Abstract
In recent years, lentiviral expression systems have gained an unmatched reputation among the gene therapy community for their ability to deliver therapeutic transgenes into a wide variety of difficult-to-transfect/transduce target tissues (brain, hematopoietic system, liver, lung, retina) without eliciting significant humoral immune responses. We have cloned a construction kit-like self-inactivating lentiviral expression vector family which is compatible to state-of-the-art packaging and pseudotyping technologies and contains, besides essential cis-acting lentiviral sequences, (i) unparalleled polylinkers with up to 29 unique sites for restriction endonucleases, many of which recognize 8 bp motifs, (ii) strong promoters derived from the human cytomegalovirus immediate-early promoter (PhCMV) or the human elongation factor 1α (PhEF1α), (iii) PhCMV– or PPGK– (phosphoglycerate kinase promoter) driven G418 resistance markers or fluorescent protein-based expression tracers and (iv) tricistronic expression cassettes for coordinated expression of up to three transgenes. In addition, we have designed a size-optimized series of highly modular lentiviral expression vectors (pLenti Module) which contain, besides the extensive central polylinker, unique restriction sites flanking any of the 5′U3, R-U5-ψ+-SD, cPPT-RRE-SA and 3′LTRΔU3 modules or placed within the 5′U3 (–78 bp) and 3′LTRΔU3 (8666 bp). pLentiModule enables straightforward cassette-type module swapping between lentiviral expression vector family members and facilitates the design of Tat-independent (replacement of 5′LTR by heterologous promoter elements), regulated and self-excisable proviruses (insertion of responsive operators or LoxP in the 3′LTRΔU3 element). We have validated our lentiviral expression vectors by transduction of a variety of insect, chicken, murine and human cell lines as well as adult rat cardiomyocytes, rat hippocampal slices and chicken embryos. The novel multi-purpose construction kit-like vector series described here is compatible with itself as well as many other (non-viral) mammalian expression vectors for straightforward exchange of key components (e.g. promoters, LTRs, resistance genes) and will assist the gene therapy and tissue engineering communities in developing lentiviral expression vectors tailored for optimal treatment of prominent human diseases.
INTRODUCTION
Gene therapy strategies rely on efficient transfer of therapeutic transgenes into desired target cells. A variety of viral and non-viral vectors and expression concepts have been designed and evaluated for their safety, high-level transduction, tropism and sustained expression in a variety of therapeutically relevant cells and tissues (1,2). Retroviral vectors derived from oncoretroviruses such as the murine leukemia virus (MLV) emerged as the most widely used gene therapy tools for transgene delivery currently in the clinics (3). The attractiveness of oncoretroviral transduction technologies resides in: (i) their ability to mediate stable integration in the target chromosomes likely promoting long-term expression of delivered transgenes; (ii) their large cloning capacity sufficient for most foreseeable clinical situations; (iii) their compatibility with pseudotyping strategies which extend the tropism of gene delivery; and (iv) the exclusive delivery of therapeutic transgenes in the absence of viral genes which precludes any potent humoral immune response eliminating transduced cells and enables recurring treatments (3,4).
In addition to these characteristics common to all retroviruses, lentiviruses such as the human immunodeficiency virus type 1 (HIV-1) can replicate in non-mitotic cells owing to their so-called pre-integration complex, a macromolecular structure comprising the viral genome, a few structural proteins and the enzymes responsible for reverse transcription and integration (5–7). Transduction of proliferation-incompetent cells is a decisive asset for molecular interventions in tissues considered key targets for future gene therapy including the brain, the heart, the hematopoietic system, the liver, the lungs and the retina (8–14). Like all retroviruses, the HIV-1 genome contains the gag, pol and env regions, which encode the core proteins, the virion-associated enzymes and the envelope (Env) glycoprotein (13,15). This coding region is flanked by the long terminal repeats (LTRs; 5′ and 3′LTR) and cis-acting sequences essential for integration, transcription and polyadenylation. In addition, HIV-1 contains two regulatory genes, tat and rev, required for viral replication (LTR transactivation and nuclear export of viral RNA) and four accessory genes, vif, vpr, vpu and nef, which are dispensable for viral growth but critical for in vivo replication and pathogenesis (15). Basal transcription of the HIV-1 provirus’ 5′LTR initially results in small amounts of multiply spliced transcripts encoding Tat, Rev and Nef. Tat transactivates 5′LTR-mediated transcription until Rev reaches a threshold concentration and mediates cytoplasmic accumulation of unspliced and singly spliced viral transcripts followed by production of the late viral proteins (15).
Capitalizing on a refined understanding of HIV-1 molecular biology following decades of intensive research on acquired immunodeficiencies, a group of researchers pioneered the transition of this well-evolved pathogen into a high leverage gene therapy tool (16–18). HIV-1-based vectors have been generated using a multiply attenuated packaging system, which is activated in its most advanced third generation configuration by transient transfection of four plasmids encoding: (i) gag (coding for the virion main structural proteins) and pol (responsible for lentivirus-specific enzymes); (ii) rev (a post-transcriptional regulator for gag and pol expression as well as nuclear RNA export); (iii) vsv-g (required for pseudotyping); and (iv) the desired transgene in a lentiviral expression configuration (lentiviral expression vector or lentivector) (19). Latest developments include packaging cell lines, which express all packaging components and require exclusive transfection of the lentiviral expression vector (20–23). The lentiviral expression vector, which can be combined with any generation of packaging system, is the only genetic material transferred to the target cell. It typically comprises the transgene cassette flanked by cis-acting elements required for encapsidation, reverse transcription and integration including the packaging signal (ψ+), the polypurine tracts (PPT), 5′ and 3′LTRs as well as env-derived sequences encompassing the Rev response element (RRE) (16–19,24). Latest generation HIV-1-based expression technologies include self-inactivating (SIN) vectors, which loose the transcriptional capacity of the LTR once integrated into target cells. SIN vectors show improved performance and are devoid of transcriptional interference and in vivo suppression associated with standard vectors and enable construction of more-stringent tissue-specific and/or regulatable expression configurations (25,26).
Unfortunately, current lentiviral expression vectors are the result of a progressive development rather than a bottom-up rational design and are therefore often lacking convenient multiple cloning sites (MCS) and a modular set-up to increase compatibility with existing expression technologies (gene regulation, recombination) (16,17,19). This situation has significantly delayed the dissemination of lentiviral expression technology beyond the pioneering laboratories to a broad scientific community. We describe here a size-optimized highly modular construction kit of SIN lentiviral expression vectors containing extended MCS, multicistronic expression cassettes as well as various promoter and resistance elements.
MATERIALS AND METHODS
Cell culture and fluorescence microscopy
Chinese hamster ovary cells (CHO-K1; ATCC CCL-61), baby hamster kidney cells BHK-21 (ATCC CCL-10), human fibrosarcoma cells HT-1080 (ATCC CCL-121), human cervical adenocarcinoma cells HeLa (ATCC CCL-2), human hepatocellular carcinoma cells HepG2 (ATCC HB-8065) and human chronic myelogenous leukemia cell line K-562 (ATCC-243) were cultivated in FMX-8 (Cell Culture Technologies GmbH, Switzerland), Dulbecco’s modified Eagle medium (DMEM) (BHK-21, HT-1080, HeLa, HepG2; catalog no. 52100-039; Life Technologies AG, Basel, Switzerland) or Iscove’s modified Dulbecco’s medium (K-562, catalog no. 4220-022; Life Technologies AG) supplemented with 10% fetal calf serum (all other cell lines; PAA Vienna, Austria; Lot. no. A01129-242). The chicken bursal cell line DT40 was cultivated as described before (27). Adult rat cardiomyocytes (ARC) were prepared and cultivated as described previously (28). Hippocampal slices prepared from 6-day postnatal rats were cultured using the roller-tube technique (29).
For DRAQ5-mediated DNA-specific staining of transduced cells, 4 × 103 CHO-K1 cells were seeded into chamber slides (Lab-Tek, Nalge Nunc International, IL) and grown for 24 h before they were infected with 2 × 106 c.f.u./ml of desired lentiviruses (pMF365-, pBM40- and pBM45-derived). After 48 h, DRAQ5 (Biostatus Ltd, Leicestershire, UK) was added to the culture at a final concentration of 25 µM for 7 min. The medium was removed and cells washed twice with PBS (Dulbecco’s phosphate-buffered saline, Sigma, catalog no. D5773) before they were fixed with 4% paraformaldehyde in PBS for 8 min and washed another five times with PBS. After removing the PBS, the cells were covered with a drop of Lisbeth’s medium [Tris-buffered glycerol: 3:7 mixture of 0.1 M Tris–HCl (pH 9.5) and glycerol plus 50 mg/ml n-propyl-gallat] and sealed with a cover slip for confocal microscopy.
Expression of fluorescent proteins was visualized using a Leica DM-RB fluorescence microscope (Heerbrugg, Switzerland) or a confocal microscope set-up (Zeiss Axioplan fluorescence microscope, Biorad MRC-600 confocal scanner, Silicon Graphics workstation) equipped with appropriate filters. The fluorescence of the enhanced green fluorescent protein (EGFP) and the enhanced yellow fluorescent protein (EYFP) were visualized using the XF114 filter (Omega Optical Inc., Brattleboro, VT), the enhanced cyan fluorescent protein (ECFP) was detected using the XF105 filter (Omega Optical Inc.) and the red fluorescent protein was monitored using the N2.1 filter by Leica Inc (Heerbrugg, Switzerland).
In vivo transduction of chicken embryos
Experiments on chicken embryos were conducted following the shell-free cultivation protocols by Djonov and co-workers (30). After 3 days of incubation at 37°C, Brown Leghorn eggs were opened, and their contents were carefully poured into plastic Petri dishes, 80 mm in diameter. The chicken embryos were incubated at 37°C in a humidified atmosphere. Recombinant lentiviruses were applied locally on the top of the growing chorioallontoic membrane (CAM) or injected intravenously at embryonic day 9. On embryonic day 11, the CAMs were examined by in vivo fluorescence microscopy following intravenous (i.v.) injection of 0.1 ml 2.5 fluoresceine isothiocyanate dextran (2 000 000; Sigma, St Louis, MO) (31). The developing blood vessels were monitored using a Polyvar-Reichert fluorescent microscope at a magnification of 25×. The microscope was equipped with a custom-built heating table to maintain the temperature of the specimens at 37°C. Blood circulation and microvascular patterns were monitored for ∼15 min for every CAM using an LE CCD Optronics video camera (Visitron system, Puchheim, Germany) and a digital video recorder (Sony, DHR-1000 VC).
Construction of lentiviral expression vectors
Lentiviral expression vectors (Table 1) containing extended multiple cloning sites. The basic multi-purpose lentiviral expression vector pMF356 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3) is derived from pNL-EGFPΔU3, a pNL series lentiviral vector containing an EcoRV/PvuII deletion in the U3 region (corresponding to 8666–9066 in Genbank accession no. AF033819) of the 3′LTR [3′LTRΔU3 (25,32)].
Table 1. Plasmids used and constructed in this study.
| Plasmid | Description | Reference or source |
|---|---|---|
| pcDNA3.1/V5-His TOPO | Mammalian expression vector | Invitrogen, Carlsbad, CA |
| pcDNA3 | Mammalian expression vector vector | Invitrogen, Carlsbad, CA |
| pcDNA3-VEGF121 | pcDNA3 encoding human VEGF121 | Weber, unpublished |
| pCF10 | Dual-regulated expression vector | Fux and Fussenegger, submitted for publication |
| pEF4/MycHisB | Expression vector containing the human elongation factor 1α promoter (PEF1α ) | Invitrogen, Carlsbad, CA |
| pEYFP-C1 | Expression vector encoding the enhanced yellow fluorescent protein under control of PhCMV | Clontech, Palo Alto, CA |
| PMSCVneo | Retroviral expression vector derived from the murine embryonic stem cell virus | Grez et al., 1990 |
| PLPCX | Retroviral expression vector derived from the Moloney MLV (MoMuLV) and Moloney murine sarcoma virus (MoMuSV) | Miller and Rosman, 1989 |
| pNL-EGFP | Lentiviral expression vector (5′LTR-ψ+-oriSV40-cPPT-RRE-3′LTR) | Mochizuki et al., 1998 |
| pNL-EGFPΔU3 | pNL-EGFP containing an EcoRV/PvuII deletion in the U3 region of the 3′LTR eliminating any enhancers (5′LTR-ψ+-oriSV40-cPPT-RRE-3′LTRΔU3) | Reiser et al., unpublished |
| pSS173 | Mammalian expression vector encoding the human placental SEAP | Schlatter et al., 2002 |
| pWW85 | Triple-fluorescent expression vector encoding ECFP, RFP and EYFP | Weber et al., 2002 |
| pWW265 | VEGF121 was excised from pcDNA-VEGF121 by HindIII/SpeI and ligated into the corresponding sites (HindIII/SpeI) of pCF10 (PhCMV-VEGF121-pA) | Weber et al., unpublished |
| pTRIDENT1 | Tricistronic mammalian expression vector harboring a tetracycline-responsive, PhCMV*-1-driven expression unit (PhCMV*-1-MCSI-IRESI-MCSII-IRESII-MCSIII-pA) (pMF125) | Fussenegger et al., 1998 |
| pTRIDENT3 | Tricistronic mammalian expression vector harboring a tetracycline-responsive, PhCMV*-1-driven expression unit (PhCMV*-1-MCSI-IRES-MCSII-CITE*-MCSIII-pA) (pMF122) | Fussenegger et al., 1998 |
| pMF201 | Dual-regulated expression vector encoding EYFP and ECFP | Fussenegger et al., 2000 |
| pMF242 | pcDNA3.1/V5-His TOPO encoding mouse erythropoietin (mEPO) | Fussenegger et al., 2000 |
| pMF320 | PEF1α was amplified from pEF4/MycHisB by OMF164/OMF165 and cloned in antisense orientation into pcDNA.3.1/V5-His TOPO | This work |
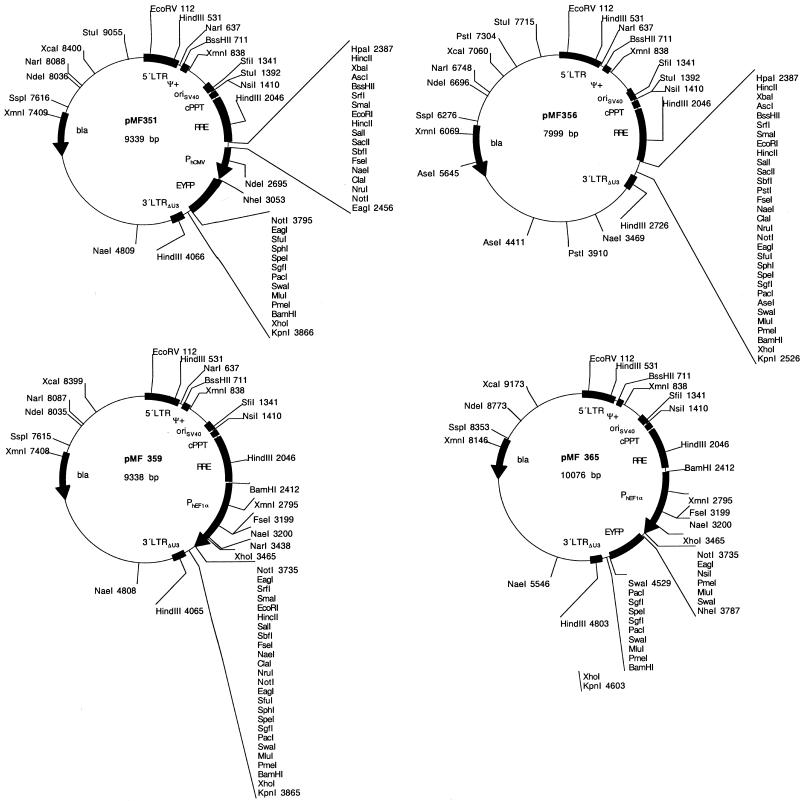
| pMF351 | PhCMV-EYFP was amplified from pEYFP-C1 with OMF179/OMF180 and cloned as HpaI/KpnI fragment into the corresponding sites (HpaI/KpnI) of pNL-EGFPΔU3 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PhCMV-EYFP-MCSII-3′LTRΔU3) | This work |
| pMF352 | U3 was amplified from pNL-EGFPΔU3 using OMF183/OMF184 and cloned into pcDNA3.1/V5-His TOPO. The U3 fragment contains a SbfI site | This work |
| pMF353 | R-U5-ψ+-SD was amplified from pNL-EGFPΔU3 using OMF185/OMF186 and cloned into pcDNA3.1/V5-His TOPO | This work |
| pMF355 | PPT-3′LTRΔU3 was amplified from pNL-EGFPΔU3 using OMF189/OMF190 and cloned into pcDNA3.1/V5-His TOPO | This work |
| pMF356 | The PhCMV-EYFP cassette of pMF351 was excised by NotI and the backbone religated (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3) | This work |
| pMF357 | U3 was excised from pMF352 by XhoI/SrfI and cloned into the corresponding sites (XhoI/SrfI) of pTRIDENT1 | This work |
| pMF358 | R-U5-ψ+-SD was excised from pMF353 by SrfI/SwaI and cloned into the corresponding sites (SrfI/SwaI) of pMF357 | This work |
| pMF358x | R-U5-ψ+-SD was excised from pMF376 by SrfI/SwaI and cloned into the corresponding sites (SrfI/SwaI) of pMF357 | This work |
| pMF359 | PEF1α was excised from pMF320 by EcoRV/XmaI and cloned into the corresponding sites (HpaI/XmaI) of pMF356 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3) | This work |
| pMF360 | cPPT-RRE-SA was excised from pMF375 by SwaI/MluI and cloned into the corresponding sites (SwaI/MluI) of pMF358 | This work |
| pMF360x | cPPT-RRE-SA was excised from pMF375 by SwaI/MluI and cloned into the corresponding sites (SwaI/MluI) of pMF358x | This work |
| pMF363 | PhCMV was excised from pLPCX using StuI/EcoRI and cloned into the compatible sites (HpaI/EcoRI of pMF356 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCS-3′LTRΔU3) | This work |
| pMF365 | EYFP was excised from pMF201 by EcoRV/SpeI and ligated to the compatible sites (SmaI/SpeI) of pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EYFP-3′LTRΔU3) | This work |
| pMF371 | ECFP was excised from pMF201 by EcoRI/NotI and ligated to the corresponding sites (EcoRI/NotI) of pMF363 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-3′LTRΔU3) | This work |
| pMF375 | cPPT-RRE-SA was amplified from pNL-EGFPΔU3 using OMF191/OMF192 and cloned into pcDNA3.1/V5-His TOPO | This work |
| pMF376 | R-U5-ψ+-SD was amplified from pNL-EGFPΔU3 using OMF185/OMF193 and cloned into pcDNA3.1/V5-His TOPO | This work |
| pMF378 | OriSV40 was amplified from pNL-EGFPΔU3 using OMF195/OMF196 and cloned into pcDNA3.1/V5-His TOPO | This work |
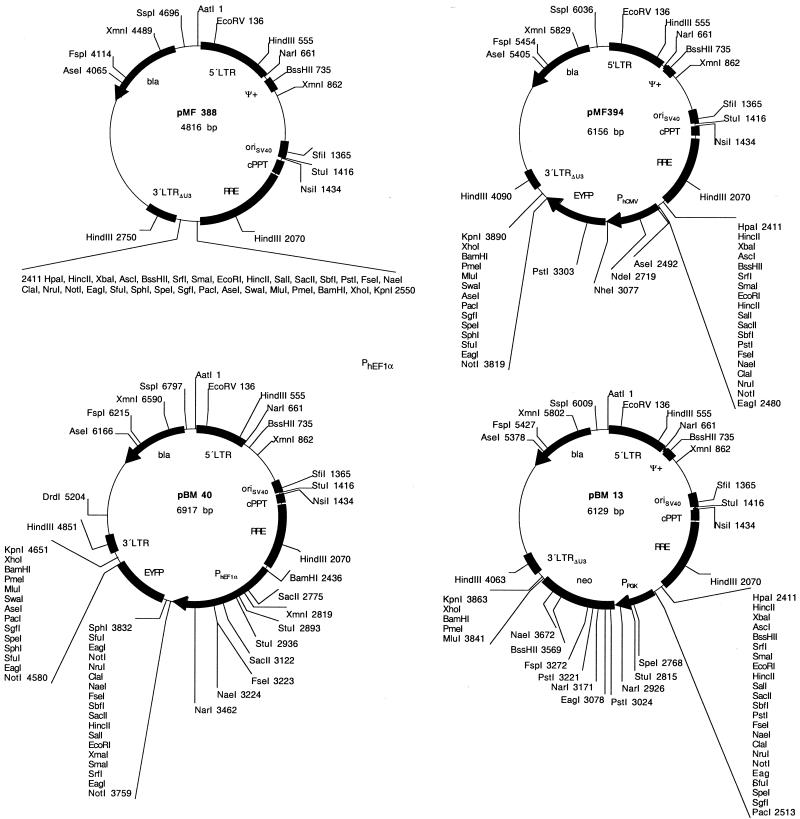
| pMF388 | The 5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3 was amplified from pMF356 by OMF199/OMF200 and cloned as AatII/NheI fragment to the compatible sites (AatII/XbaI) of the pTRIDENT1 backbone (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3) | This work |
| pMF394 | PhCMV-EYFP was excised from pMF351 using HpaI/KpnI and cloned into the corresponding sites (HpaI/KpnI) of pMF388 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-EYFP-3′LTRΔU3) | This work |
| pMF402 | PPT-3′LTRΔU3 was excised from pMF355 by MluI/XbaI and cloned into the corresponding sites (MluI/XbaI) of pMF360 | This work |
| pBM1 | The ECFP-IRESI-RFP cassette was excised from pWW85 by EcoRI/AscI and cloned into the corresponding sites of pLentiTRIDENT2 (EcoRI/AscI) 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-IRESI-RFP-IRESII-MCSIII-3′LTRΔU3 | This work |
| pBM6 | The PPGK-neo cassette was amplified from pMSCVneo using OBM1/OBM2 and cloned in sense orientation into pcDNA3.1/V5-His TOPO | This work |
| pMB7 | PEF1α was excised from pMF320 using EcoRV/XmaI and cloned into the compatible sites (HpaI/XmaI) of pMF388 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3) | This work |
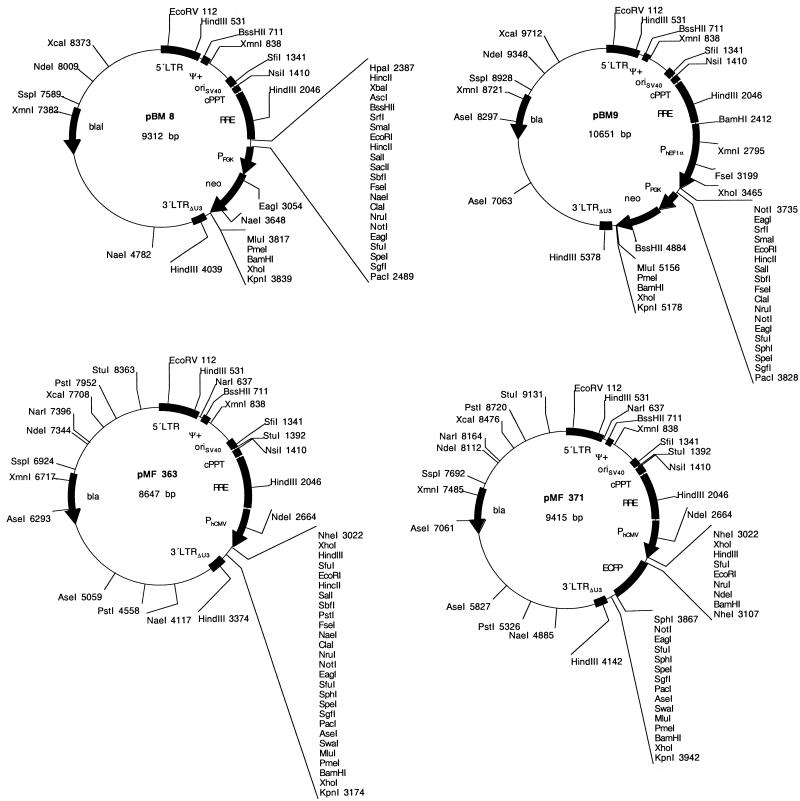
| pBM8 | The PPGK-neo cassette was excised from pBM6 by PacI/MluI and cloned into the corresponding sites (PacI/MluI) of pMF356 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3) | This work |
| pBM9 | The PPGK-neo cassette was excised from pBM6 by PacI/MluI and inserted into the corresponding sites (PacI/MluI) of pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3) | This work |
| pBM13 | The PPGK-neo cassette was excised from pBM6 using PacI/MluI and cloned into the corresponding sites (PacI/MluI) of pMF388 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3) | This work |
| pBM14 | The PPGK-neo cassette was excised from pBM6 using PacI/MluI and cloned into the corresponding sites (PacI/MluI) of pBM7 (5′LTR-ψ+-oriSV40-cPPT-RRE-PEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3) | This work |
| pBM40 | EYFP was excised from pLentiModule4 by NheI/MluI and cloned into the compatible sites (SpeI/MluI) of pBM7 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EYFP-3′LTRΔU3) | This work |
| pBM42 | mEPO was excised from pMF242 by EcoRI/EcoRV and cloned into the compatible sites (EcoRI/PmeI) of pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EPO-3′LTRΔU3) | This work |
| pBM43 | VEGF121 was excised from pWW265 by SalI/MluI and ligated into the corresponding sites (SalI/MluI) of pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-VEGF121-3′LTRΔU3) | This work |
| pBM44 | SEAP was excised from pSS173 by EcoRI/EcoRV and ligated into the compatible sites (EcoRI/PmeI) of pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-SEAP-3′LTRΔU3) | This work |
| pLentiTRIDENT1 | The MCSI-IRESI-MCSII-IRESII-MCSIII cassette was excised from pTRIDENT1 by EcoRI/BglII and cloned into the compatible sites (EcoRI/BamHI) of pMF363 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-IRESI-MCSII-IRESII-MCSIII-3′LTRΔU3) (pMF370) | This work |
| pLentiTRIDENT2 | The MCSI-IRES-MCSII-CITE*-MCSII cassette was excised from pTRIDENT3 by EcoRI/BglII and cloned into the compatible sites (EcoRI/BamHI) of pMF363 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-IRES-MCSII-CITE*-MCSIII-3′LTRΔU3) (pMF369) | This work |
| pLentiTFT1 | The triple-fluorescent expression cassette ECFP-IRESI-RFP-IRESII-EYFP was excised from pWW85 using EcoRI/MluI and cloned into the corresponding sites (EcoRI/MluI) of pLentiTRIDENT1 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-IRESI-RFP-IRESII-EYFP-3′LTRΔU3) (pMB2) | This work |
| pLentiTFT2 | EYFP was excised from pWW85 by SpeI/MluI and cloned to the corresponding sites (SpeI/MluI) of pBM1 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-IRES-RFP-CITE*-EYFP-3′LTRΔU3) (pMB3) | This work |
| pLentiModule1 | PPT-3′LTRΔU3 was excised from pMF355 by MluI/XbaI and cloned into the corresponding sites (MluI/XbaI) of pMF360x (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-MCS-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM4) | This work |
| pLentiModule2 | The PhCMV-EYFP cassette was excised from pMF351 by EcoRI/MluI and cloned into the corresponding sites (EcoRI/MluI) of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhCMV-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM5) | This work |
| pLentiModule3 | oriSV40 was excised from pMF378 by XbaI/NheI and cloned into the XbaI sites of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-MCS-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI-oriSV40) (pBM15) | This work |
| pLentiModule4 | oriSV40 was excised from pMF378 by XbaI/NheI and cloned into the XbaI sites of pLentiModule2 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhCMV-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI-oriSV40) (pBM12) | This work |
| pLentiModule5 | The PhEF1α-EYFP cassette was excised from pMF365 by BamHI and ligated in sense orientation into the corresponding site (BamHI) of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM45) | This work |
| pLentiModule6 | The PhEF1α-VEGF121 cassette was excised from pBM43 by BamHI and ligated in sense oritentation into the corresponding site (BamHI) of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-VEGF121-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM46) | This work |
| pLentiModule7 | The PhEF1α-EPO cassette was excised from pBM42 by BamHI and ligated in sense orientation into the corresponding site (BamHI) of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-EPO-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM47) | This work |
| pLentiModule8 | The PhEF1α-SEAP cassette was excised from pBM44 by BamHI and ligated in sense orientation into the corresponding site (BamHI) of pLentiModule1 (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-SEAP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI) (pBM48) | This work |
pMF356 was constructed by a two-step procedure as follows. (i) The PhCMV-EYFP cassette (PhCMV, promoter of the human cytomegalovirus; EYFP) was amplified from pEYFP-C1 (Clontech, Palo Alto, CA) using oligonucleotides OMF179: GATCGTTAACTCTAGAGGCGCGCCCGGGC GAATTCGTCGACCCGCGGCCTGCAGGCCGGCCATCtagttattaatagtaatc [annealing sequence in lower case, restriction sites HpaI/HincII/XbaI/AscI/SrfI/SmaI/EcoRI/SalI/SacII/SbfI/FseI/ClaI (GmATC sensitive) /NruI/NotI are underlined] and OMF180: GATC GGTACCTCGAGGATCCGTTTAAACGCGTATTTAAA TTAATTAGCGATCGCACTAGTGCATGCTTCGAAGC GGCCGCttacttgtacagctcgtc (annealing sequence in lower case, restriction sites KpnI/XhoI/BamHI/PmeI/MluI/SwaI/PacI/SgfI/SpeI/SphI/SfuI/NotI are underlined) and ligated as HpaI/KpnI fragment into pNL-EGFPΔU3 resulting in pMF351 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PhCMV-EYFP-MCSII-3′LTRΔU3). (ii) The PhCMV-EYFP cassette was eliminated from pMF351 by NotI restriction and religation, which resulted in pMF356 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3).
pMF356 was further digested with PacI/MluI and the PacI/MluI PPGK-neo (PPGK, promoter of the phosphoglycerate kinase; neo, G418 resistance gene) cassette of pBM6 was inserted (pBM8; 5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3). pBM6 was constructed by amplifying PPGK-neo from pMSCVneo (Clontech) using oligonucleotides OBM1: CGACTAGTTTAATTAAaattctaccgggtagggg (annealing sequence in lower case, SpeI/PacI sites are underlined) and OBM2: CGGGTACCACGCGTcctcagaagaac tcgtcaag (annealing sequence in lower case, KpnI/MluI sites are underlined) and cloned in sense orientation into pcDNA3.1/V5-His TOPO (Invitrogen, Carlsbad, CA).
pMF356 derivatives containing either PhEF1α (PhEF1α, promoter of the human elongation factor 1α; pMF359) or PhCMV (pMF363) promoters were generated as follows.
pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3). (i) PhEF1α was amplified from pEF4/MycHisB (Invitrogen) using oligonucleotides OMF164: GATC GGATCCtgcaaagatggataaagt (annealing sequence in lower case, BamHI site is underlined) and OMF165: GATC AAGCTTTTAATTAAGCGATCGCGCCCGGGCGCGGC CGCaactagccagcttgggtc (annealing sequence in lower case, HindIII/PacI/SgfI/SrfI/SmaI/NotI sites are underlined) and cloned in antisense orientation into pcDNA3.1/V5-His TOPO (Invitrogen) to result in pMF320. PhEF1α was excised from pMF320 by EcoRV/XmaI and cloned into the compatible sites (HpaI/XmaI) of pMF356 thereby resulting in pMF359 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3). (ii) EYFP was excised from pMF201 (33) by EcoRV/SpeI and cloned into the compatible sites (SmaI/SpeI) of pMF359 to result in pMF365 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EYFP-3′LTRΔU3). (iii) pMF359 was restricted using PacI/MluI and the PPGK-neo cassette excised from pBM6 by PacI/MluI was inserted (pBM9; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3). pMF359 derivatives include: (i) pBM42: pMF359 was restricted with EcoRI/PmeI and EPO (erythropoietin) excised from pMF242 (33) by EcoRI/EcoRV was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EPO-3′LTRΔU3). (ii) pBM43: pMF359 was restricted with SalI/MluI and VEGF121 (variant 121 of the vascu lar endothelial growth factor) excised from pWW265 (C.Weber and A.Zisch, unpublished data) by SalI/MluI was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-VEGF121-3′LTRΔU3). (iii) pBM44: pMF359 was restricted with EcoRI/PmeI and SEAP (human placental secreted alkaline phosphatase) excised from pSS173 (34) by EcoRI/EcoRV was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-SEAP-3′LTRΔU3).
pMF363 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCS-3′LTRΔU3): (i) PhCMV was excised from pLPCX (Clontech) with StuI/EcoRI and ligated to the compatible HpaI/EcoRI sites of pMF356 to result in pMF363 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCS-3′LTRΔU3). (ii) ECFP was excised from pMF201 (33) by EcoRI/NotI and ligated to the corresponding sites (EcoRI/NotI) of pMF363 thereby resulting in pMF371 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-3′LTRΔU3).
Tricistronic pLentiTRIDENT expression vectors. pLenti TRIDENT vectors contain a pTRIDENT-derived tricistronic expression cassette inserted in the MCS of pMF363. pLentiTRIDENT1 (pMF370; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-IRESI-MCSII-IRESII-MCSIII-3′LTRΔU3) was constructed by excising the MCSI-IRESI-MCSII-IRESII-MCSIII cassette from pTRIDENT1 [pMF125 (35)] by EcoRI/BglII and cloning it into the compatible sites (EcoRI/BamHI) of pMF363. pLentiTRIDENT1 was further restricted using EcoRI/MluI and the triple-fluorescent expression cassette (ECFP-IRESI-RFP-IRESII-EYFP) excised from pWW85 (36) by EcoRI/MluI was inserted [pLentiTFT1 (pBM2); 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-IRESI-RFP-IRESII-EYFP-3′LTRΔU3]. pLentiTRIDENT2 (pMF369; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-IRES-MCSII- CITE*-MCSIII-3′LTRΔU3) was constructed by excising the MCSI-IRES-MCSII-CITE*-MCSIII cassette from pTRIDENT3 [pMF122 (35)] by EcoRI/BglII and cloning it into the compatible sites (EcoRI/BamHI) of pMF363. pLentiTRIDENT2 was further restricted using EcoRI/AscI and the ECFP-IRESI-RFP cassette excised from pWW85 (36) by EcoRI/AscI was inserted to result in pBM1 (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-ECFP-IRES-RFP-CITE*-MCSIII-3′LTRΔU3). Subsequently, pBM1 was digested using SpeI/MluI and the EYFP cassette excised from pWW85 by SpeI/MluI was inserted [pLentiTFT2 (pBM3); 5′LTR-ψ+- oriSV40-cPPT-RRE-PhCMV-ECFP-IRES-RFP-CITE*-EYFP- 3′LTRΔU3].
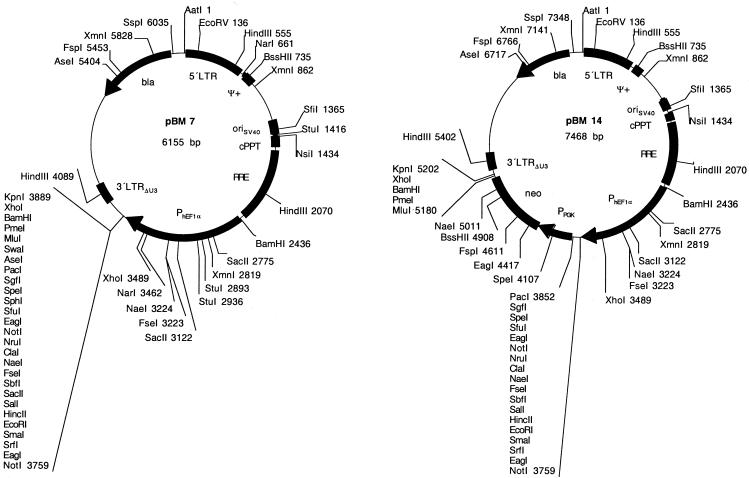
Highly compact lentiviral expression vectors. pNL-derived lentiviral expression vectors contain superfluous chromosomal sequences flanking the lentiviral expression unit (32). In order to eliminate these chromosomal sequences, the 5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3 cassette was amplified from pMF356 using oligonucleotides OMF199: GATCGACGTCacttacaccaggaaaggc (annealing sequence in lower case, AatII site is underlined) and OMF200: GATCGCTAGCacctctacctcctggggg (annealing sequence in lower case, NheI site is underlined) and cloned as AatII/NheI fragment to the compatible, AatII/XbaI-restricted pTRIDENT1 [pMF125 (35)] backbone thereby resulting in pMF388 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3). pMF388 derivatives include: (i) pMF394: pMF388 was restricted with HpaI/KpnI and the PhCMV-EYFP cassette excised from pMF351 by HpaI/KpnI was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-EYFP-3′LTRΔU3). (ii) pBM7: PhEF1α was excised from pMF320 by EcoRV/XmaI and cloned into the compatible HpaI/XmaI sites of pMF388 (5′LTR-ψ+-oriSV40-cPPT-RRE-PEF1α-MCS-3′LTRΔU3). (iii) pBM13: pMF388 was restricted with PacI/MluI and the PacI/MluI PPGK-neo cassette excised from pBM6 was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3). (iv) pBM14: pBM7 was restricted with PacI/MluI and the PacI/MluI PPGK-neo cassette excised from pBM6 was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3). (v) pBM40: pBM7 was restricted with SpeI/MluI and EYFP excised from pBM12 by NheI/MluI was inserted (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EYFP-3′LTRΔU3).
pLentiModule, a modular lentiviral expression vector (all sites indicated correspond to GenBank accession no. AF033819). pLentiModule consists of four minimal lentiviral modules [U3, R-U5-ψ+-SD, cPPT-RRE-SA, PPT-3′LTRΔU3 (ΔU3-R-U5)] containing unique restriction sites at their joints. These modules were independently amplified from pNL-EGFPΔU3 and cloned into pcDNA3.1/V5-His TOPO (Invitrogen). (i) pMF352: U3 (8631–9085) was amplified using oligonucle otides OMF183, GATCCTCGAGTCGCGActggaagggctaatttgg (annealing sequence lower case, XhoI/NruI sites are under lined); and OMF184, GATCGCCCGGGCCAGTACAGGC AAAAAGCAGCTGCTTATATGTAGCATCTGAG GGCTCGCCACTCCCCAGTCCCGCCCAGGCCACACCTCCCTGCAGGcctggaaagtccccagcg (annealing sequence lower case, SrfI and SbfI are underlined; this primer inserts a SbfI at position 9007 of U3) (XhoI/NruI-U31-SbfI-U32-SrfI). (ii) pMF376: R-U5-ψ+-SD (1–793) was amplified from pNL-EGFPΔU3 using oligonucleotides OMF185, GATCGCC CGGGCggtctctctggttagacc (annealing sequence lower case, SrfI site is underlined); and OMF193, GATCATT TAAATtttaaagttctaggtgat (annealing sequence lower case, SwaI site is underlined) (SrfI-R-U5-ψ+-SD-SwaI). pMF353: a shorter R-U5-ψ+-SD module (1–377) was amplified using oligos OMF185 and OMF186: GATCATTTAAAT tcattaatctaattctcc (annealing sequence lower case, SwaI site is underlined). (iii) pMF375: cPPT-RRE-SA [4330–4475 (cPPT), 7200–8024 (RRE)] was amplified using oligonucleotides OMF191, GATCATTTAAATatgcataaaagaaaaggg (annealing sequence lower case, SwaI site is underlined); and OMF192, CTAGGATCACGCGTTTAATTAAGCGATCG CACTAGTATCGATGGCGCGCCGGCCGGCCAGGCCT GCGGCCGCGAATTCGACGTCatccgttcactaatcgaa (annealing sequence lower case, MluI/PacI/SgfI/SpeI/ClaI/AscI/FseI/StuI/NotI/EcoRI/AatII are underlined) (SwaI-cPPT-RRE-SA-MluI/PacI/SgfI/SpeI/ClaI/AscI/FseI/StuI/NotI/EcoRI/AatII). (iv) pMF355: PPT-3′LTRΔU3 (ΔU3-R-U5) (8615–9181 and 1–181) was amplified using oligonucleotides OMF189, GATCACGCGTGGATCCAAAAGAAAAGGGGGGACT GGAAGGGCTAATTCACTCCCAAAGAAGACAAGATG TTTAAACGTTAACTGATCActgctttttgcctgtact [annealing sequence lower case, MluI/BamHI is underlined, PPT is shown in bold; PmeI/HpaI/BclI are underlined and placed between the EcoRV/PvuII (GAT … CTG, italics) ligation sites flanking the ΔU3 deletion (corresponding to 8666–9066) of the 3′LTR (25,32)]; and OMF190, GATCTCTAG AGTCGACGCTAGCCATATGctgctagagattttccaca (annealing sequence lower case, XbaI/SalI/NheI/NdeI are underlined) (MluI/BamHI-PPT-ΔU31-PmeI/HpaI/BclI-ΔU32-R-U5-NdeI/NheI/SalI/XbaI).
pLentiModule was assembled on a pTRIDENT1 backbone following a four-step procedure. (i) U3 was excised from pMF352 using XhoI/SrfI and ligated to the corresponding sites (XhoI/SrfI) of pTRIDENT1 to result in pMF357. (ii) R-U5-ψ+-SD was excised from pMF353 and pMF376 by SrfI/SwaI and ligated into the corresponding sites (SrfI/SwaI) of pMF357 to result in pMF358 and pMF358x. (iii) cPPT-RRE-SA was excised from pMF375 using SwaI/MluI and ligated into the corresponding sites (SwaI/MluI) of pMF358 and pMF358x to result in pMF360 and pMF360x. (iv) PPT-3′LTRΔU3 was excised from pMF355 by MluI/XbaI and ligated into the corresponding sites (MluI/XbaI) of pMF360 and pMF360x to result in pMF402 and pLentiModule1 (pBM4; XhoI/NruI- U31-StuI / SbfI-U32-SrfI-R-U5-ψ + -SD-SwaI-cPPT-RRE-SA- AatII/EcoRI/NotI/EagI/StuI/FseI/EagI/AscI/BssHII/ClaI/SpeI/ SgfI/PacI/MluI/BamHI-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/ SalI/XbaI). plentiModule1 derivatives include the following. (i) pLentiModule2: pLentiModule1 was cut with EcoRI/MluI and the PhCMV-EYFP cassette excised from pMF351 by EcoRI/MluI was inserted to result in pLentiModule2 (pBM5) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhCMV-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI). (ii) pLentiModule5: pLenti Module1 was cut with BamHI and the PhEF1α-EYFP-encoding BamHI cassette of pMF365 was inserted (pBM45) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI). (iii) pLentiModule6: pLentiModule1 was cut with BamHI and the PhEF1α-VEGF121-encoding BamHI cassette of pBM43 was inserted (pBM46) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-VEGF121-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI). (iv) pLentiModule7: pLentiModule1 was cut with BamHI and the PhEF1α-EPO-encoding BamHI cassette of pBM42 was inserted (pBM47) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-EPO-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI). (v) pLentiModule8: pLentiModule1 was cut with BamHI and the PhEF1α-SEAP-encoding BamHI cassette of pBM44 was inserted (pBM48) XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhEF1α-SEAP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI).
In order to place the oriSV40 on pLentiModule1, this plasmid was restricted with XbaI and the oriSV40 XbaI/NheI fragment excised from pMF378 was inserted to result in pLentiModule3 (pBM15) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-MCS-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI-oriSV40). pMF378 was constructed by amplifying oriSV40 from pNL-EGFPΔU3 using oligonucleotides OMF195: GATCTCTAGAtatgactccgcccatccc (annealing sequence lower case, XbaI site is underlined) and OMF196: GATCGCTAGCttgcaaaagcctaggcc (annealing sequence lower case, NheI sequence is underlined) and ligating it into pcDNA3.1/V5-His TOPO. Furthermore, pLentiModule2 was restricted with XbaI and the oriSV40 XbaI/NheI fragment excised from pMF378 was inserted to result in pLentiModule4 (pBM12) (XhoI/NruI-U31-StuI/SbfI-U32-SrfI-R-U5-ψ+-SD-SwaI-cPPT-RRE-SA-PhCMV-EYFP-ΔU31-PmeI/HpaI/BclI-ΔU32-NdeI/NheI/SalI/XbaI-oriSV40).
Lentivirus production and infection
For production of replication-incompetent SIN lentiviruses, a mixture containing 94 µl DMEM, 6 µl FUGENE (Roche Diagnostics AG, Rotkreuz, Switzerland), 25 µM chloroquine, 1 µg pLTR-G [encoding the pseudotyping envelope protein VSV-G of the vesicular stomatitis virus (17)], 1 µg of the helper construct pCD/NL-BH* (32) and 1 µl of the desired transgene-encoding lentiviral expression vector were transfected into human embryonic kidney cells (HEK293-T, kindly provided by Andreas Zisch). The medium was replaced after 12–15 h and virus particles were produced for another 48 h. Viral particles were collected from the HEK293-T supernatant by filtration through a 0.45 µm filter (Schleicher & Schuell GmbH, Dassel, Germany: FP 030/2) yielding typical titers of 2 × 107 c.f.u./ml when titrated on CHO-K1 cells. p24 assays were performed using a commercial kit (NEK-050, Perkin Elmer, Boston, MA) [pMF351, 104.8 ± 6.4 ng/ml p24; pLentiModule1, 109.9 ± 7.25 ng/ml p24 (triplicate mean values)]. 60 000 target cells per 6-well were infected with 200 µl viral supernatant. Hippocampal slice cultures were infected as described by Ehrengruber and co-workers (37).
RESULTS
Design of multi-purpose lentiviral expression vectors containing extensive multiple cloning sites
Most lentiviral expression vectors currently available from the research groups, which have pioneered lentiviral transduction technologies are lacking extensive MCS rendering the straightforward design of HIV-1-based expression vectors complicated (16). We have constructed a versatile lentiviral expression vector family derived from the HIV-1-based SIN pNL-EGFPΔU3 (J.Reiser, unpublished data; 38) which contains up to 29 unique sites for restriction endonucleases, many of which are rare-cutting 8 bp-recognizing enzymes. pNL-EGFPΔU3 has a classical lentiviral expression vector set-up consisting of 5′LTR, ψ+, splice donor (SD), origin of replication of the simian virus 40 (oriSV40), central polypurine tract (cPPT), RRE, splice acceptor (SA) and a 3′LTRΔU3. The PhCMV-EGFP expression cassette is inserted between the SA and the 3′LTRΔU3 using the unique HpaI and KpnI sites (5′LTR-ψ+-SD-oriSV40-cPPT-RRE-SA-HpaI-PhCMV-EGFP-KpnI-3′LTRΔU3). The oriSV40 is boosting the vector copy number in transiently transfected cells producing SV40 large T antigen, such as HEK293-T cells (17). A 400 bp EcoRV/PvuII deletion within the 3′LTR (3′LTRΔU3) inactivates the 5′LTR promoter upon reverse transcription and integration when the ΔU3 deletion is transferred to the 5′LTR in a self-sufficient manner, a process also referred to as SIN (25,26).
In order to extend the MCS of pNL-EGFPΔU3, a PCR-amplified PhCMV-EYFP cassette flanked by 26 unique restriction sites was cloned into the HpaI/KpnI sites of pNL-EGFPΔU3 and resulted in pMF351 (5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PhCMV-EYFP-MCSII-3′LTRΔU3; Fig. 1, Table 1). The PhCMV-EYFP was subsequently excised by a NotI deletion resulting in pMF356, a lentiviral expression vector containing 28 unique restriction sites (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3; Fig. 1, Table 1). As non-exhaustive examples for the construction kit-like lentiviral expression vector platform a variety of pMF356 derivatives have been designed which contain: (i) a PPGK-driven neomycin resistance gene (neo) (pBM8; 5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3), (ii) a strong constitutive human elongation factor 1α promoter (PhEF1α; pMF359; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3), (iii) a PhEF1α-driven EYFP cassette (pMF365; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCSI-EYFP-MCSII-3′LTRΔU3), (iv) a PhEF1α promoter plus a PPGK-neo cassette (pBM9; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3), (v) a human cytomegalovirus immediate early promoter (PhCMV; pMF363; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCS-3′LTRΔU3) or (vi) a PhCMV-ECFP cassette (pMF371; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-ECFP-MCSII-3′LTRΔU3) (see Fig. 1 and Table 1 for all plasmids).
Figure 1.
(Previous page and above) SIN lentivectors containing optimized MCS. Generic HIV-1-derived lentivectors containing the 5′LTR, the extended packaging signal (ψ+), the Simian virus 40 origin of replication (oriSV40), the cPPT, the RRE and the 3′LTR with deleted enhancer sequences (3′LTRΔU3). The MCS was placed between RRE and 3′LTRΔU3. Besides this basic lentivector configuration a variety of derivatives containing different mammalian/human promoters (PPGK, phosphoglycerate kinase promoter; PhEF1α, human elongation factor 1α promoter; PhCMV, human immediate early cytomegalovirus promoter) and reporter (EYFP and ECFP) or resistance genes (neo, G418 resistance gene) have been designed.
In order to validate this class of multi-purpose lentivectors, a variety of pMF359 derivatives were constructed which encode EPO (pBM42), the vascular endothelial growth factor (VEGF121, pBM43) or the SEAP (pBM44) under control of PhEF1α. pBM42-, pBM43- and pBM44-derived lentiviruses were able to drive high-level protein secretion following transduction of CHO-K1 (Table 2). Also, pMF351- and pMF365-based lentivectors displayed similar transduction efficiencies compared with their parental constructs of the pNL-EGFP series (38) (Fig. 2, Table 3).
Table 2. EPO, VEGF121 and SEAP production in CHO-K1 cells following lentiviral transduction.
| Lentivector | Production (ng/ml) | ||
|---|---|---|---|
| EPO | VEGF121 | SEAP | |
| pBM42 | 65.4 ± 9.2 | ||
| pBM43 | 20.0 ± 0.5 | ||
| pBM44 | 2.1 × 103 ± 80.6 | ||
| pLentiModule7 | 37.1 ± 5.5 | ||
| pLentiModule6 | 11.3 ± 1.1 | ||
| pLentiModule8 | 1.1 × 103 ± 23.6 | ||
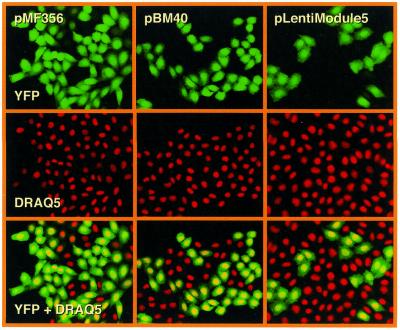
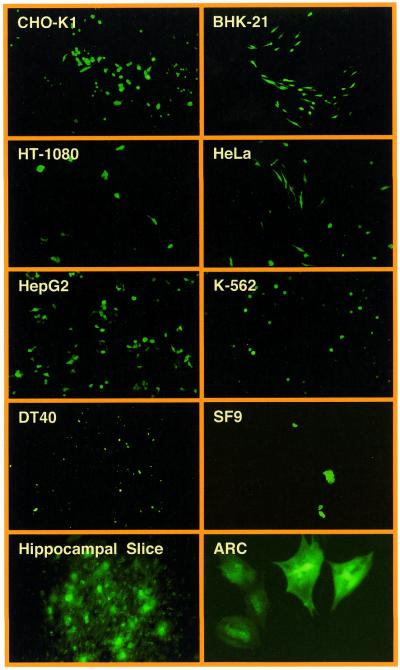
Figure 2.
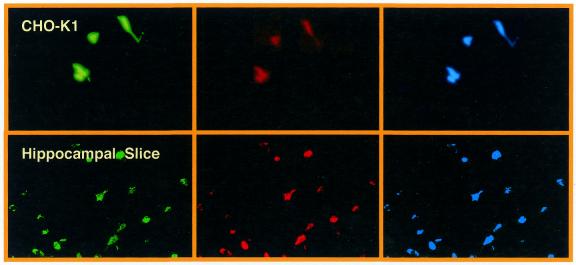
Confocal microscopy analysis of transduction efficiencies of key lentivectors. CHO-K1 were transduced with 2 × 106 EYFP-encoding pMF365-, pBM40- and pLentiModule-derived lentiviruses. Transduced cells were stained with the DNA-specific DRAQ5 dye and analyzed for YFP- and DRAQ5-mediated fluorescence by confocal microscopy (magnification 200×).
Table 3. Titer and p24 production of various lentivectors.
| Lentivector | Titer (c.f.u./ml)a | p24 (ng/ml) |
|---|---|---|
| pNL-EGFPb | 2 × 107 | 95.0 ± 5.3 |
| pNL-EGFPΔU3 | 4 × 107 | 165.3 ± 5.1 |
| pMF351 | 2.5 × 107 | 104.8 ± 6.4 |
| pMF365 | 2 × 107 | 97.3 ± 8.5 |
| pBM2 | ND | 158.7 ± 7.3 |
| pBM40 | 2 × 107 | 97.3 ± 9.6 |
| pBM42 | ND | 66.1 ± 8.3 |
| pBM43 | ND | 70.6 ± 7.8 |
| pBM44 | ND | 63.5 ± 8.0 |
| pLentiModule1 | 2.5 × 107 | 110 ± 7.2 |
| pLentiModule2 | 2.5 × 107 | 110 ± 7.3 |
| pLentiModule4 | 2 × 107 | 97.2 ± 12.3 |
| pLentiModule5 | 1.2 × 107 | 48.6 ± 5.4 |
| pLentiModule6 | ND | 64.9 ± 6.7 |
| pLentiModule7 | ND | 58.8 ± 8.6 |
| pLentiModule8 | ND | 72.3 ± 8.1 |
Although the basic lentiviral expression units contained in these expression vectors (pMF351, pMF356, pBM8, pMF359, pMF365, pBM9, pBM363, pMF371; Fig. 1) are only ∼3.5 kb in size, they are flanked by superfluous chromosomal regions that are remnants of the integration sites of the first HIV proviruses cloned from the human chromosome [molecular clone HXB2 (39)]. As these chromosomal sites are not packaged and transferred to the target cells, they are not limiting the cargo size of the lentiviral expression units. However, the entire expression vectors may reach the replicative limit when amplified in Escherichia coli.
Elimination of any chromosomal sequences was achieved by PCR-mediated amplification of the lentiviral expression units (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3). AatII and NheI sites engineered in the primer extensions enabled ligation to the pTRIDENT-derived minimal high-copy number vector backbone fragment generated by AatII/XbaI digestion (35,40). This cloning procedure resulted in pMF388, a 4.8 kb vector containing a lentiviral expression module with 29 unique restriction sites for convenient integration of desired transgenes (Fig. 3, Table 1; pMF388, 5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-3′LTRΔU3). Several ex emplary pMF388 derivatives have been designed which contain in addition to the basic lentiviral expression unit: (i) a PhCMV-driven EYFP cassette (pMF394; 5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PhCMV-EYFP-MCSII-3′LTRΔU3), (ii) a PhEF1α promoter (pBM7; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCS-3′LTRΔU3), (iii) a PhEF1α-driven EYFP cas sette (pBM40; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-EYFP- 3′LTRΔU3), (iv) a PPGK-driven neomycin resistance cassette (PPGK-neo; pBM13; 5′LTR-ψ+-oriSV40-cPPT-RRE-MCSI-PPGK-neo-MCSII-3′LTRΔU3) or (v) a PhEF1α promoter plus a PPGK-neo cassette (pBM14; 5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-MCSI-PPGK-neo-MCSII-3′LTRΔU3) (see Fig. 3 and Table 1 for all plasmids).
Figure 3.
(Previous page and above) Size-optimized SIN lentivectors. These lentivectors are derivatives of the ones shown in Figure 1 and are devoid of any chromosomal sequences flanking the lentiviral expression vectors.
Besides the great flexibility to accommodate almost any desired transgene, the extended MCS of the lentiviral expression vectors shown in Figures 1 and 3 are fully compatible with pQuattro, pTRIDENT, pRetroTRIDENT, pTWIN, pRetroTWIN and pDuoRex type constructs and enable one-step exchange of multicistronic and regulated expression units among these different expression vector families (35,40–42). In addition, these vectors facilitate straightforward swapping of cis-acting elements and transgene expression units between each other using: (i) remaining polylinker sites; (ii) unique restriction sites within the (5′) β-lactamase coding region (bla; AseI, FspI, XmnI, SspI, AatII); and/or (iii) corresponding cis-acting elements (EcoRV, NarI, BssHII, XmnI, SfiI, StuI, NsiI).
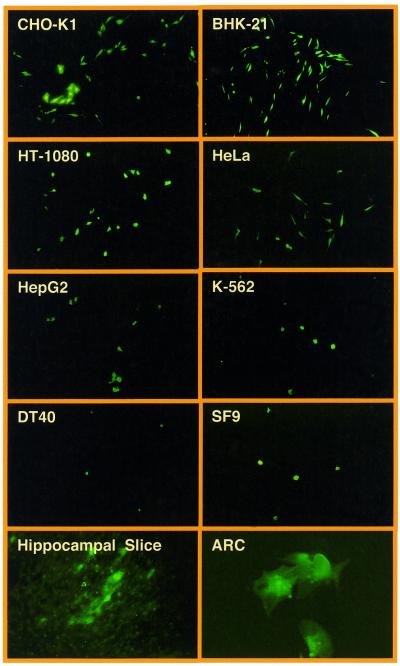
We have tested the construction kit-like lentiviral expression vector platform by infecting various insect (SF9), chicken (DT40), murine (BHK-21, CHO-K1) and human (HeLa, HT-1080, HepG2, K-562) cell lines as well as ARC and hippocampal slices using the pMF351-derived lentivirus (5′LTR-ψ+-oriSV40-cPPT-RRE-MCS-PhCMV-EYFP-MCSII-3′LTRΔU3). All cell lines transduced with pMF351-derived lentiviruses show high-level expression of the EYFP while EYFP expression is absent in mock-transduced control cultures. Even ARCs, which are known to be refractory to a variety of transfection/transduction technologies, were readily transduced by the pMF351-derived lentivirus providing an alternative to the recently described Sindbis-based transduction systems (28). Also, this lentivirus transduced glial cells in rat hippocampal slices (Fig. 4). In CHO-K1 cells the transduction efficiency of pBM4-derived lentiviruses was similar compared with the parental pNL-EGFP and pMF351 vector series (38) (see above and Fig. 2, Table 3).
Figure 4.
Transduction of optimized lentivectors into various insect, chicken, murine and human cell types as exemplified by VSV-G-pseudotyped pMF351-derived lentiviruses (Fig. 1). The gene encoding the EYFP was successfully transduced into Chinese hamster ovary cells (CHO-K1), baby hamster kidney cells (BHK-21), human fibrosarcoma cells (HT-1080), human cervial adenocarcinoma cells (HeLa), human hepatocellular carcinoma cells (HepG2), human chronic myelogenous leukemia cells (K-562), bursal chicken cells (DT40) as well as insect cells (SF9). pMF351-derived lentiviruses also transduced glial cells in hippocampal slice cultures (hippocampal slice) and primary ARC (magnification 100×, hippocampal slice 50×; ARC 400×).
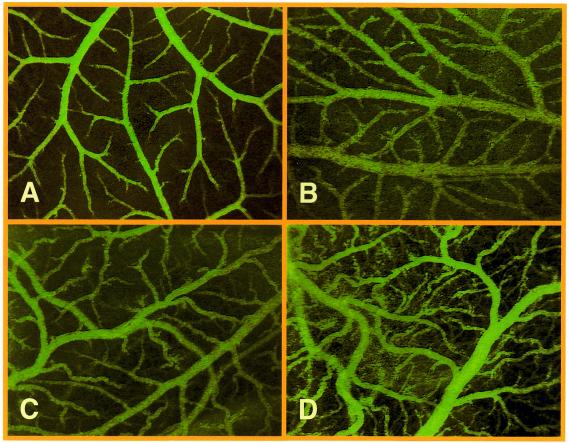
The biological potential of lentiviral transduction was further exemplified by expression of VEGF in chicken embryos. Infection of the chick embryos’ CAM with increasing concentrations of pBM43-derived lentiviruses (5′LTR-ψ+-oriSV40-cPPT-RRE-PhEF1α-VEGF121-3′LTRΔU3) resulted in a dose-dependent VEGF121-mediated local induction of new blood vessels demonstrated by increased vascular density, enhanced formation of numerous arterioles and venules as well as by a boost in vessel endpoint and branching density (Fig. 5). When overdosing VEGF121 (5 × 106 c.f.u./ml producing 35.5 ± 3.8 ng/ml VEGF121 in 48 h; not shown) or following i.v. application of pBM43-derived lentivirus (1.5 × 106 c.f.u./ml producing 10.6 ± 0.9 ng/ml VEGF121 in 48 h), the hierarchical, tree-like structure of the supplying vessels disappears and the multitude of arterioles and venules adopt an irregular tortuous shape associated with atypical delta- or brush-like vessel endpoints (Fig. 5D).
Figure 5.
In vivo examination of microvascular growth in the CAM of 11 day old chicken embryos 48 h after pBM43-based lentiviral VEGF121 transduction. Whereas the regular vessel pattern observed on the CAM remained intact in mock transductions (A), dose-dependent angiogenic response resulted in increased vascular density and atypical (brush- and delta-like) endpoint patterns following spotting of 0.5 × 106 c.f.u./ml (producing 3.4 ± 0.3 ng/ml VEFF121 in 48 h) (B), 3 × 106 c.f.u./ml (producing 21.2 ± 1.7 ng/ml VEGF121 in 48 h) or i.v. injection of 1.5 × 106 c.f.u./ml (producing 10.6 ± 0.9 ng/ml VEGF121 in 48 h) (C) (magnification 25×).
Construction of pLentiTRIDENT vectors for tricistronic expression of up to three transgenes
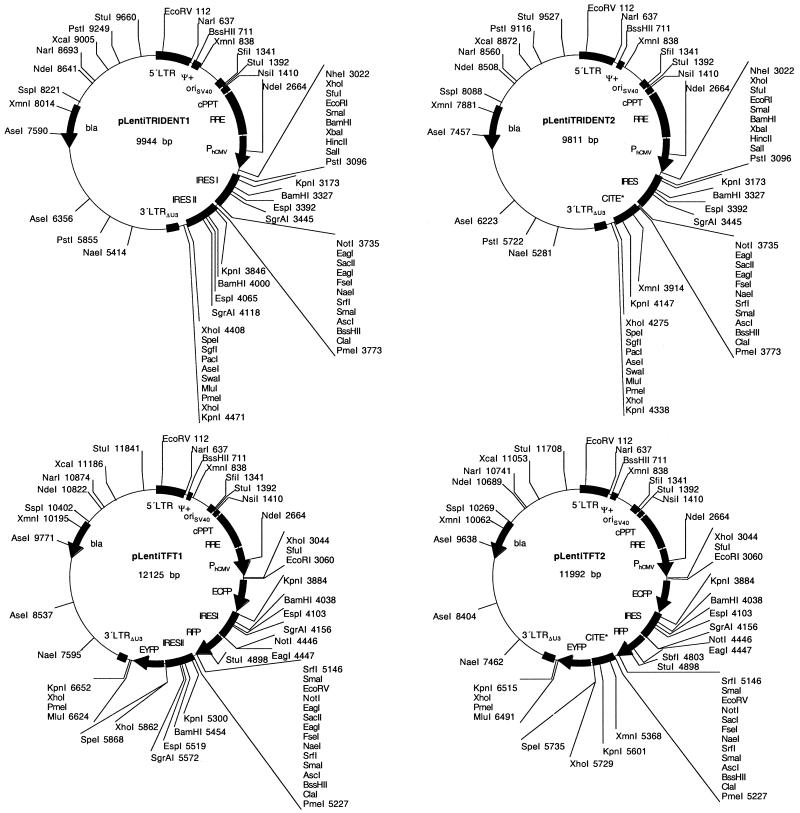
Multicistronic expression technologies for coordinated expression of several transgenes from a single artificial eukaryotic operon have generated unprecedented impact in rational reprogramming of mammalian production cell lines and anti-inflammation engineering (43,44). Particularly in a therapeutic setting when the therapeutic proteins consist of multiple subunits or multiple target/strategy approaches involving ribozymes, antisense RNA, transdominant pro teins and intracellular antibodies, multicistronic expression technologies are highly desirable (45). We have recently pioneered a multicistronic expression vector family, pTRIDENT, which enables tricistronic expression of up to three transgenes from a single constitutive or regulatable promoter. Whereas the first cistron is translated in a classical cap-dependent manner, translation initiation of the second and third cistrons rely on viral cap-independent internal ribosome entry sites of poliovirus (IRES) or encephalomyocarditis virus (CITE) which had been optimized for maximum translation enhancement (CITE*) (35,40,42).
In order to merge multicistronic expression and lentiviral transduction technology, we have designed the pLenti TRIDENT vector series, which contains a PhCMV-driven tricistronic eukaryotic operon within a pMF356-derived lentiviral expression cassette (Fig. 6, Table 1). pLenti TRIDENT1/(pLentiTRIDENT2) was constructed by excising the MCSI-IRESI-MCSII-IRESII/(CITE*)-MCSIII cassette of pTRIDENT1/(pTRIDENT3) by EcoRI/BglII and ligating it into the lentiviral transduction unit of pMF363 (EcoRI/BamHI) [5′LTR-ψ+-oriSV40-cPPT-RRE-PhCMV-MCSI-IRESI-MCSII-IRESII/(CITE*)-MCSIII-3′LTRΔU3]. The pLenti TRIDENT vectors were validated by inserting the genes encoding the (enhanced) cyan, red and yellow fluorescent proteins into the first, second and third cistrons, respectively, which resulted in the triple-fluorescent pLentiTRIDENT vectors (pLentiTFT1/(pLentiTFT2). CHO-K1 cells and glial cells in hippocampal slices transduced with pLentiTFT vectors showed simultaneous high-level expression of all three fluorescent marker proteins (Fig. 7).
Figure 6.
pLentiTRIDENT, tricistronic lentiviral expression vectors for simultaneous expression of up to three transgenes. Besides the basic lentiviral cis-acting elements (see Fig. 1) pLentiTRIDENT contain a tricistonic expression unit consisting of two IRES or encephalomyocarditis viral origin (CITE*) flanked by extensive MCS for integration of desired transgenes, for example the ones encoding the cyan (ECFP), red (RFP) and yellow (EYFP) fluorescent genes (pLentiTFT1 and pLentiTFT2).
Figure 7.
Transduction of Chinese hamster ovary (CHO-K1) cell and glial cells in rat hippocampal slice cultures using pLentiTRIDENT1-derived lentiviruses. Simultanous expression of the genes encoding the cyan, red and yellow fluorescent proteins could be observed (magnification 200×).
pLentiModule, an advanced highly modular lentiviral expression system
Previous lentiviral expression vectors have been designed for optimal integration of desired transgenes by engineering of extended MCS. However, lentiviral transduction units have been successfully modified in the past years to optimize specific cis-acting modules for: (i) Tat-independent production of lentiviruses by replacement of the 5′LTR enhancer region (19,23,26,46); (ii) optimization of packaging signal and Rev-responsive elements (47,48); and (iii) replacement of the enhancer sequences of the 3′LTR by LoxP sequences for conditional site-specific excision of transduced expression units (49) or by antibiotic-responsive operator modules for conditional proviral gene expression (46).
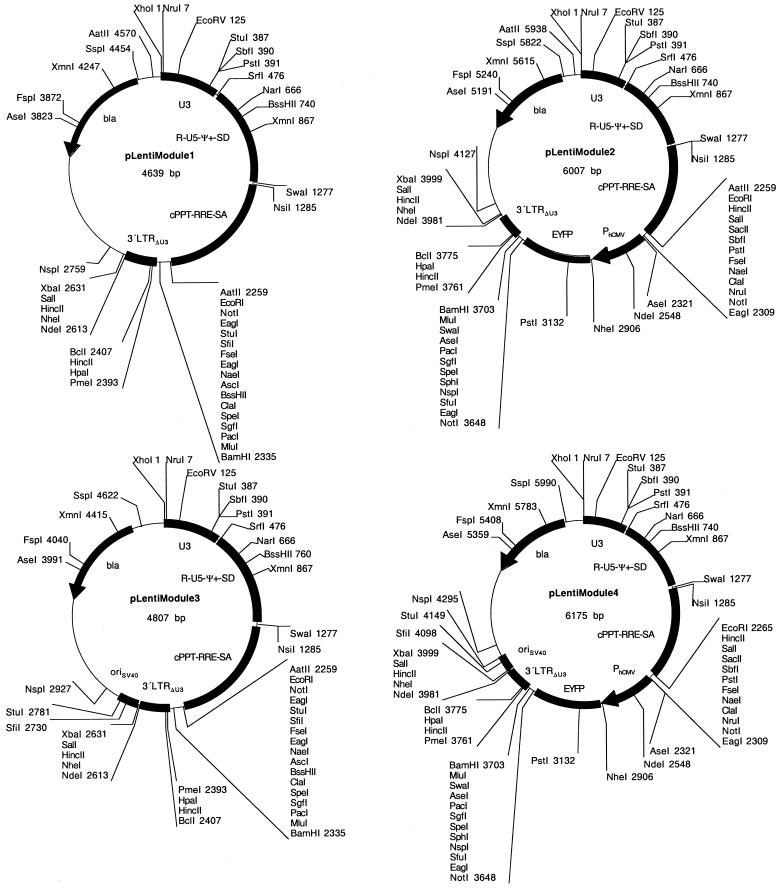
In order to combine minimal lentiviral transduction modules with extended polylinkers and the highest flexibility for advanced modification of the cis-acting elements we designed a novel modular lentivector (pLentiModule) which consists of four modules: (i) XhoI/NruI-U31-StuI/SbfI/PstI-U32-SrfI, (ii) SrfI-R-U5-ψ+-SD-SwaI/NsiI, (iii) SwaI/NsiI-cPPT-RRE-SA-MCSI and (iv) MCSI-3′LTRΔU3(1)-MCSII-3′LTRΔU3(2)-MCSIII. These modules are flanked by unique restriction sites and were sequentially assembled into a minimal pTRIDENT-derived prokaryotic vector backbone to result in pLentiModule1 [XhoI/NruI-U31-StuI/SbfI/PstI-U32-SrfI-R-U5-ψ+-SD-SwaI/NsiI-cPPT-RRE-SA-MCSI-3′LTRΔU3(1)-MCSII-3′LTRΔU3(2)-MCSIII; Fig. 8, Table 1]. In contrast to the previously described lentivectors pLentiModule is devoid of the oriSV40 since transfer of this simian virus element into target cells is incompatible with current therapeutic modalities.
Figure 8.
Modular SIN lentiviral expression vectors. In order to facilitate the design of advanced lentiviral expression vectors, all cis-acting lentiviral sequences are flanked by unique restriction sites in pLentiModule vectors (XhoI/NruI-U3-SrfI; SrfI-R-U5-ψ+-SD-SwaI/NsiI; SwaI/NsiI-cPPT-RRE-SA-MCS; MCS-3′LTRΔU3-NdeI/NheI/SalI/XbaI). Unique restriction sites have also been placed within U3 (StuI, SbfI, PstI) and at the site of the deletion within 3′LTRΔU3 (PmeI, HpaI, BclI). In order to enhance virus production an oriSV40 (origin of replication of the Simian virus 40) has been placed 3′ of the 3′LTR outside of the lentiviral expression unit. Test lentivectors pLentiModule2 and pLentiModule3 contain a PhCMV-driven EYFP expression cassette in the central MCS.
pLentiModule1 displays a construction kit-like highly modular structure and enables design of the following lentivector characteristics. (i) Transfer of the entire lentiviral expression cassette using the flanking unique sites XhoI/NruI and MCSIII, (ii) exchange of the 5′U3 region by XhoI/NruI and SrfI, (iii) switching of the 5′U3 enhancer (Tat-binding) sequences by XhoI/NruI and StuI/SbfI/PstI, (iv) substitution of the R-U5-ψ+-SD module using SrfI and SwaI/NsiI, (v) replacement of the cPPT-RRE-SA module by modified counterparts using the SwaI/NsiI and MCSI sites, (vi) cloning of desired transgenes, promoters and resistance cassettes into MCSI which is also compatible with the pLentiTRIDENT vectors and all of the previously described lentivectors (Figs 1, 3 and 6), (vii) swapping of the 3′LTRΔU3 element using sites contained in MCSI and MCSIII and (viii) integration of functional units (for example, LoxP, tissue-specific or regulatable operators, insulator sequences) into MCSII which are copied into the 5′LTR upon reverse transcription and integration. In addition, (ix) cassette swapping with all other vectors described here is further facilitated using any of the sites within the bla region (AseI/FspI/XmnI/SspI/AatII) and the R-U5-ψ+-SD module (NarI/BssHII/XmnI).
pLentiModule1 was tested by insertion of a PhCMV-EYFP cassette into its MCS (pLentiModule2; Fig. 6, Table 1) and transduction into insect (SF9), chicken (DT40), murine (BHK-21, CHO-K1) and human (HeLa, HT-1080, HepG2, K-562) cell lines as well as into ARCs and glial cells of hippocampal slice cultures (Fig. 9). Furthermore, pLentiModule1-derived lentivectors encoding EPO (pLentiModule7), VEGF121 (pLentiModule6) or SEAP (pLentiModule8) under control PhEF1α display high-level expression of these therapeutic/reporter proteins in CHO-K1 cells (Tables 2 and 3).
Figure 9.
Transduction of pLentiModule-derived lentiviruses into various insect, chicken, murine and human cell types as exemplified by a VSV-G-pseudotyped pLentiModule3-derived lentivirus (pLentiModule4). The gene coding for the EYFP was successfully transduced into Chinese hamster ovary cells (CHO-K1), baby hamster kindney cells (BHK-21), human fibrosarcoma cells (HT-1080), human cervial adenocarcinoma cells (HeLa), human hepatocellular carcinoma cells (HepG2), human chronic myelogenous leukemia cells (K-562), bursal chicken cells (DT40) as well as insect cells (SF9). pLentiModule4-derived lentiviruses also transduced glial cells in hippocampal slice cultures (hippocampal slice) and primary ARC (magnification 100×, hippocampal slice 50×; ARC 400×).
As pLentiModule1 and pLentiModule2 are devoid of oriSV40, the virus titer is significantly reduced to the ones generated using an intact oriSV40/large T antigen replication system (Table 3). We have therefore inserted a 168 bp fragment spanning the oriSV40 into MCSIII of pLentiModule1 and pLentiModule2, respectively, resulting in pLentiModule3 and pLentiModule4. As quantified from CHO-K1 transductions, oriSV40-containing pLentiModule derivatives achieve virus titers, which compares to aforementioned lentivectors (Fig. 2, Table 3).
DISCUSSION
Since their discovery in the early 1980s, HIV-1 lentiviruses were known to cause one of the most devastating epidemics in industrialized countries in recent history (50,51). However, the unique characteristic of HIV-1 to transduce and integrate into the human genome owing to a set of accessory genes managing nuclear import has brought this deadly virus into the limelight of the gene therapy arena as most genetic defects in humans reside in non-proliferating terminally differentiated cells. As the first development of replication-incompetent HIV-1-based vectors to transduce mitotically inert rat neurons, human skin fibroblasts and CD34+ cells (16,17), lentiviral transduction technologies have witnessed a progress to the top of currently most promising gene therapy initiatives.
Elimination of some 60% of its genome (including all accessory genes) and re-assembly in multiply attenuated split-genome packaging systems successfully addressed legitimate initial safety concerns. The most advanced lentiviral transduction systems include a third-generation packaging system consisting of a (i) gag-pol, (ii) rev and (iii) a vesicular stomatitis virus-derived envelope protein (VSV-G) as well as (iv) a SIN lentiviral expression vector. The split-genome strategy dramatically reduces the emergence of replication-competent recombinants and pseudotyping eliminates targeted infection of CD4+ T lymphocytes as well as extension of the cell tropism for therapeutic interventions (19). However, for successful infection of some target tissues including the liver some accessory proteins such as Vpr and Vif may be essential and should be considered for the packaging set-up (9). Typically, replication-incompetent lentiviral particles are produced by co-transfecting a helper cell line with all packaging and the lentiviral expression vector. However, with pharmaceutical production of therapeutic lentiviruses in mind a variety of different stable packaging cell lines have been designed which produced replication-incompetent transgenic lentiviruses in a tetracycline-responsive manner (20–23).
The lentiviral expression unit is the only genetic material transferred to the target cells and encodes the desired therapeutic transgene. This expression vector contains only cis-acting sequences and is devoid of any lentiviral coding sequences, which prevents significant humoral immune responses. Major improvements of lentiviral expression vectors include: (i) elimination of enhancer sequences from the 3′LTR which results in complete loss of the 5′LTR’s transcriptional capacity and the viral mobilization competence upon integration of the provirus in the target chromosome thereby increasing safety and minimizing interferences with internal and chromosomal promoters in vivo and (ii) replacement of 5′LTR promoters by heterologous promoters to achieve Tat-independent and/or tetracycline-responsive virus production (23,25,26,49). Many of these state-of-the-art lentivectors have been successfully used in pioneering gene therapy studies for the treatment of many prominent human diseases in animal models including Parkinson’s disease and metachromatic leukodystrophy (8,10).
Despite the dramatic progress in lentivector development in recent years lentiviral expression configurations available from the pioneering research groups have been rather specialized and inconvenient for straightforward design of multi-purpose lentivectors by a broad scientific community. We have therefore designed a highly modular construction kit-like expression platform based on the latest generation SIN lentivectors for mono- and multicistronic expression in a variety of cell lines and primary cells including, for the first time, ARCs. Also, to our knowledge, we report here the first lentiviral transduction into chicken embryos. This simple-to-use multi-purpose lentivector expression platform, which is compatible with any of the aforementioned packaging concepts is expected to greatly facilitate the use of lentiviral transduction technologies by a wider scientific community to accelerate the advancement in gene therapy and tissue engineering.
Acknowledgments
ACKNOWLEDGEMENTS
We thank H. M. Eppenberger for providing adult rat cardiomyocytes, J. Reiser for plasmid pNL-EGFPΔU3 as well as a set of helper vectors, L. Rietschin and A. Nussbaumer for preparation of the hippocampal slice cultures and B. Lennon, S. Schlatter and W. Weber for critical comments on the manuscript. This work was supported by the Novartis Research Foundation, the Roche Research Foundation and the Bundesamt für Bildung und Wissenschaft within the Framework V Programme of the European Commission. Work in the labs of M.U.E. and M.F. was supported by the Swiss National Science Foundation (grant nos 31-57125.99 and 631-065946, respectively).
REFERENCES
- 1.Crystal R.G. (1995) Transfer of genes to humans: early lessons and obstacles to success. Science, 270, 404–410. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa M. and Huang,L. (2001) Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum. Gene Ther., 12, 861–870. [DOI] [PubMed] [Google Scholar]
- 3.Verma I.M. and Somia,N. (1997) Gene therapy—promises, problems and prospects. Nature, 389, 239–242. [DOI] [PubMed] [Google Scholar]
- 4.Williams R.S. (1995) Human gene therapy—of tortoises and hares. Nature Med., 1, 1137–1138. [DOI] [PubMed] [Google Scholar]
- 5.Follenzi A., Ailles,L.E., Bakovic,S., Geuna,M. and Naldini,L. (2000) Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nature Genet., 1, 566–573. [DOI] [PubMed] [Google Scholar]
- 6.Turelli P., Doucas,V., Craig,E., Mangeat,B., Klages,N., Evans,R., Kalpana,G. and Trono,D. (2001) Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complex: interference with early steps of viral replication. Mol. Cell, 7, 1245–1254. [DOI] [PubMed] [Google Scholar]
- 7.Zennou V., Petit,C., Guetard,D., Nerhbass,U., Montagnier,L. and Charneau,P. (2000) HIV-1 genome nuclear import is mediated by a central DNA flap. Cell, 101, 173–185. [DOI] [PubMed] [Google Scholar]
- 8.Consiglio A., Quattrini,A., Martino,S., Bensadoun,J.C., Dolcetta,D., Trojani,A., Benaglia,G., Marchesini,S., Cestari,V., Oliverio,A., Bordignon,C. and Naldini,L. (2001) In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nature Med., 7, 310–316. [DOI] [PubMed] [Google Scholar]
- 9.Kafri T., Blömer,U., Peterson,A., Gage,F.H. and Verma,I.M. (1997) Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nature Genet., 17, 314–317. [DOI] [PubMed] [Google Scholar]
- 10.Kordower J.H., Emborg,M.E., Bloch,J., Ma,S.Y., Chu,Y., Leventhal,L., McBride,J., Chen,E.Y., Palfi,S., Roitberg,B.Z. et al. (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science, 290, 767–773. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi H., Takahashi,M., Gage,F.H. and Verma,I.M. (1997) Stable and efficient transfer into the retina using an HIV-based lentiviral vector. Proc. Natl Acad. Sci. USA, 94, 10319–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park F., Ohashi,K., Chiu,W., Naldini,L. and Kay,M.A. (2000) Efficient lentiviral transduction of liver requires cell cycling in vivo. Nature Genet., 24, 49–52. [DOI] [PubMed] [Google Scholar]
- 13.Vigna E. and Naldini,L. (2000) Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med., 2, 308–316. [DOI] [PubMed] [Google Scholar]
- 14.Zennou V., Serguera,C., Sarkis,C., Colin,P., Perret,E., Mallet,J. and Charneau,P. (2001) The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat. Biotechnol., 19, 446–450. [DOI] [PubMed] [Google Scholar]
- 15.Luciw P.A. (1996) Human immunodeficiency viruses and their replication. In Fields,B.N., Knipe,D.M., Howley,P.M., Chanock,R.M., Melnick,J.L., Monath,T.P., Roizman,B. and Straus,S.E. (eds), Fields Virology, 3rd Edn. Lippincott-Raven Publishers, Philadelphia, PA, pp. 1881–1975.
- 16.Naldini L., Blömer,U., Gallay,P., Ory,D., Mulligan,R., Gage,F.H., Verman,I.M. and Trono,D. (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 272, 263–267. [DOI] [PubMed] [Google Scholar]
- 17.Reiser J., Harnison,G., Kluepfel-Stahl,S., Brady,R.O., Karlsson,S. and Schubert,M. (1996) Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc. Natl Acad. Sci. USA, 93, 15266–15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zufferey R., Nagy,D., Mandel,R.J., Naldini,L. and Trono,L. (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol., 15, 871–874. [DOI] [PubMed] [Google Scholar]
- 19.Dull T., Zufferey,R., Kelly,R., Mandel,R.J., Nguyen,M., Trono,D. and Naldini,L. (1998) A third-generation lentiviral vector with a conditional packaging system. J. Virol., 72, 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farson D., Witt,R., McGuinness,R., Dull,T., Kelly,M., Song,J., Radeke,R., Bukovsky,A., Consiglio,A. and Naldini,L. (2001) A new-generation stable inducible packaging cell line for lentiviral vectors. Hum. Gene Ther., 12, 981–997. [DOI] [PubMed] [Google Scholar]
- 21.Kafri T., van Praag,H., Zoyang,L., Gage,F.H. and Verma,I.M. (1999) A packaging cell line for lentivirus vectors. J. Virol., 73, 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klages N., Zufferey,R. and Trono,D. (2000) A stable system for high-titer production of multiply attenuated lentiviral vectors. Mol. Ther., 2, 170–176. [DOI] [PubMed] [Google Scholar]
- 23.Xu K., Ma,H., McCown,T.J., Verma,I.M. and Kafri,T. (2001) Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol. Ther., 3, 97–104. [DOI] [PubMed] [Google Scholar]
- 24.Park F. and Kay,M.A. (2001) Modified HIV-1-based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol. Ther., 4, 164–173. [DOI] [PubMed] [Google Scholar]
- 25.Zufferey R., Dull,T., Mandel,R.J., Bukovsky,A., Quiroz,D., Naldini,L. and Trono,D. (1998) Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol., 72, 9873–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi H., Blomer,U., Takahashi,M., Gage,F.H. and Verma,I.M. (1998) Development of a self-inactivating lentivirus vector. J. Virol., 72, 8150–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezzubova O., Silbergleit,A., Yamaguchi-Iwai,Y., Takeda,S. and Buerstedde,J.M. (1997) Reduced X-ray resistance and homologous recombination frequencies in a RAD54–/– mutant of the chicken DT40 cell line. Cell, 89, 185–193. [DOI] [PubMed] [Google Scholar]
- 28.Dätwyler D.A., Eppenberger,H.M., Koller,D., Bailey,J.E. and Magyar,J.P. (1999) Efficient gene delivery into adult cardiomyocytes by recombinant Sindbis virus. J. Mol. Med., 77, 859–864. [DOI] [PubMed] [Google Scholar]
- 29.Gähwiler B.H. (1981) Organotypic monolayer cultures of nervous tissue. J. Neurosci. Methods, 4, 329–342. [DOI] [PubMed] [Google Scholar]
- 30.Djonov V., Schmid,M., Tschanz,S.A. and Burri,P.H. (2000) Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ. Res., 86, 286–292. [DOI] [PubMed] [Google Scholar]
- 31.Djonov V.G., Galli,A.B. and Burri,P.H. (2000) Intussusceptive aborization contributes to vascular tree formation in the chick chorio-allantoic membrane. Anat. Embryol., 202, 347–357. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki H., Schwartz,J.P., Tanaka,K., Brady,R.O. and Reiser,J. (1998) High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol., 72, 8873–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fussenegger M., Morris,R.P., Fux,C., Rimann,M., von Stockar,B., Thompson,C.J. and Bailey,J.E. (2000) Streptogramin-based gene regulation systems for mammalian cells. Nat. Biotechnol., 18, 1203–1208. [DOI] [PubMed] [Google Scholar]
- 34.Schlatter S., Rimann,M., Kelm,J. and Fussenegger M. (2002) SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus α-amylase. Gene, 282, 19–31. [DOI] [PubMed] [Google Scholar]
- 35.Fussenegger M., Mazur,X. and Bailey,J.E. (1998) pTRIDENT, a novel vector family for tricistronic gene expression in mammalian cells. Biotechnol. Bioeng., 57, 1–10. [DOI] [PubMed] [Google Scholar]
- 36.Weber W., Marty,R.R., Keller,B., Rimann,M., Kramer,B.P. and Fussenegger,M. (2002) Versatile macrolide-responsive mammalian expression vectors for multiregulated multigene metabolic engineering. Biotechnol. Bioeng., 80, 691–705. [DOI] [PubMed] [Google Scholar]
- 37.Ehrengruber M.U., Hennou,S., Büeler,H., Naim,H.Y., Déglon,N. and Lundstrom,K. (2001) Gene transfer into neurons from hyppocampal slices: comparison of recombinant semliki forest virus, adenovirus, adeno-associated virus, lentivirus and measles virus. Mol. Cell. Neurosci., 17, 855–871. [DOI] [PubMed] [Google Scholar]
- 38.Reiser J., Lai,Z., Zhang,X.Y. and Brady,R.O. (2000) Development of multigene and regulated lentivirus vectors. J. Virol., 74, 10589–10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratner L., Fisher,A., Jagodzinski,L.L., Mitsuya,H., Liou,R.-S., Gallo,R. and Wong-Staal,F. (1987) Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res. Hum. Retroviruses, 3, 57–69. [DOI] [PubMed] [Google Scholar]
- 40.Moser S., Schlatter,S., Fux,C., Rimann,M., Bailey,J.E. and Fussenegger,M. (2000) An update of pTRIDENT multicistronic expression vectors: pTRIDENTs containing novel streptogramin-responsive promoters. Biotechnol. Prog., 16, 724–735. [DOI] [PubMed] [Google Scholar]
- 41.Fussenegger M., Moser,S. and Bailey,J.E. (1998) pQuattro vectors allow one-step transfection and auto-selection of quattrocistronic artificial mammalian operons. Cytotechnology, 28, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moser S., Rimann,M., Fux,C., Schlatter,S., Bailey,J.E. and Fussenegger,M. (2001) Dual-regulated expression technology: a new era in adjustment of heterologous gene expression in mammalian cells. J. Gene Med., 3, 529–549. [DOI] [PubMed] [Google Scholar]
- 43.Fussenegger M., Schlatter,S., Daetwyler,D., Mazur,X. and Bailey,J.E. (1998) Higher productivity of growth-arrested Chinese hamster ovary (CHO) cells expressing the cyclin-dependent kinase inhibitor p27. Nat. Biotechnol., 16, 468–472. [DOI] [PubMed] [Google Scholar]
- 44.Prati E.G.P., Matasci,M., Suter,T.B., Dinter,A., Sburlati,A.R. and Bailey,J.E. (2000) Engineering of coordinated up- and down-regulation of two glycosyltransferases of the O-glycosylation pathway in Chinese hamster ovary (CHO) cells. Biotechnol. Bioeng., 68, 239–244. [PubMed] [Google Scholar]
- 45.Dropulic B. and Jeang,K.T. (1994) Gene therapy for human immunodeficiency virus infection: genetic antiviral strategies and targets for intervention. Hum. Gene Ther., 5, 927–939. [DOI] [PubMed] [Google Scholar]
- 46.Marzio G., Verhoef,K., Vink,M. and Berkhout,B. (2001) In vitro evolution of a highly replicating, doxycycline-dependent HIV for applications in vaccine studies. Proc. Natl Acad. Sci. USA, 98, 6342–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lever A., Gottlinger,H., Haseltine,W. and Sodroski,J. (1989) Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J. Virol., 63, 4085–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam Y.S., Petrovic,A., Jeong,K.S. and Venkatesan,S. (2001) Exchange of the basic domain of human immunodeficiency virus type 1 Rev for a polyarginine stretch expands the RNA binding specificity and a minimal arginine cluster is required for optimal RRE RNA binding affinity, nuclear accumulation and trans-activation. J. Virol., 75, 2957–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmon P., Oberholzer,J., Occhiodoro,T., Morel,P., Lou,J. and Trono,D. (2000) Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol. Ther., 4, 404–414. [DOI] [PubMed] [Google Scholar]
- 50.Barre-Sinoussi F., Chermann,J.C., Rey,F., Nugeyre,M.T., Chamaret,S., Gruest,J., Dauguet,C., Axler-Blin,C., Vezinet-Brun,F., Rouzioux,C., Rozenbaum,W. and Montagnier,L. (1993) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 220, 868–871. [DOI] [PubMed] [Google Scholar]
- 51.Gallo R.C. and Montagnier,L. (1987) The chronology of AIDS research. Nature, 326, 435–436. [DOI] [PubMed] [Google Scholar]