Abstract
Dendritic cells isolated from lymphoid tissues are potent stimulators of primary allogeneic T-cell responses in vitro and in vivo. Similar major histocompatibility complex class II-bearing dendritic-shaped leukocytes are contained within transplanted organs and these are thought to be important passenger leukocytes that trigger rejection. Recent findings on the migration, phenotype, and function of cardiac dendritic leukocytes (DLs) are reviewed. After transplantation donor DLs migrate rapidly from mouse cardiac allografts into the recipients's spleens. Within the spleens donor DLs associate with recipient CD4+ T cells. Isolated cardiac DLs, like lymphoid dendritic cells, are potent stimulators of T-cell proliferation in vitro. This suggests that DLs function as passenger leukocytes by migrating from grafts into the lymphoid tissues of the recipient and that sensitization to vascularized organ allografts may occur centrally within lymphoid tissues rather than peripherally in the graft itself.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Kupiec-Weglinski J. W., Hankins D. F., Morris P. J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988 Feb 1;167(2):646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. T., Buckley G., Jenkinson E. J., Owen J. J. Survival of deoxyguanosine-treated fetal thymus allografts is prevented by priming with dendritic cells. Immunology. 1987 Apr;60(4):593–596. [PMC free article] [PubMed] [Google Scholar]
- Bolton E. M., Gracie J. A., Briggs J. D., Kampinga J., Bradley J. A. Cellular requirements for renal allograft rejection in the athymic nude rat. J Exp Med. 1989 Jun 1;169(6):1931–1946. doi: 10.1084/jem.169.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer Y., Palmer A., Taube D., Welsh K., Bewick M., Bindon C., Hale G., Waldmann H., Dische F., Parsons V. Effect of graft perfusion with two CD45 monoclonal antibodies on incidence of kidney allograft rejection. Lancet. 1989 Oct 21;2(8669):935–937. doi: 10.1016/s0140-6736(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Burris D. E., Gruel S. M., Rao U. K. Persistence of dendritic cells and allograft antigenicity despite prolonged interim hosting of cardiac allografts in rats. Transplantation. 1989 Jun;47(6):1085–1087. [PubMed] [Google Scholar]
- Chen H. D., Ma C. L., Yuan J. T., Wang Y. K., Silvers W. K. Occurrence of donor Langerhans cells in mouse and rat chimeras and their replacement in skin grafts. J Invest Dermatol. 1986 Jun;86(6):630–633. doi: 10.1111/1523-1747.ep12275627. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Fabre J. W., Batchelor J. R. The role of the spleen in the rejection and enhancement of renal allografts in the rat. Transplantation. 1975 Sep;20(3):219–226. doi: 10.1097/00007890-197509000-00006. [DOI] [PubMed] [Google Scholar]
- Fabre J. W., Morris P. J. The role of passenger leucocytes in the rejection of renal allografts in the rat. Transplantation. 1973 Jun;15(6):631–633. doi: 10.1097/00007890-197306000-00020. [DOI] [PubMed] [Google Scholar]
- Faustman D. L., Steinman R. M., Gebel H. M., Hauptfeld V., Davie J. M., Lacy P. E. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes R. D., Parfrey N. A., Gomersall M., Darden A. G., Guttmann R. D. Dendritic cell-lymphoid cell aggregation and major histocompatibility antigen expression during rat cardiac allograft rejection. J Exp Med. 1986 Oct 1;164(4):1239–1258. doi: 10.1084/jem.164.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum S. Lymph-borne dendritic leucocytes do not recirculate, but enter the lymph node paracortex to become interdigitating cells. Scand J Immunol. 1988 Jan;27(1):97–105. doi: 10.1111/j.1365-3083.1988.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Fryd D. S., Sutherland D. E., Simmons R. L., Ferguson R. M., Kjellstrand C. M., Najarian J. S. Results of a prospective randomized study on the effect of splenectomy versus no splenectomy in renal transplant patients. Transplant Proc. 1981 Mar;13(1 Pt 1):48–56. [PubMed] [Google Scholar]
- Gores P. F., Sutherland D. E., Platt J. L., Bach F. H. Depletion of donor Ia+ cells before transplantation does not prolong islet allograft survival. J Immunol. 1986 Sep 1;137(5):1482–1485. [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M. D., Inaba M., Muramatsu S., Steinman R. M. The function of Ia+ dendritic cells and Ia- dendritic cell precursors in thymocyte mitogenesis to lectin and lectin plus interleukin 1. J Exp Med. 1988 Jan 1;167(1):149–162. doi: 10.1084/jem.167.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H., Kuma S., Inaba M. M., Good R. A., Yamashita T., Kumazawa T., Ikehara S. Acceptance of murine thyroid allografts by pretreatment of anti-Ia antibody or anti-dendritic cell antibody in vitro. Transplantation. 1989 Jan;47(1):45–49. doi: 10.1097/00007890-198901000-00011. [DOI] [PubMed] [Google Scholar]
- Juhlin L., Shelley W. B. New staining techniques for the Langerhans cell. Acta Derm Venereol. 1977;57(4):289–296. [PubMed] [Google Scholar]
- Kelly R. H. Localization of afferent lymph cells within the draining node during a primary immune response. Nature. 1970 Aug 1;227(5257):510–513. doi: 10.1038/227510a0. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S., Steinman R. M. Induction of murine interleukin 1: stimuli and responsive primary cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3802–3806. doi: 10.1073/pnas.84.11.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G. G., Daynes R. A., Emam M. Biology of Langerhans cells: selective migration of Langerhans cells into allogeneic and xenogeneic grafts on nude mice. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1650–1654. doi: 10.1073/pnas.80.6.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Austyn J. M., Morris P. J. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J Exp Med. 1988 Feb 1;167(2):632–645. doi: 10.1084/jem.167.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Lee F., Birchall N., Clark S., Dower S. Interleukin 1 binds to specific receptors on human keratinocytes and induces granulocyte macrophage colony-stimulating factor mRNA and protein. A potential autocrine role for interleukin 1 in epidermis. J Clin Invest. 1988 Nov;82(5):1787–1792. doi: 10.1172/JCI113792. [DOI] [PMC free article] [PubMed] [Google Scholar]
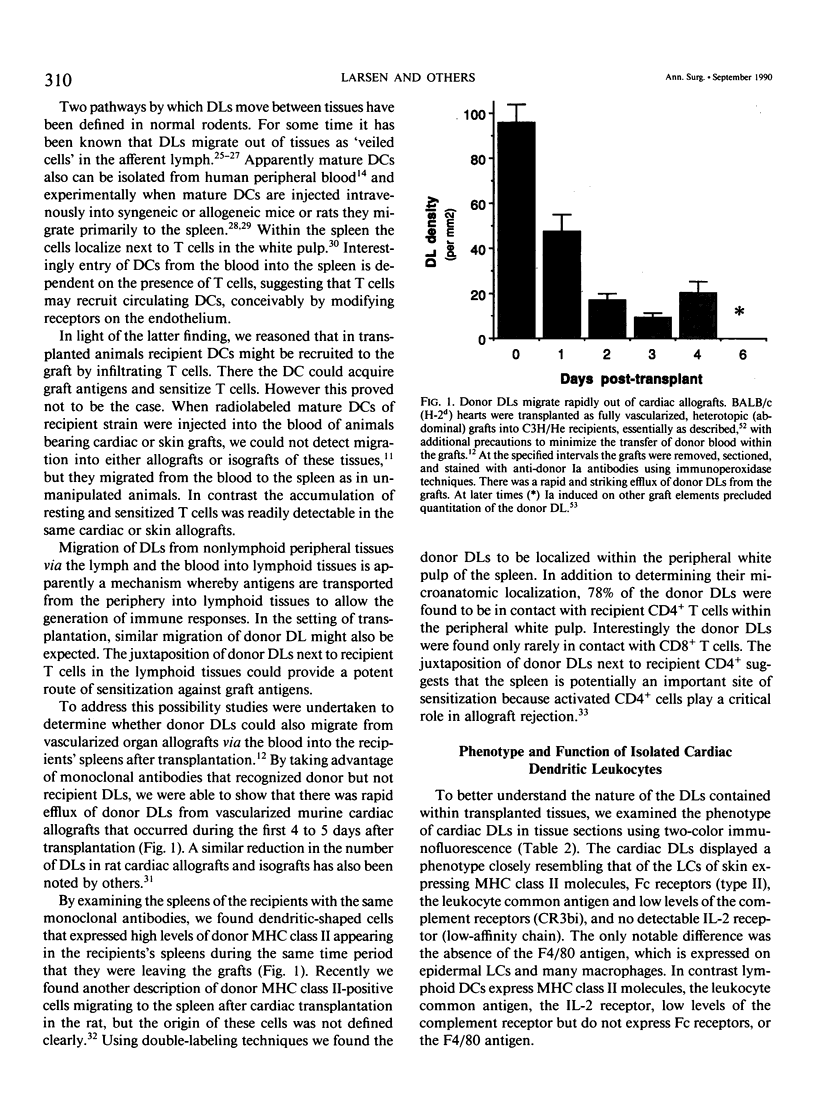
- Larsen C. P., Morris P. J., Austyn J. M. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager I., Halttunen J., Häyry P. Characteristics of dendritic cells in rat liver. Transplantation. 1988 May;45(5):936–939. doi: 10.1097/00007890-198805000-00019. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Batchelor J. R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982 Jan 1;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Morris P. J. Effector mechanisms in allograft rejection. Annu Rev Immunol. 1986;4:119–145. doi: 10.1146/annurev.iy.04.040186.001003. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Beard M. E., Hart D. N. The effect of donor pretreatment on interstitial dendritic cell content and rat cardiac allograft survival. Transplantation. 1984 Oct;38(4):371–376. doi: 10.1097/00007890-198410000-00011. [DOI] [PubMed] [Google Scholar]
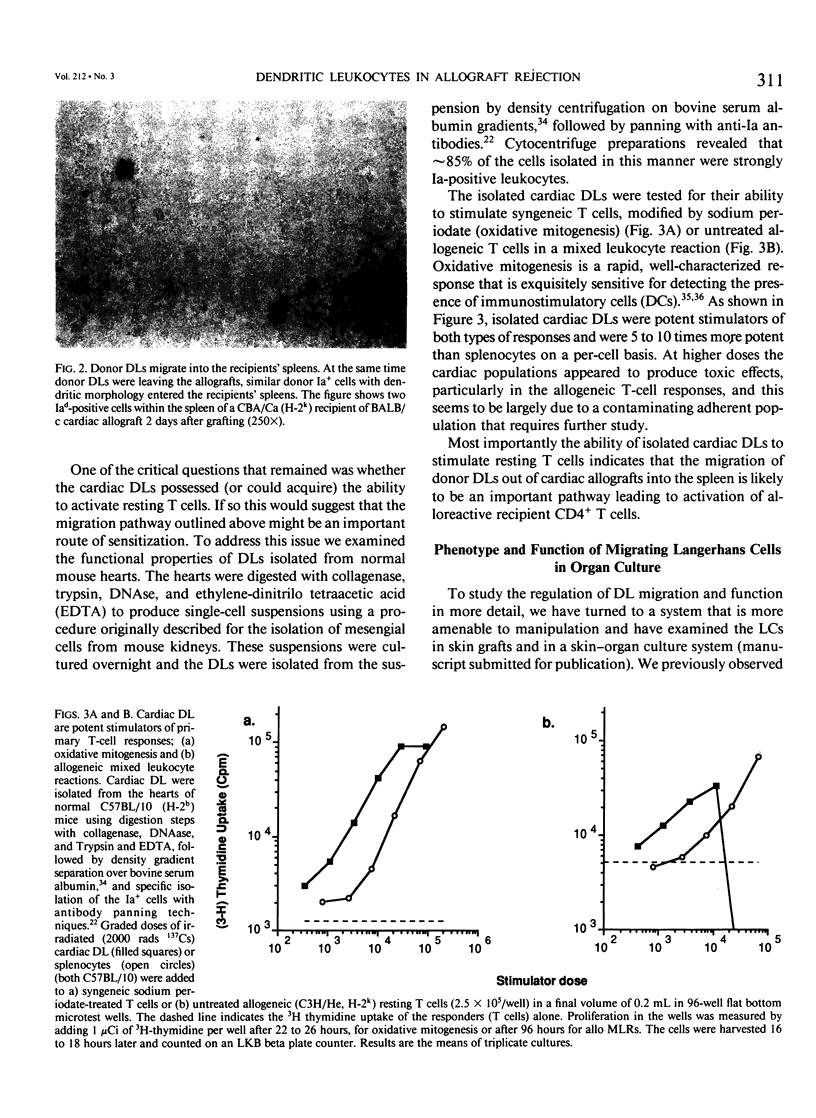
- McKenzie J. L., Prickett T. C., Hart D. N. Human dendritic cells stimulate allogeneic T cells in the absence of IL-1. Immunology. 1989 Jul;67(3):290–297. [PMC free article] [PubMed] [Google Scholar]
- Milton A. D., Fabre J. W. Massive induction of donor-type class I and class II major histocompatibility complex antigens in rejecting cardiac allografts in the rat. J Exp Med. 1985 Jan 1;161(1):98–112. doi: 10.1084/jem.161.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Pedersen N. C., Morris B. The role of the lymphatic system in the rejection of homografts: a study of lymph from renal transplants. J Exp Med. 1970 May 1;131(5):936–969. doi: 10.1084/jem.131.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak R., Blanchard J. M., Mozes M. F. Lack of effect of splenectomy on the influence of pretransplant blood transfusions on cardiac allograft survival in histoincompatible rats. Transplantation. 1986 Apr;41(4):527–529. [PubMed] [Google Scholar]
- Prickett T. C., McKenzie J. L., Hart D. N. Characterization of interstitial dendritic cells in human liver. Transplantation. 1988 Nov;46(5):754–761. doi: 10.1097/00007890-198811000-00024. [DOI] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A. M., Fathman C. G., Inaba K., Steinman R. M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989 Mar 1;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R. A., Brent L., Rayfield L. S. Presentation of alloantigens by host cells. Eur J Immunol. 1986 May;16(5):569–574. doi: 10.1002/eji.1830160519. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J. Donor-type MHC-positive cells in the host spleen after rat organ transplantation. Differences between pancreas and heart allograft recipients. Transplantation. 1986 Jun;41(6):787–789. [PubMed] [Google Scholar]
- Steinman R. M., Lustig D. S., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974 Jun 1;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart F. P., Reckard C. R., Ketel B. L., Schulak J. A. Effect of splenectomy on first cadaver kidney transplants. Ann Surg. 1980;192(4):553–561. doi: 10.1097/00000658-198010000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E., Fryd D. S., So S. K., Bentley F. R., Ascher N. L., Simmons R. L., Najarian J. S. Long-term effect of splenectomy versus no splenectomy in renal transplant patients. Reanalysis of a randomized prospective study. Transplantation. 1984 Dec;38(6):619–624. doi: 10.1097/00007890-198412000-00015. [DOI] [PubMed] [Google Scholar]
- Talmage D. W., Dart G., Radovich J., Lafferty K. J. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976 Jan 30;191(4225):385–388. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- Taube D., Welsh K. I., Bewick M., Dische F. E., Palmer A., Parsons V., Snowden S. Pretreatment of human renal allografts with monoclonal antibodies to induce long-term tolerance. Transplant Proc. 1987 Feb;19(1 Pt 3):1961–1963. [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]