Abstract
Partially-deleted mitochondrial DNA (ΔmtDNA) accumulates during aging of postmitotic tissues. This accumulation has been linked to decreased metabolic activity, increased reactive oxygen species formation and the aging process. Taking advantage of cell lines with heteroplasmic mtDNA mutations, we showed that, after severe mtDNA depletion, organelles are quickly and predominantly repopulated with ΔmtDNA, whereas repopulation with the wild-type counterpart is slower. This behavior was not observed for full-length genomes with pathogenic point mutations. The faster repopulation of smaller molecules was supported by metabolic labeling of mtDNA with [3H]thymidine during relaxed copy number control conditions. We also showed that hybrid cells containing two defective mtDNA haplotypes tend to retain the smaller one as they adjust their normal mtDNA copy number. Taken together, our results indicate that, under relaxed copy number control, ΔmtDNAs repopulate mitochondria more efficiently than full-length genomes.
INTRODUCTION
The human mitochondrial genome is a 16 569 bp circular molecule, containing genes that are necessary for the synthesis of the catalytic components of the oxidative phosphorylation (OXPHOS) system. Although these components are essential for mitochondrial respiration and ATP production, they are intrinsically dependent on factors encoded by the nuclear DNA, synthesized in cytosolic ribosomes and imported into the mitochondria. These include factors that regulate mitochondrial DNA (mtDNA) replication, such as the mtDNA polymerase γ. Although several additional protein factors associated with mtDNA replication have been described, it is still unclear how mtDNA replication and copy number are controlled (1,2).
Mitochondrial genomes harboring large deletions are known to accumulate both in patients with heteroplasmic mtDNA mutations and in normal individuals during aging, particularly in postmitotic tissues such as muscle and brain (3). These observations support the mitochondrial theory of aging, which states that the slow accumulation of impaired mitochondria is the driving force of the aging process. This idea is attractive because it can be reconciled with the free radical theory of aging, which argues that oxidative damage plays a key role in senescence. Among the numerous mechanisms known to generate oxidants, leakage of superoxide anion and hydrogen peroxide from the mitochondrial electron transport chain are the chief candidates. Increased damage to mtDNA could exacerbate this leakage of reactive oxygen species (ROS) (4).
It is not known how mtDNA deletions accumulate during aging. Although the smaller size of partially-deleted molecules suggested early on that they could have a replicative advantage (5,6), direct evidence of this phenomenon has been lacking. In most cases, partially-deleted mtDNAs (ΔmtDNAs) contain the same number of replication origins as the wild-type genome but they can be up to 50% shorter (7). We have previously shown that cells harboring homoplasmic levels of ΔmtDNA repopulated their organelles with mtDNA faster than cells containing wild-type mitochondrial genomes (8). In these cells, however, there was no competition between mutated and wild-type genomes, as they were present in a homoplasmic state. Therefore, we could not rule out that differences in mtDNA repopulation were due to different metabolic states of these cells. In the present study, we addressed this issue by studying heteroplasmic cells. Our results showed that mtDNA with large deletions, but not with pathogenic point mutations, repopulates organelles significantly faster than wild-type genomes in the same cell, particularly during relaxed copy number control.
MATERIALS AND METHODS
Characterization of human cells containing different pathogenic mtDNA mutations
We have used several characterized cell lines containing the osteosarcoma 143B(TK–) nuclear background and various mtDNAs. Cell lines harboring heteroplasmic mtDNA deletions were obtained by fusing 143B/206ρ° with: (i) an enucleated HeLa/fibroblast hybrid line harboring ∼50% of the 4.9 kb ‘common deletion’ [lines ΔBH.10.5.n, spanning mtDNA positions 8482–13 459; (9)]; (ii) a patient-derived fibroblast cell line harboring a 7.5 kb partial deletion in the mtDNA was used to generate transmitochondrial cybrids (ΔHH6.1 and ΔH2.1, spanning positions 7982–15 504). The cell line Δ16.10.40 had the same deletion in a homoplasmic state (8,10). Two cell lines harboring a heteroplasmic mtDNA point mutation in the tRNAAsn gene (G5703A, lines W91 and W76) and two cell lines harboring a heteroplasmic mtDNA point mutation in the tRNALeu(UUR) gene (C3256T, lines SUA72 and SUB43) were previously characterized (11,12). A cell line harboring a homoplasmic 4 bp apocytochrome b gene deletion was obtained as previously described (13).
mtDNA depletion and repopulation analysis
The various cell lines were grown in complete medium (DMEM supplemented with 4.5 mg/ml glucose, 50 µg/ml uridine, 10% FCS, 1 mM pyruvate and 10 µg/ml gentamycin) in the presence of 50 ng/ml ethidium bromide (EtBr). EtBr was kept in the media for 15 days, after which cells were fed with complete media without EtBr. Cells were kept between 30 and 80% confluence with excess fresh medium to assure exponential growth. At selected days (0, 6, 15, 22, 30 and 45) cells were harvested for DNA extraction.
For each cell line (obtained from all selected treatment days) DNA samples (∼5 µg) were digested with PvuII and analyzed by Southern blot using two probes. The first probe corresponded to a non-deleted region (mtDNA position 3305– 4261), whereas the second probe corresponded to the nuclear 18S rDNA gene (14). All probes were prepared by the random primer method. Hybridizing fragments were quantitated in a Cyclone Phosphoimager (Packard Instruments). Signals were used to determine the ratios between mtDNA/nDNA corresponding to the three concentrations. The intensity of the signal was normalized to day zero in the repopulation analyses.
mtDNA synthesis analysis
Selected hybrid lines were labeled with [3H]thymidine (20 µCi/ml; Perkin Elmer) for the times indicated. Mito chondria were purified by nitrogen cavitation as described (15) and DNA purified by phenol/chlorophorm extraction. Purified mtDNA was digested with PvuII and separated by electrophoresis on a 0.8% agarose gel. The gel was stained with EtBr and the fragments corresponding to mtDNA were visualized under UV light. Approximately 0.2 cm segments were sliced from the agarose lane and dissolved with 200 µl of 7 M NaClO4 for 30 min at 50°C. The solubilized agarose slice was added to scintillation liquid (10 ml) and c.p.m. measured in a scintillation counter. Cells were either directly labeled in medium containing 10% dialyzed serum or pre-treated with 50 ng/ml of EtBr in complete medium for 3 days, allowed to recover for 24 h and pulse labeled for the times indicated. When necessary, the label was chased for 4 additional days in complete medium. The incorporated c.p.m. were normalized by the number of thymidines in each molecule and a correction factor (1.87) was applied to the ΔmtDNA counts before the percentage labeled mutated mtDNA was calculated.
mtDNA haplotype analysis
Total DNA from different cell lines was purified as described (14). For the differentiation between partially-deleted or partially-duplicated mtDNA, Southern blots of PvuII and SnaBI digests were performed as described above, with the exception that the probe outside the deleted region corresponded to positions 140–585. The analyses of point mutations in the tRNALeu(UUR) and tRNAAsn genes were performed by ‘last cycle hot’ PCR as previously described (11,12). For the 4 bp deletion in the apocytochrome b gene (Δ4bp cyt b) analysis, ∼250 ng of DNA were used to amplify a 93 bp fragment by PCR. We used oligonucleotide primers corresponding to mtDNA position 14 747–14 770 (F) and 14 840– 14 817 (B). The forward primer was end-labeled with [γ-32P]ATP. Labeled products were separated by electrophoresis on a 6% denaturing polyacrylamide gel, dried and exposed to a phosphoimager screen. Radiolabeled fragments were identified and quantitated in a Cyclone (Packard Instruments) phosphoimager storage system (13).
Establishment and genetic characterization of hybrid lines
The cell lines 143B, Δ16.10.40 and Δ4cytb3.E were transfected with the respective plasmids pIRESneo, pZEO and pIRESpuro, and stable transfectants isolated by selection with the respective drugs. Transfection was performed with lipofectamine as suggested by the manufacturer (Life Technologies). These cells were fused to each other with polyethylene glycol as described (9). Fusion products were subjected to double selection for the nuclear markers. Individual hybrid clones were isolated by the cloning ring method, expanded and their mtDNA analyzed for (i) the Δ4cyt b by PAGE of PCR-amplified fragments as described above, (ii) large deletions by Southern blot also as described above.
RESULTS
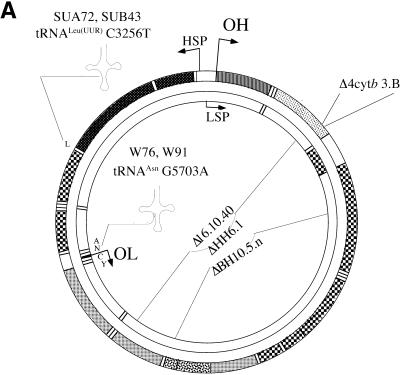
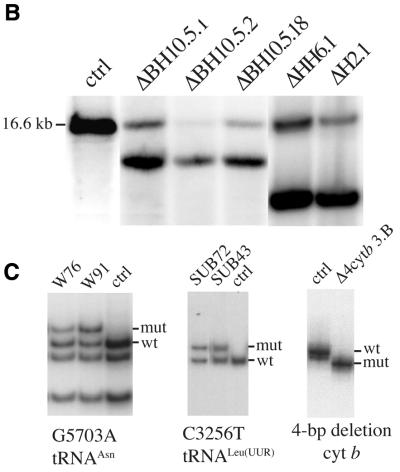
Observations that mtDNA with large-scale deletions accumulate with age prompted us to study the relative organelle repopulation rate of such molecules. To better understand this process we analyzed several cell lines containing different heteroplasmic mtDNA mutations but having a similar nuclear background, derived from the human osteosarcoma 143B(TK–) cell line. These cell lines were obtained by fusing the mtDNA-less 143B derivative (206ρ°) to cytoplasts obtained from fibroblasts of patients with mitochondrial disorders. Figure 1 shows the location of the mutations in the mitochondrial genome and their level of heteroplasmy. We investigated for the presence of deletion dimers or partial duplications in the cell lines described above by a series of Southern blots. PvuII and SnaBI digests using probes either inside or outside the deletion region showed that the cell lines used in these experiments contained essentially only the wild-type mtDNA and deletion monomers (not shown).
Figure 1.
Characterization of cell lines with mtDNA mutations used in this study. (A) Diagram illustrating the location of two point mutations, two large deletions and a 4 bp deletion in the apocytochrome b gene in the human mtDNA. (B) Southern blot showing the heteroplasmic mtDNA deletions in some of the cell lines used. Total DNA extracted from the different cell lines was digested with PvuII and analyzed by Southern hybridization to a probe corresponding to the ND1 gene. (C) PCR/RFLP analyses of the two pathogenic point mutations and PCR/LP of the apocytochrome b 4 bp deletion (A). The cell lines with tRNA mutations used in this study were heteroplasmic. Cell lines harboring homoplasmic levels of apocytochrome b 4 bp deletion, a 7.5 kb deletion and wild-type genomes were used in the hybrid experiments described below.
mtDNA harboring deletions repopulate mitochondria faster than the full-length counterparts in the same cell
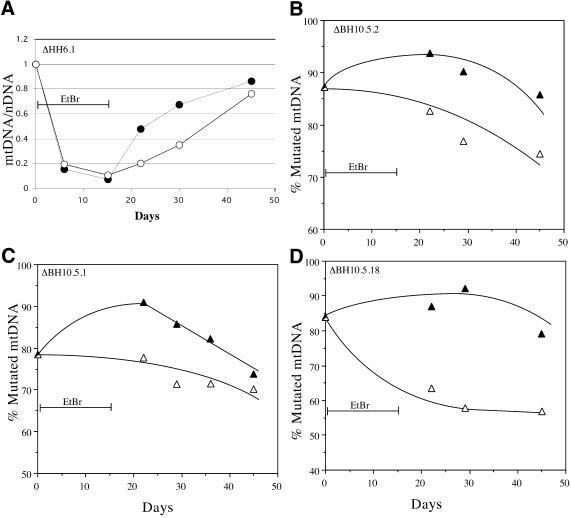
EtBr has been used extensively to reduce the mtDNA copy number in proliferating cells (16). We treated osteosarcoma cell lines with 50 ng/ml EtBr for 15 days, after which the drug was removed from the medium and the cells allowed to grow for 30 additional days. Throughout the experiment, the medium was supplemented with pyruvate and uridine to avoid a strong selection against cells defective in respiration, which can be auxotrophic for these substrates (17). DNA was obtained at different days and analyzed for the ratio mtDNA/nuclear gene. Figure 2A shows that the EtBr treatment led to a marked depletion of mtDNA followed by a repopulation phase.
Figure 2.
ΔmtDNA heteroplasmy fluctuations after induced mtDNA depletion. (A) An osteosarcoma cell line containing high levels of a ΔmtDNA was treated with 50 ng/ml EtBr for 15 days and allowed to recover for 30 additional days. Cells were harvested at the indicated times and their DNA purified and analyzed for relative levels of mtDNA (mutated or wild-type, closed and open circles, respectively) / nDNA as described in Materials and Methods. (B–D) Three cell lines harboring ΔmtDNA were subjected to a 15 day EtBr treatment followed by a 30 day recovery period. Parallel cultures growing in the same medium, but without EtBr were also analyzed to assess heteroplasmy fluctuations related to time in culture. Total DNA extracted at different times was analyzed by Southern blot and the percentage mutated mtDNA represented as filled triangles in the EtBr treated series and as open triangles in the control series. The mtDNA levels during EtBr treatment (days 7 and 15) were very low and did not allow a reliable estimation of heteroplasmy. There was a reduction in the percentage mutated mtDNA during normal growth conditions, but EtBr treatment was consistently associated with an increase in percentage mutated mtDNA immediately after the treatment.
Selected cell lines were divided into two groups, one containing heteroplasmic mtDNA large deletions and one containing heteroplasmic mtDNA point mutations. In all cases they were treated with EtBr for 15 days at which point the drug was removed and the cells allowed to grow for 30 additional days. Parallel cultures that were not treated with EtBr were also analyzed to assess fluctuations in mtDNA heteroplasmy that were independent of EtBr treatment. The EtBr-induced mtDNA depletion was confirmed for all the treated samples by Southern blot (not shown). The percentages of mutated and wild-type mtDNA were not calculated during days 6 and 15 of EtBr treatment because mtDNA levels were markedly decreased, affecting the reliability of the Southern blot assay.
In the heteroplasmic mtDNA large deletions group, untreated cells showed a small drift towards lower levels of mtDNA mutations (Fig. 2B–D, open triangles). After 45 days in culture (under non-selective conditions) the percentage of mutated mtDNA decreased by 10–20% depending on the cell line. In the EtBr-treated group, there was an increase in the mutated mtDNA population immediately following the removal of EtBr from the medium (Fig. 2B–D, solid triangles). Seven days after EtBr removal, the percentage of ΔmtDNA increased by 6.2 ± 4.8% (mean ± SD) whereas during the same time in the untreated group the percentage of ΔmtDNA decreased by 9.1 ± 8.8%. Therefore the mean difference in the percent mutated mtDNA between treated and untreated, 7 days after EtBr withdrawal was 15.3% (P = 0.011). The increase in percent of ΔmtDNA observed in the EtBr treated cells was followed by a drift towards slightly lower levels, confirming the trend observed in untreated cells (Fig. 2B–D).
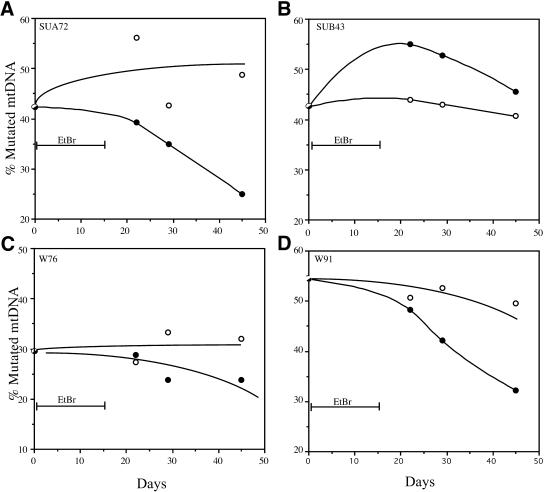
In contrast to the mtDNA large deletions group, increases in the percentage of mutated mtDNA after EtBr treatment/withdrawal could not be observed in cells heteroplasmic for pathogenic point mutations (Fig. 3). Overall, there was also a trend for a small reduction in the percentage of point mutated mtDNA with time in culture without treatment. EtBr treatment/withdrawal was not associated with an increase in the percentage of mutated mtDNA. With exception of the cell line SUB43, the other three cell lines showed a decrease in the percentage of mutated mtDNA following the EtBr treatment/withdrawal (Fig. 3).
Figure 3.
Point mutant mtDNA heteroplasmy fluctuations after induced mtDNA depletion. Four cell lines harboring two different pathogenic mtDNA point mutations in tRNA genes were subjected to a 15 day EtBr treatment followed by a 30 day recovery period. Parallel cultures growing in the same medium, but without EtBr were performed to assess heteroplasmy fluctuations during time in culture. Total DNA was extracted at different times and analyzed by PCR/RFLP. The percentage mutated mtDNA is represented as filled circles in the EtBr treated series and as open circles in the control series (A–D). With the exception of one cell line (B), the percentage of point mutated mtDNA either did not change significantly or decreased from pretreatment levels. The treatment with EtBr did not seem to alter this natural trend.
mtDNA harboring large deletions are preferentially replicated under relaxed copy number control
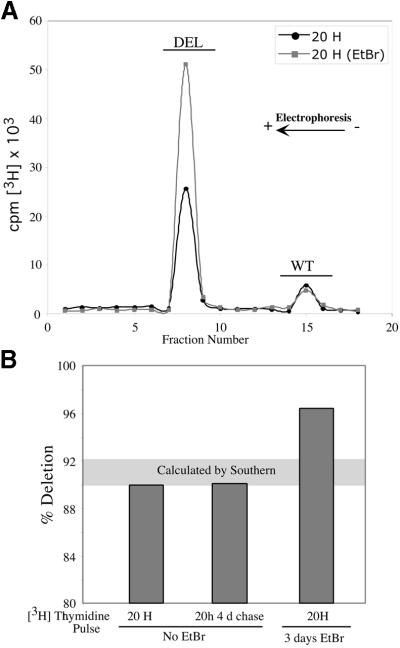
We measured the rate of [3H]thymidine incorporation into cell line ΔDH2.1, which harbors a heteroplasmic 7.5 kb mtDNA deletion. Cells were pulse-labeled with [3H]thymidine for 20 h both without EtBr and after 3 days EtBr (50 ng/ml) treatment. The EtBr treatment was shorter than in other experiments because a relatively high level of mtDNA was necessary to obtain reliable results with this method. mtDNA purified from labeled cells was digested with PvuII and fractionated by agarose gel electrophoresis. Figure 4A shows that the relative rate of [3H]thymidine incorporation was significantly increased in the partially-deleted molecule after EtBr treatment. Bars on Figure 4B represent the percentage ΔmtDNA calculated from the [3H]thymidine-labeled fraction after correction for the number of thymidines (mutated molecule values were multiplied by 1.87). Twenty hours labeling followed by a 4 day chase showed a 3H-labeled fraction of ΔmtDNA that was similar to the one obtained by Southern blot data (i.e. ∼89–92% deleted, horizontal grey bar on Fig. 4B). The EtBr pre-treatment led to an over-representation of 3H-labeled fraction of ΔmtDNA (96% of total, Fig. 4B).
Figure 4.
Metabolic labeling of mtDNA with [3H]thymidine. The cell line ΔDH2.1 was incubated with 20 µCi/ml of [3H]thymidine for the times indicated. In one group, cells were pretreated with EtBr for 3 days, allowed to recover without the drug for 24 h and labeled with [3H]thymidine for the periods indicated. mtDNA from labeled cells was purified from mitochondria isolated by N2 cavitation as described in Materials and Methods. mtDNA was digested with PvuII and separated by electrophoresis on a 0.8% agarose gel. The gel lane was sliced and the gel fractions analyzed in a scintillation counter (A). Percentage mutated mtDNA was calculated from the incorporated 3H after correction for the number of thymidines in each molecule. (B) Comparison of these values with the percentage mutation obtained from Southern blots (grey horizontal area).
mtDNA harboring large deletions are preferentially maintained in hybrids with competing mitochondrial genomes
In order to study mtDNA heteroplasmic dynamics in an independent system, we created hybrids containing different types of mitochondrial genomes and assessed if there were specific haplotypes that were preferentially maintained. To do so, we used three cell lines that had 143B-derived nuclear background, but each one tagged with a different dominant nuclear marker, namely neomycin-, zeocin- or puromycin-resistance. Figure 5A illustrates the procedure and describes the following cell lines: (i) a neo-resistant cell line with wild-type mtDNA; (ii) a zeo-resistant cell line with a large mtDNA deletion (Δ7.5 kb mtDNA deletion, Δ16.10.40); (iii) a puro-resistant cell line with a small 4 bp deletion in the apocytochrome b gene. In contrast to the cell lines used in the previous experiments, these cell lines were homoplasmic for the respective mtDNA haplotypes. Cell fusions were performed and hybrid clones were isolated by double selection according to the nuclear markers present in their nuclei. All media were supplemented with pyruvate and uridine. The expanded clones had their DNA extracted and characterized as described below.
Figure 5.
Heteroplasmy levels of somatic hybrids generated without selection for OXPHOS function. Three different cell lines were fused to each other as depicted in (A). One contained wild-type mtDNA and a neomycin-resistance nuclear marker (N), a second was homoplasmic for a mtDNA 7.5 kb deletion containing a zeocin-resistance nuclear marker (Z) and a third was homoplasmic for a pathogenic 4 bp deletion in cytochrome b gene containing a puromycin-resistance nuclear marker (P). Fusion products were grown in high glucose media containing two of the respective selection drugs (i.e. G418, zeocin or puromycin) and supplemented with uridine. Surviving clones were isolated and their DNA analyzed by Southern blot as described in Materials and Methods. (B) Analysis of the cytochrome b 4 bp deletion in hybrids of PN cell lines. A 32P-labeled PCR amplicon, corresponding to the 5′ end of the apocytochrome b gene, was separated by electrophoresis on a 6% denaturing polyacrylamide gel and analyzed in a phosphoimager. (C) Phosphoimager signal from a Southern blot of DNA extracted from the hybrids between cells containing the 7.5 kb deletion and wt (NZ lines) or the 4 bp deletion in apocytochrome b (ZP lines).
To differentiate mtDNA haplotypes in PN hybrids [i.e. puro (harboring Δ4 bp) and neo (harboring wt mtDNA)], we amplified a 93 bp fragment flanking the 4 bp deletion and analyzed the amplicons by PAGE. Surprisingly, PN clones were homoplasmic for the wild-type mtDNA, losing the Δ4 bp mtDNA (Fig. 5B). As expected, amplifications from ZP clones [i.e. zeo (harboring mtDNA with a Δ7.5 kb deletion) and puro (harboring Δ4 bp)] gave rise exclusively to the Δ4 bp cyt b in this test, as the large deletion present in the zeo-resistant cell line spanned the PCR target (i.e. the Δ4 bp cyt b region; Fig. 5B). mtDNA with a large 7.5 kb deletion were distinguished from the wild type and the Δ4 bp cyt b by Southern analysis. With the exception of one clone (NZ1) NZ clones [i.e. neo (harboring wt mtDNA) and zeo (harboring Δ7.5 kb mtDNA)] showed a higher percentage of wild type than ΔmtDNA. In contrast, ZP clones [i.e. zeo (harboring Δ7.5 kb mtDNA) and puro (harboring Δ4 bp)] had consistently high levels of the mitochondrial genome harboring a large deletion (Fig. 5C). Taken together, these results suggest that the hybrid lines tended to preserve the functional mitochondrial genome, probably because of better growth performance. In the cases where both mitochondrial genomes were defective, the smaller one prevailed.
DISCUSSION
mtDNA heteroplasmy under tight copy number control
A preferential maintenance of ΔmtDNA was not observed during normal cell growth conditions (Fig. 2) (18). In heteroplasmic cells harboring ΔmtDNA, the fraction of partially-deleted genomes actually showed a small decrease (10–20%) with time under non-selective culture conditions. This reduction was probably observed because cells with relatively higher levels of the wild-type mtDNA have slightly better growth performance, even in rich medium (i.e. media supplemented with high glucose, pyruvate and uridine). Osteosarcoma cybrids with homoplasmic levels of ΔmtDNA have been shown to grow slightly more slowly than their wild-type counterparts (18). Hayashi et al. showed that HeLa cybrids with different levels of ΔmtDNA also had slightly slower population doubling times, with the slowest being the cell line with the highest percentage of mutated mtDNA (19). Tang et al. (18) found that the ΔmtDNA fraction decreased by only ∼10% during prolonged culture, and found it to be not significant. Hayashi et al. found that, after producing cybrids, there was a bias towards an increase in the ΔmtDNA population after which the levels ‘stabilized’. However, examination of their data showed a consistent small reduction (5–15%) in the percentage of ΔmtDNA after 10 weeks post-fusion (19). Differences in nuclear background may affect the balance between mutated and wild-type mtDNA in non-selective conditions.
mtDNA heteroplasmy under relaxed copy number control
Treatment with EtBr, which reduced the mtDNA copies to very low levels, had a significant effect on the mtDNA repopulation rates within organelles. When EtBr was removed from the media, mtDNA replication ensued at possibly maximum speed, as the still poorly characterized copy number control mechanism attempted to repopulate organelles with the normal copy number or mtDNA mass (20). Under these conditions of fast replicative activity, ΔmtDNAs fared significantly better, repopulating organelles ∼2–3 times faster than their wild-type counterparts. The present results suggest that increased repopulation rates were not due to an OXPHOS response, as the mutated mtDNA harboring point mutations (but still having an OXPHOS defect) did not show changes in repopulation rates relative to the wild-type mtDNA. Differences in cellular growth were also minimized by the fact that mutated and wild-type genomes were present in the same cell. [3H]Thymidine incorporation was significantly increased in the partially-deleted genomes after mtDNA copy number reduction, corroborating the concept that fast replicative rates favor smaller molecules. These results are compatible with a previous report showing that the rates of [3H]thymidine incorporation were similar between partially-deleted and wild-type mtDNA when cells were labeled without previous mtDNA depletion (21). The replicative advantage of smaller mitochondrial genomes may be masked by the slow replication rates in copy number-controlled cells as well as by the cellular selection observed in proliferating cells.
Our observation that replication between the two mtDNA haplotypes was not synchronized suggests that, even though human mitochondrial genomes are associated with proteins and membranes (22), possibly resembling the yeast nucleoids (23), they do not appear to behave as a group of molecules segregating as a unit as previously suggested (24). Therefore, in a putative mammalian nucleoid, individual molecules have the potential to replicate independently from others. The nucleoid concept described above (24) was proposed to explain mtDNA heteroplasmy stability. However, our results show that heteroplasmy is not stable during fast mtDNA replication.
mtDNA heteroplasmy under copy number re-setting
The proliferating hybrid results suggest that when a double dose of mtDNA is introduced in cells, they tend to maintain functional mitochondrial genomes. This conclusion is based on the observation that wild-type genomes were preferentially maintained in hybrids of 143B and either Δ16.10.40 or Δ4cyt b. In contrast, in hybrids between Δ16.10.40 and Δ4cyt b, the mtDNA with the largest deletion was preferentially maintained. It is unclear why the wild-type mitochondrial genomes were retained at such high relative levels. In particular, the absence of genomes with the cyt b 4 bp deletion in the PN (wt + Δ4cyt b) clones was surprising. Increased ROS previously associated with this cyt b mutation may make this mtDNA haplotype particularly unfavorable to organelles or cells during the resetting of mtDNA copy number (13).
Factors influencing mtDNA repopulation rates
It is unlikely that the processivity of the DNA polymerase γ differs between mutated and wild-type mtDNA. A ‘normal’ replication rate has been reported for mtDNA with point mutations (25), and it is likely to be unchanged in partially-deleted mitochondrial genomes as well. It is more likely that the increased repopulation rate is due to faster completion of the relatively slow mtDNA replication process (26).
Seidel-Rogol and Shadel recently showed that during the EtBr treatment recovery phase, mtTFA (which is known to be unstable in the absence of mtDNA) and mtRNA polymerase increased to normal levels at a significantly slower rate than mtDNA (27). Transcripts for these genes have been previously shown to be unaffected by mtDNA depletion (8). It is likely that such replication factors will be limiting during the repopulation phase and the small amounts of replication factors available after depletion of mtDNA can be re-engaged only after a mtDNA molecule finishes its replication cycle. Therefore, replication factors would be available to replicate another molecule sooner when the target molecule is smaller.
Insights into ΔmtDNA accumulation during aging
The results described in this report provide direct evidence that under conditions of relaxed copy number control, ΔmtDNAs have an advantage over wild-type molecules in the same cell. This phenomenon, previously suggested based on indirect evidence (28,29), has important implications for the mechanisms of mtDNA deletions accumulation during the aging and disease processes.
Although no consensus has been reached, it is generally believed that accumulation of mtDNA deletions is more prevalent than accumulations of mtDNA point mutations during aging of postmitotic tissues (30). Recent models showed that relaxed replication of mtDNA alone could lead, through random genetic drift, to the clonal expansion of single mutant events during human life (31). This mechanism could lead to the accumulation or disappearance of a specific mtDNA haplotype in some cases, but in our view, genetic drift does not explain the consistent increase of ΔmtDNA nor excludes the contributing effect of faster repopulation of organelles with smaller genomes during active replication. There is also evidence that mtDNA with partial duplications may accumulate with age (32–34). The mechanism for the accumulation of partial duplications is unclear and cannot be explained by our results. Although mtDNA molecules with partial duplications have additional replication origins that could compensate for the increased length, earlier work showed that mtDNA dimers replicate slower than monomers (35).
Although there may be important differences between postmitotic tissues and our culture cell system, the observation of heteroplasmy fluctuations during rapid mtDNA repopulation allows us to draw some conclusions regarding the molecular aspect of differential repopulation rates. Our results are in agreement with previous in situ hybridization experiments that showed that most age-related mtDNA deletions in muscle are caused by clonal expansion of deletions (36,37). In muscle, mitochondria with defective function are stimulated to proliferate, and that may increase mtDNA replication, mimicking a relaxed copy number control situation. It also strengthened the view that age-related mtDNA deletions are probably generated at random but their levels gradually increase with time. Our results also raise the possibility that the accumulation of ΔmtDNAs may be accelerated by metabolic or environmental changes leading to either a transient reduction in mtDNA levels or a relaxation in copy number control.
CONCLUSION
In summary, our results provide evidence that under proliferating conditions, cells harboring relatively high levels of ΔmtDNAs have a slight reduction in the mutated fraction, probably because of better growth of cells with lower mtDNA mutation loads. This is compatible with the relatively low percentage of mutated mtDNA in proliferating peripheral blood cells and fibroblasts of patients with mitochondrial diseases (38). On the other hand, at the molecular level, smaller mtDNAs containing the major two replication origins have a repopulating advantage over the wild-type counterpart that operates most efficiently during relaxed copy number control. This situation parallels the accumulation of large-scale mtDNA deletions in postmitotic tissues, where selection based on cellular growth or survival does not take place, and abnormal organelle proliferation would lead to an increase in mtDNA replication rates (36,37).
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants EY10804 and GM55766 and by the Muscular Dystrophy Association.
REFERENCES
- 1.Shadel G.S. and Clayton,D.A. (1997) Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem., 66, 409–435. [DOI] [PubMed] [Google Scholar]
- 2.Moraes C.T. (2001) What regulates mitochondrial DNA copy number in animal cells? Trends Genet., 17, 199–205. [DOI] [PubMed] [Google Scholar]
- 3.Wallace D.C. (1997) Mitochondrial DNA in aging and disease. Sci. Am., 277, 40–47. [DOI] [PubMed] [Google Scholar]
- 4.Raha S. and Robinson,B.H. (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci., 25, 502–508. [DOI] [PubMed] [Google Scholar]
- 5.Corral-Debrinski M., Horton,T., Lott,M.T., Shoffner,J.M., Beal,M.F. and Wallace,D.C. (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nature Genet., 2, 324–329. [DOI] [PubMed] [Google Scholar]
- 6.Cortopassi G.A., Shibata,D., Soong,N.W. and Arnheim,N. (1992) A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc. Natl Acad. Sci. USA, 89, 7370–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mita S., Rizzuto,R., Moraes,C.T., Shanske,S., Arnaudo,E., Fabrizi,G.M., Koga,Y., DiMauro,S. and Schon,E.A. (1990) Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res., 18, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moraes C.T., Kenyon,L. and Hao,H. (1999) Mechanisms of human mitochondrial DNA maintenance: the determining role of primary sequence and length over function. Mol. Biol. Cell, 10, 3345–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancho S., Moraes,C.T., Tanji,K. and Miranda,A.F. (1992) Structural and functional mitochondrial abnormalities associated with high levels of partially deleted mitochondrial DNAs in somatic cell hybrids. Somat. Cell Mol. Genet., 18, 431–442. [DOI] [PubMed] [Google Scholar]
- 10.King M.P. (1996) Use of ethidium bromide to manipulate ratio of mutated and wild-type mitochondrial DNA in cultured cells. Methods Enzymol., 264, 339–344. [DOI] [PubMed] [Google Scholar]
- 11.Hao H. and Moraes,C.T. (1996) Functional and molecular mitochondrial abnormalities associated with a C → T transition at position 3256 of the human mitochondrial genome. The effects of a pathogenic mitochondrial tRNA point mutation in organelle translation and RNA processing. J. Biol. Chem., 271, 2347–2352. [DOI] [PubMed] [Google Scholar]
- 12.Hao H. and Moraes,C.T. (1997) A disease-associated G5703A mutation in human mitochondrial DNA causes a conformational change and a marked decrease in steady-state levels of mitochondrial tRNA(Asn). Mol. Cell. Biol., 17, 6831–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana M., de Coo,I., Diaz,F., Smeets,H. and Moraes,C.T. (2000) An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann. Neurol., 48, 774–781. [PubMed] [Google Scholar]
- 14.Moraes C.T., Shanske,S., Tritschler,H.J., Aprille,J.R. Andreetta,F., Bonilla,E., Schon,E.A. and DiMauro,S. (1991) mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am. J. Hum. Genet., 48, 492–501. [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb R.A. and Granville,D.J. (2002) Analyzing mitochondrial changes during apoptosis. Methods, 26, 341–347. [DOI] [PubMed] [Google Scholar]
- 16.Desjardins P., de Muys,J.M. and Morais,R. (1986) An established avian fibroblast cell line without mitochondrial DNA. Somat. Cell Mol. Genet., 12, 133–139. [DOI] [PubMed] [Google Scholar]
- 17.King M.P. and Attardi,G. (1989) Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science, 246, 500–503. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y., Manfredi,G., Hirano,M. and Schon,E.A. (2000) Maintenance of human rearranged mitochondrial DNAs in long-term cultured transmitochondrial cell lines. Mol. Biol. Cell., 11, 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi J., Ohta,S., Kikuchi,A., Takemitsu,M., Goto,Y. and Nonaka,I. (1991) Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc. Natl Acad. Sci. USA, 88, 10614–10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y., Schon,E.A., Wilichowski,E., Vazquez-Memije,M.E., Davidson,E. and King,M.P. (2000) Rearrangements of human mitochondrial DNA (mtDNA): new insights into the regulation of mtDNA copy number and gene expression. Mol. Biol. Cell, 11, 1471–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moraes,C.T. and Schon,E.A. (1995) In Palmieri,F., Papa,S., Saccone,C. and Gadaleta,M.N. (eds), Progress in Cell Research: Symposium on ‘Thirty years of Progress in Mitochondrial Bioenergetics and Molecular Biology’. Elsevier, Vol. 5, pp. 209–215.
- 22.Albring M., Griffith,J. and Attardi,G. (1977) Association of a protein structure of probable membrane derivation with HeLa cell mitochondrial DNA near its origin of replication. Proc. Natl Acad. Sci. USA, 74, 1348–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyakawa I., Sando,N., Kawano,S., Nakamura,S. and Kuroiwa,T. (1987) Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. J. Cell Sci., 88, 431–439. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs H.T., Lehtinen,S.K. and Spelbrink,J.N. (2000) No sex please, we’re mitochondria: a hypothesis on the somatic unit of inheritance of mammalian mtDNA. Bioessays, 22, 564–572. [DOI] [PubMed] [Google Scholar]
- 25.Emmerson C.F., Brown,G.K. and Poulton,J. (2001) Synthesis of mitochondrial DNA in permeabilised human cultured cells. Nucleic Acids Res., 29, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton D.A. (1982) Replication of animal mitochondrial DNA. Cell, 28, 693–705. [DOI] [PubMed] [Google Scholar]
- 27.Seidel-Rogol B.L. and Shadel,G.S. (2002) Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res., 30, 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Prosser,R., Simonetti,S., Sadlock,J., Jagiello,G. and Schon,E.A. (1995) Rearranged mitochondrial genomes are present in human oocytes. Am. J. Hum. Genet., 57, 239–247. [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson N.G., Holme,E., Kristiansson,B., Oldfors,A. and Tulinius,M. (1990) Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr. Res., 28, 131–136. [DOI] [PubMed] [Google Scholar]
- 30.Pallotti F., Chen,X., Bonilla,E. and Schon,E.A. (1996) Evidence that specific mtDNA point mutations may not accumulate in skeletal muscle during normal human aging. Am. J. Hum. Genet., 59, 591–602. [PMC free article] [PubMed] [Google Scholar]
- 31.Elson J.L., Samuels,D.C., Turnbull,D.M. and Chinnery,P.F. (2001) Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am. J. Hum. Genet., 68, 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodyak N.D., Nekhaeva,E., Wei,J.Y. and Khrapko,K. (2001) Quantification and sequencing of somatic deleted mtDNA in single cells: evidence for partially duplicated mtDNA in aged human tissues. Hum. Mol. Genet., 10, 17–24. [DOI] [PubMed] [Google Scholar]
- 33.Kajander O.A., Rovio,A.T., Majamaa,K., Poulton,J., Spelbrink,J.N., Holt,I.J., Karhunen,P.J. and Jacobs,H.T. (2000) Human mtDNA sublimons resemble rearranged mitochondrial genoms found in pathological states. Hum. Mol. Genet., 9, 2821–2835. [DOI] [PubMed] [Google Scholar]
- 34.Moore C.A., Gudikote,J. and Van Tuyle,G.C. (1998) Mitochondrial DNA rearrangements, including partial duplications, occur in young and old rat tissues. Mutat. Res., 421, 205–217. [DOI] [PubMed] [Google Scholar]
- 35.Bogenhagen D., Lowell,C. and Clayton,D.A. (1981) Mechanism of mitochondrial DNA replication in mouse L-cells. Replication of unicircular dimer molecules. J. Mol. Biol., 148, 77–93. [DOI] [PubMed] [Google Scholar]
- 36.Johnston W., Karpati,G., Carpenter,S., Arnold,D. and Shoubridge,E.A. (1995) Late-onset mitochondrial myopathy. Ann. Neurol., 37, 16–23. [DOI] [PubMed] [Google Scholar]
- 37.Moslemi A.R., Melberg,A., Holme,E. and Oldfors,A. (1996) Clonal expansion of mitochondrial DNA with multiple deletions in autosomal dominant progressive external ophthalmoplegia. Ann. Neurol., 40, 707–713. [DOI] [PubMed] [Google Scholar]
- 38.Moraes C.T., DiMauro,S., Zeviani,M., Lombes,A., Shanske,S., Miranda,A.F., Nakase,H., Bonilla,E., Werneck,L.C., Servidei,S. et al. (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N. Engl. J. Med., 320, 1293–1299. [DOI] [PubMed] [Google Scholar]