels in male rats. J Steroid Biochem. 1987 Jan;26(1):67–71. doi: 10.1016/0022-4731(87)90032-x. [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Schainker B. A., Meyer E. R. Endogenous opioids participate in the regulation of the hypothalamus-pituitary-luteinizing hormone axis and testosterone's negative feedback control of luteinizing hormone. Endocrinology. 1979 May;104(5):1286–1291. doi: 10.1210/endo-104-5-1286. [DOI] [PubMed] [Google Scholar]
- Dolecek R., Dvorácek C., Jezek M., Kubis M., Sajnar J., Závada M. Very low serum testosterone levels and severe impairment of spermatogenesis in burned male patients. Correlations with basal levels and levels of FSH, LH and PRL after LHRH + TRH. Endocrinol Exp. 1983 Mar;17(1):33–45. [PubMed] [Google Scholar]
- Eskeland N. L., Lugo D. I., Pintar J. E., Schachter B. S. Stimulation of beta-endorphin secretion by corticotropin-releasing factor in primary rat Leydig cell cultures. Endocrinology. 1989 Jun;124(6):2914–2919. doi: 10.1210/endo-124-6-2914. [DOI] [PubMed] [Google Scholar]
- Evain D., Morera A. M., Saez J. M. Glucocorticoid receptors in interstitial cells of the rat testis. J Steroid Biochem. 1976 Nov-Dec;7(11-12):1135–1139. doi: 10.1016/0022-4731(76)90045-5. [DOI] [PubMed] [Google Scholar]
- Ferin M. A role for the endogenous opioid peptides in the regulation of gonadotropin secretion in the primate. Horm Res. 1987;28(2-4):119–125. doi: 10.1159/000180935. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Midgley A. R., Jr Response of the adult rat to orchidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology. 1969 Jun;84(6):1359–1364. doi: 10.1210/endo-84-6-1359. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Gunsalus G. L., Bardin C. W. The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology. 1986 May;118(5):2039–2044. doi: 10.1210/endo-118-5-2039. [DOI] [PubMed] [Google Scholar]
- Gindoff P. R., Jewelewicz R., Hembree W., Wardlaw S., Ferin M. Sustained effects of opioid antagonism during the normal human luteal phase. J Clin Endocrinol Metab. 1988 May;66(5):1000–1004. doi: 10.1210/jcem-66-5-1000. [DOI] [PubMed] [Google Scholar]
- Jacobs D. O., Evans D. A., Mealy K., O'Dwyer S. T., Smith R. J., Wilmore D. W. Combined effects of glutamine and epidermal growth factor on the rat intestine. Surgery. 1988 Aug;104(2):358–364. [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart E. D., Baxter C. R., Parker C. R., Jr Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab. 1987 Apr;64(4):842–848. doi: 10.1210/jcem-64-4-842. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Spriggs D. R., Manogue K. R., Sherman M. L., Revhaug A., O'Dwyer S. T., Arthur K., Dinarello C. A., Cerami A., Wolff S. M. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988 Aug;104(2):280–286. [PubMed] [Google Scholar]
- Milenkovic L., Rettori V., Snyder G. D., Beutler B., McCann S. M. Cachectin alters anterior pituitary hormone release by a direct action in vitro. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2418–2422. doi: 10.1073/pnas.86.7.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. R., Jr, Baxter C. R. Divergence in adrenal steroid secretory pattern after thermal injury in adult patients. J Trauma. 1985 Jun;25(6):508–510. doi: 10.1097/00005373-198506000-00006. [DOI] [PubMed] [Google Scholar]
- Rivier C., Rivier J., Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science. 1986 Feb 7;231(4738):607–609. doi: 10.1126/science.3003907. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. In the rat, interleukin-1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology. 1989 May;124(5):2105–2109. doi: 10.1210/endo-124-5-2105. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 1984 Mar;114(3):914–921. doi: 10.1210/endo-114-3-914. [DOI] [PubMed] [Google Scholar]
- Russell L. D., Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec. 1977 Mar;187(3):347–366. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- Sharp B. M., Matta S. G., Peterson P. K., Newton R., Chao C., Mcallen K. Tumor necrosis factor-alpha is a potent ACTH secretagogue: comparison to interleukin-1 beta. Endocrinology. 1989 Jun;124(6):3131–3133. doi: 10.1210/endo-124-6-3131. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Warren R. S., Jeevanandam M., Gabrilove J. L., Larchian W., Oettgen H. F., Brennan M. F. Tumor necrosis factor and the acute metabolic response to tissue injury in man. J Clin Invest. 1988 Oct;82(4):1321–1325. doi: 10.1172/JCI113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Seasholtz A. F., Herbert E. Rat corticotropin-releasing hormone gene: sequence and tissue-specific expression. Mol Endocrinol. 1987 May;1(5):363–370. doi: 10.1210/mend-1-5-363. [DOI] [PubMed] [Google Scholar]
- Tulassay Z., Viczián M., Böjthe L., Czeizel A. Quantitative histological studies on the injury of spermatogenesis induced by endotoxin in rats. J Reprod Fertil. 1970 Jun;22(1):161–164. doi: 10.1530/jrf.0.0220161. [DOI] [PubMed] [Google Scholar]