Abstract
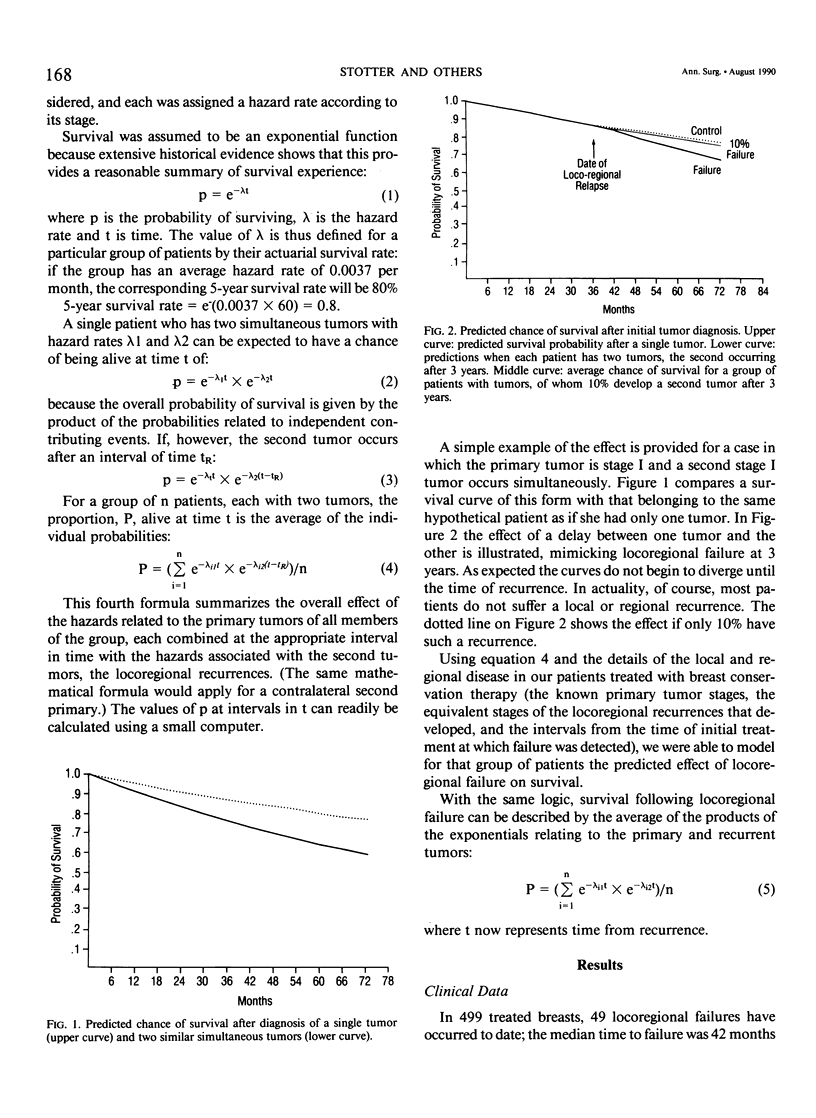
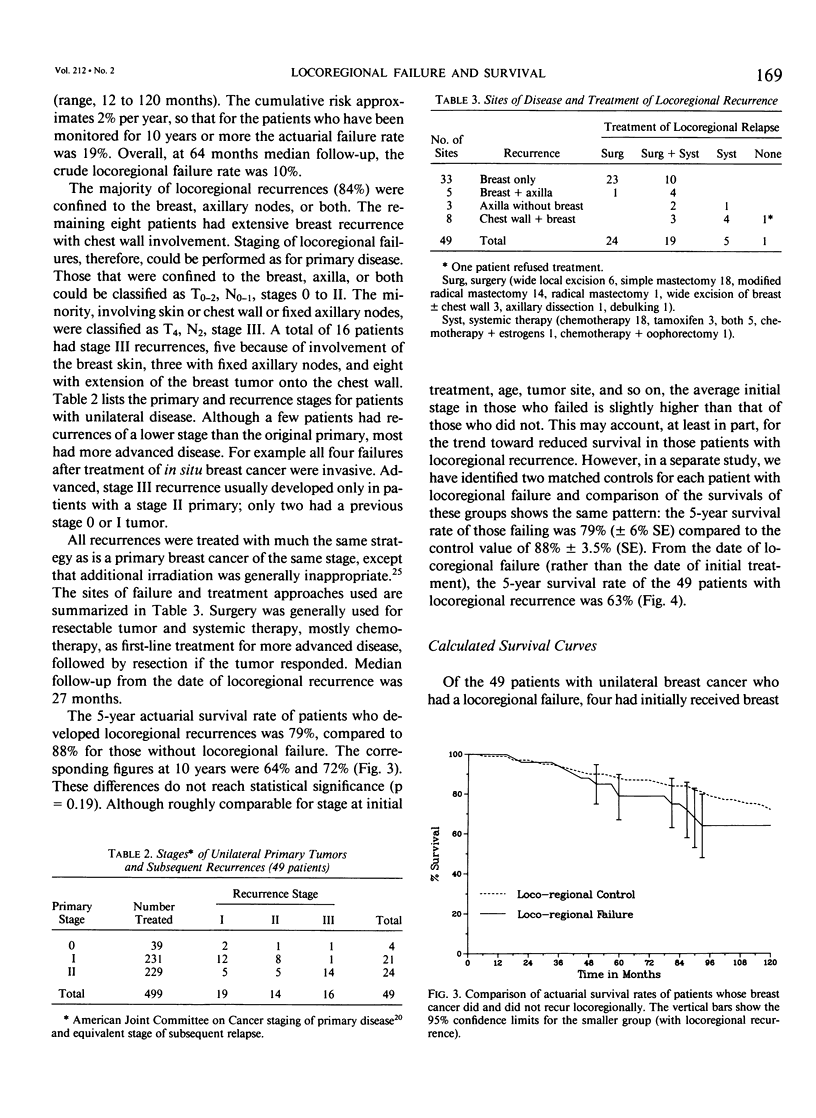
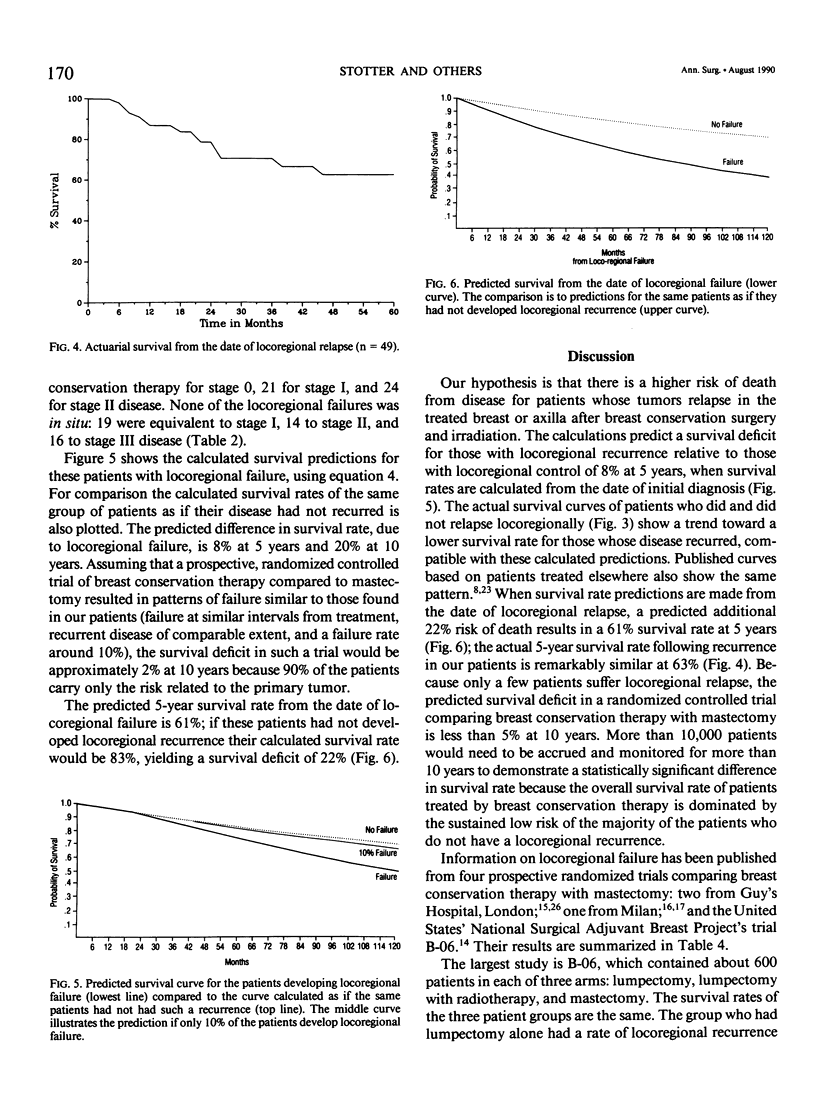
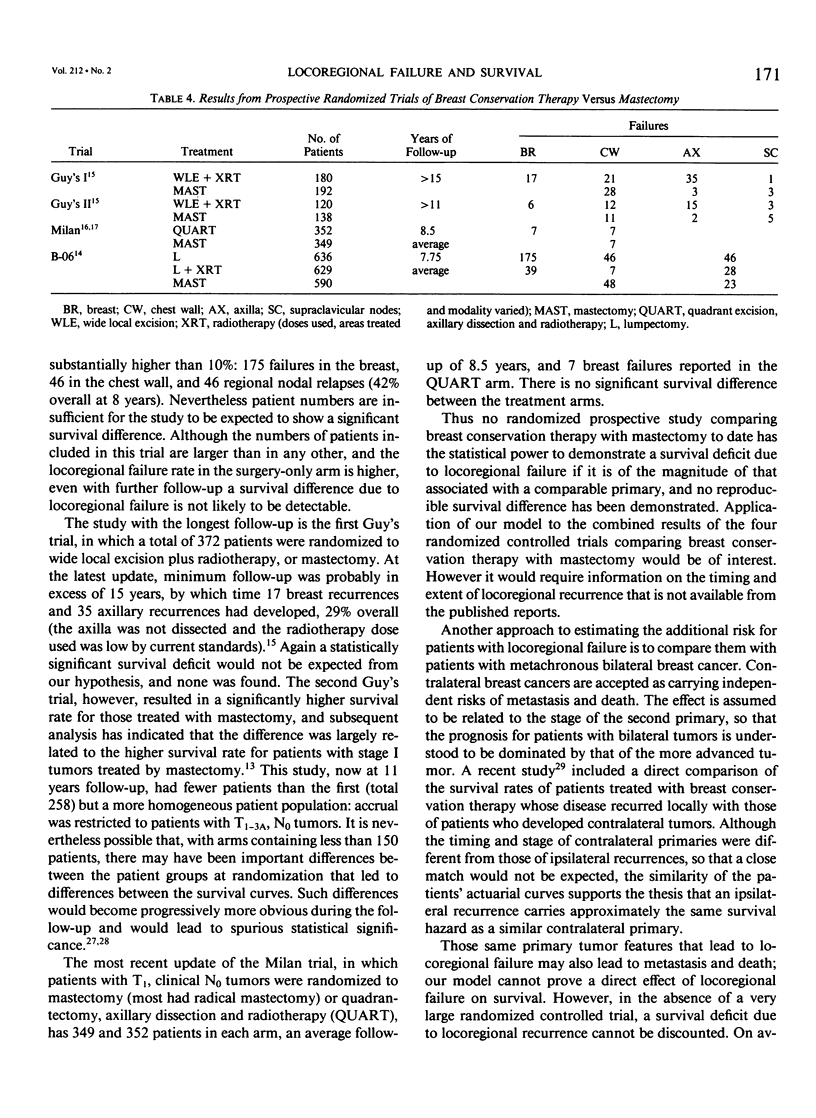
We postulated that locoregional recurrence after limited surgery and radiotherapy for breast cancer might be associated with an additional survival hazard, similar to that of a second primary tumor with the same extent of local and regional disease. Using this hypothesis we examined the likely resultant effect on survival. Our calculations indicated that no statistically significant survival deficit due to such recurrence would be detectable until a randomized controlled trial comparing breast conservation with mastectomy had monitored more than 10,000 patients for more than 10 years. A simple mathematical model predicted 5-year survival rates in a cohort of patients treated with breast conservation of 75%, compared to 83% in those without locoregional recurrence. From the date of locoregional recurrence, a 61% 5-year survival rate was predicted, compared to 83% if no hazard was associated with locoregional recurrence. These predictions were compared with the actuarial survival rates of 499 patients with unilateral breast cancer, 49 of whom had developed locoregional recurrence. From the date of initial treatment, the 5-year survival rate of those whose disease recurred was 79%, compared to 88% for those without locoregional recurrence (p = 0.19). The actuarial 5-year survival rate from the date of locoregional recurrence was 63%. The similarity between the patient data and the predictions of the mathematical model indicates that locoregional failure after breast conservation therapy may result in reduced survival. The lack of a significant survival deficit in our cohort or in controlled trials comparing breast conservation therapy with mastectomy is compatible to the small size of the overall effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson N. K., Bartelink H., van Dongen J. A., van Dam F. S. Evaluation of breast conserving therapy: clinical, methodological and psychosocial perspectives. Eur J Surg Oncol. 1988 Apr;14(2):133–140. [PubMed] [Google Scholar]
- Amalric R., Santamaria F., Robert F., Seigle J., Altschuler C., Kurtz J. M., Spitalier J. M., Brandone H., Ayme Y., Pollet J. F. Radiation therapy with or without primary limited surgery for operable breast cancer: a 20-year experience at the Marseilles Cancer Institute. Cancer. 1982 Jan 1;49(1):30–34. doi: 10.1002/1097-0142(19820101)49:1<30::aid-cncr2820490107>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bross I. D., Blumenson L. E. Predictive design of experiments using deep mathematical models. Cancer. 1971 Dec;28(6):1637–1646. doi: 10.1002/1097-0142(197112)28:6<1637::aid-cncr2820280645>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Clark R. M., Wilkinson R. H., Mahoney L. J., Reid J. G., MacDonald W. D. Breast cancer: a 21 year experience with conservative surgery and radiation. Int J Radiat Oncol Biol Phys. 1982 Jun;8(6):967–979. doi: 10.1016/0360-3016(82)90163-8. [DOI] [PubMed] [Google Scholar]
- Cuzick J., Stewart H., Peto R., Baum M., Fisher B., Host H., Lythgoe J. P., Ribeiro G., Scheurlen H., Wallgren A. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987 Jan;71(1):15–29. [PubMed] [Google Scholar]
- Cuzick J., Stewart H., Peto R., Fisher B., Kaae S., Johansen H., Lythgoe J. P., Prescott R. J. Overview of randomized trials comparing radical mastectomy without radiotherapy against simple mastectomy with radiotherapy in breast cancer. Cancer Treat Rep. 1987 Jan;71(1):7–14. [PubMed] [Google Scholar]
- Fisher B., Redmond C., Poisson R., Margolese R., Wolmark N., Wickerham L., Fisher E., Deutsch M., Caplan R., Pilch Y. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989 Mar 30;320(13):822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- Freiman J. A., Chalmers T. C., Smith H., Jr, Kuebler R. R. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978 Sep 28;299(13):690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- Gilliland M. D., Barton R. M., Copeland E. M., 3rd The implications of local recurrence of breast cancer as the first site of therapeutic failure. Ann Surg. 1983 Mar;197(3):284–287. doi: 10.1097/00000658-198303000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J., Caleffi M. The significance of local control in the primary treatment of breast cancer. Lucy Wortham James clinical research award. Arch Surg. 1987 Nov;122(11):1244–1247. doi: 10.1001/archsurg.1987.01400230030004. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Amalric R., Brandone H., Ayme Y., Spitalier J. M. Contralateral breast cancer and other second malignancies in patients treated by breast-conserving therapy with radiation. Int J Radiat Oncol Biol Phys. 1988 Aug;15(2):277–284. doi: 10.1016/s0360-3016(98)90005-0. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Amalric R., Brandone H., Ayme Y., Spitalier J. M. Results of wide excision for mammary recurrence after breast-conserving therapy. Cancer. 1988 May 15;61(10):1969–1972. doi: 10.1002/1097-0142(19880515)61:10<1969::aid-cncr2820611006>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Spitalier J. M., Amalric R., Brandone H., Ayme Y., Bressac C., Hans D. Mammary recurrences in women younger than forty. Int J Radiat Oncol Biol Phys. 1988 Aug;15(2):271–276. doi: 10.1016/s0360-3016(98)90004-9. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Spitalier J. M., Amalric R. Late breast recurrence after lumpectomy and irradiation. Int J Radiat Oncol Biol Phys. 1983 Aug;9(8):1191–1194. doi: 10.1016/0360-3016(83)90179-7. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Spitalier J. M., Amalric R. Results of salvage surgery for local failure following conservative therapy of operable breast cancer. Front Radiat Ther Oncol. 1983;17:84–90. doi: 10.1159/000407281. [DOI] [PubMed] [Google Scholar]
- Lachin J. M. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981 Jun;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Maddox W. A., Carpenter J. T., Jr, Laws H. L., Soong S. J., Cloud G., Urist M. M., Balch C. M. A randomized prospective trial of radical (Halsted) mastectomy versus modified radical mastectomy in 311 breast cancer patients. Ann Surg. 1983 Aug;198(2):207–212. doi: 10.1097/00000658-198308000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P., Ferguson D. J., Karrison T. A controlled trial of extended radical mastectomy. Cancer. 1985 Feb 15;55(4):880–891. doi: 10.1002/1097-0142(19850215)55:4<880::aid-cncr2820550429>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht A., Silver B., Schnitt S., Connolly J., Hellman S., Harris J. R. Breast relapse following primary radiation therapy for early breast cancer. I. Classification, frequency and salvage. Int J Radiat Oncol Biol Phys. 1985 Jul;11(7):1271–1276. doi: 10.1016/0360-3016(85)90241-x. [DOI] [PubMed] [Google Scholar]
- Sarrazin D., Dewar J. A., Arriagada R., Benhamou S., Benhamou E., Lasser P., Fontaine F., Travagli J. P., Spielmann M., Le Chevalier T. Conservative management of breast cancer. Br J Surg. 1986 Aug;73(8):604–606. doi: 10.1002/bjs.1800730804. [DOI] [PubMed] [Google Scholar]
- Stotter A. T., McNeese M. D., Ames F. C., Oswald M. J., Ellerbroek N. A. Predicting the rate and extent of locoregional failure after breast conservation therapy for early breast cancer. Cancer. 1989 Dec 1;64(11):2217–2225. doi: 10.1002/1097-0142(19891201)64:11<2217::aid-cncr2820641106>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Banfi A., Del Vecchio M., Saccozzi R., Clemente C., Greco M., Luini A., Marubini E., Muscolino G., Rilke F. Comparison of Halsted mastectomy with quadrantectomy, axillary dissection, and radiotherapy in early breast cancer: long-term results. Eur J Cancer Clin Oncol. 1986 Sep;22(9):1085–1089. doi: 10.1016/0277-5379(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Zucali R., Luini A. Local control and survival in early breast cancer: the Milan trial. Int J Radiat Oncol Biol Phys. 1986 May;12(5):717–720. doi: 10.1016/0360-3016(86)90027-1. [DOI] [PubMed] [Google Scholar]


