Abstract
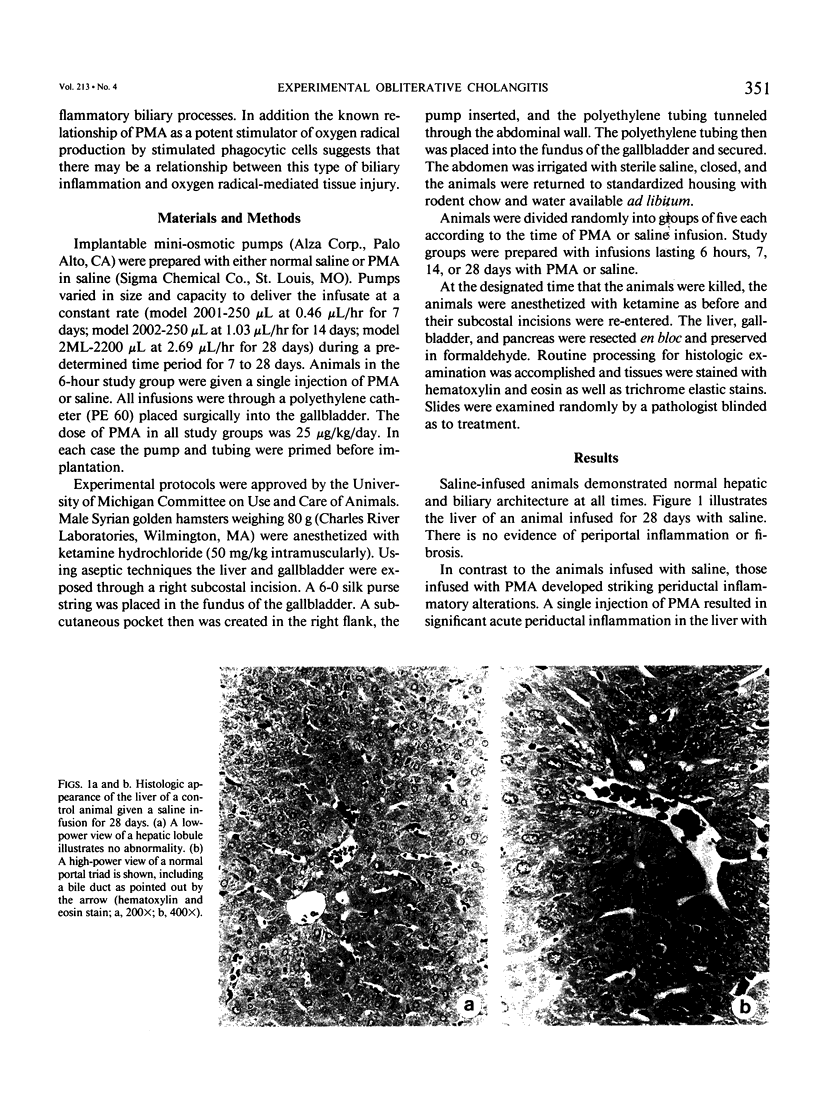
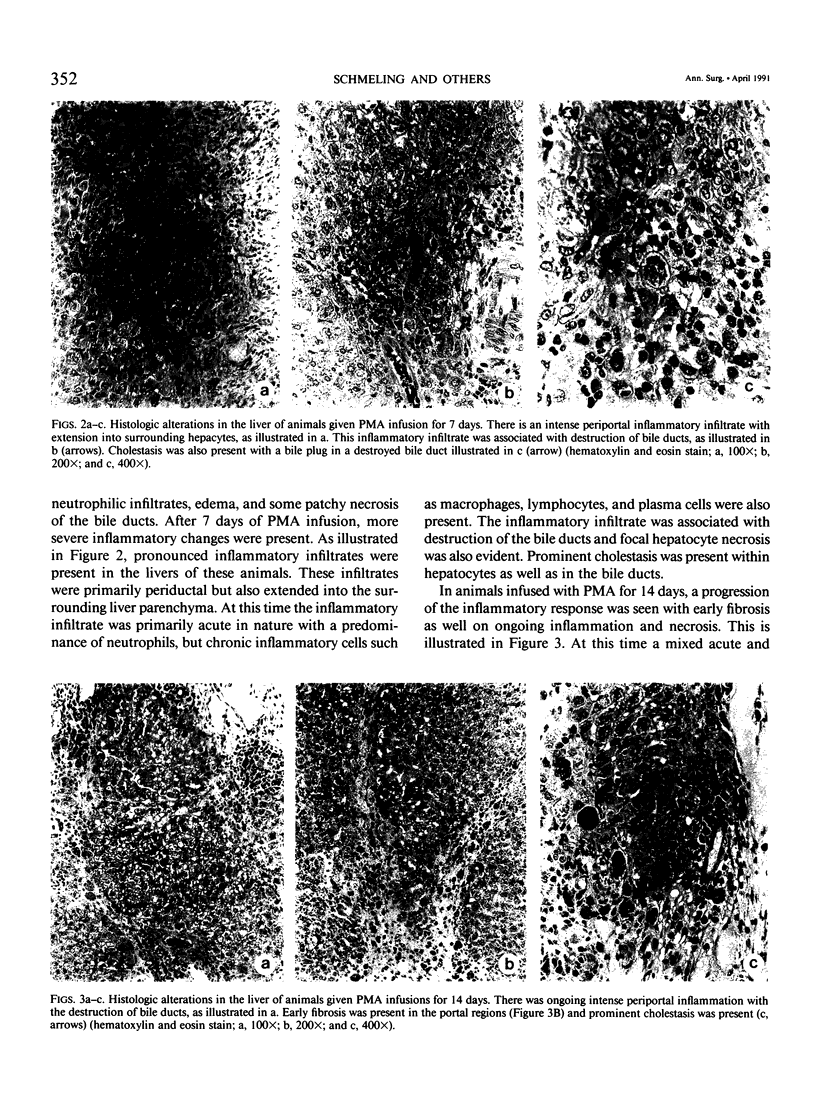
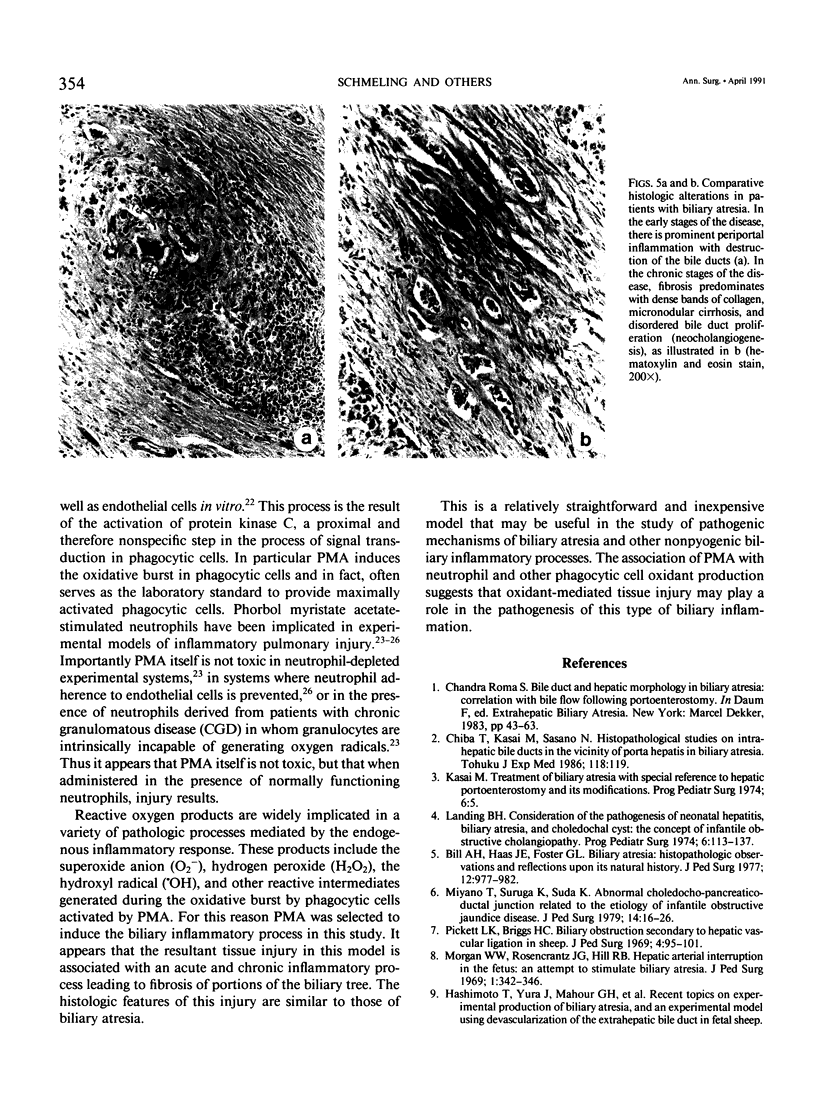
Noninfectious obliterative cholangitis results from biliary tract inflammation in clinical conditions such as biliary atresia and sclerosing cholangitis. The purpose of this study was to develop an animal model of noninfectious biliary tract inflammation and fibrosis. An implantable osmotic pump was connected to a catheter placed into the gallbladder of hamsters. Phorbol myristate acetate (PMA) was infused into the biliary tract for periods of 6 hours to 28 days. After 7 days the animals developed neutrophil infiltration, cellular necrosis, and edema of the biliary ducts. After 14 days, the animals demonstrated intrahepatic cholestasis with bile duct fibrosis and acute and chronic inflammatory cell infiltration. By 28 days pronounced portal fibrosis was present, some of which created an early bridging cirrhosis pattern. In addition there was evidence of neocholangiogenesis. We conclude that long-term PMA infusion into the biliary tract generates an inflammatory response characterized by obliterative cholangitis and fibrosis, sharing many of the histologic features of human biliary atresia. This model may provide a relatively simple technique for investigating the process of nonpyogenic biliary tract inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill A. H., Haas J. E., Foster G. L. Biliary Atresia: histopathologic observations and reflections upon its natural history. J Pediatr Surg. 1977 Dec;12(6):977–982. doi: 10.1016/0022-3468(77)90609-1. [DOI] [PubMed] [Google Scholar]
- DANKS D., BODIAN M. A GENETIC STUDY OF NEONATAL OBSTRUCTIVE JAUNDICE. Arch Dis Child. 1963 Aug;38:378–390. doi: 10.1136/adc.38.200.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer E. L., Snapper J. R. Role of circulating granulocytes in sheep lung injury produced by phorbol myristate acetate. J Appl Physiol (1985) 1986 Feb;60(2):576–589. doi: 10.1152/jappl.1986.60.2.576. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Caty M. G., Johnson K. J., Ward P. A. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989 Dec;210(6):740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult J. R., Nourshargh S. Phorbol myristate acetate enhances human polymorphonuclear neutrophil release of granular enzymes but inhibits chemokinesis. Br J Pharmacol. 1985 Nov;86(3):533–537. doi: 10.1111/j.1476-5381.1985.tb08928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail G., Morganroth M. L., Todd R. F., 3rd, Boxer L. A. Prevention of pulmonary injury in isolated perfused rat lungs by activated human neutrophils preincubated with anti-Mo1 monoclonal antibody. Blood. 1987 Apr;69(4):1167–1174. [PubMed] [Google Scholar]
- Kasai M. Treatment of biliary atresia with special reference to hepatic porto-enterostomy and its modifications. Prog Pediatr Surg. 1974;6:5–52. [PubMed] [Google Scholar]
- Landing B. H. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst--the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1974;6:113–139. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- Miyano T., Suruga K., Suda K. Abnormal choledocho-pancreatico ductal junction related to the etiology of infantile obstructive jaundice diseases. J Pediatr Surg. 1979 Feb;14(1):16–26. doi: 10.1016/s0022-3468(79)80570-9. [DOI] [PubMed] [Google Scholar]
- Morecki R., Glaser J. H., Cho S., Balistreri W. F., Horwitz M. S. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1982 Aug 19;307(8):481–484. doi: 10.1056/NEJM198208193070806. [DOI] [PubMed] [Google Scholar]
- Morgan W. W., Jr, Rosenkrantz J. C., Hill R. B., Jr Hepatic arterial interruption in the fetus--an attempt to simulate biliary atresia. J Pediatr Surg. 1966 Aug;1(4):342–346. doi: 10.1016/0022-3468(66)90336-8. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Cousart S., Lineberger A. S., Bond E., Bass D. A., DeChatelet L. R., Leake E. S., McCall C. E. Phorbol myristate acetate: in vivo effects upon neutrophils, platelets, and lung. Am J Pathol. 1980 Oct;101(1):79–92. [PMC free article] [PubMed] [Google Scholar]
- Pickett L. K., Briggs H. C. Biliary obstruction secondary to hepatic vascular ligation in fetal sheep. J Pediatr Surg. 1969 Feb;4(1):95–101. doi: 10.1016/0022-3468(69)90188-2. [DOI] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Schmeling D. J., Caty M. G., Oldham K. T., Guice K. S., Hinshaw D. B. Evidence for neutrophil-related acute lung injury after intestinal ischemia-reperfusion. Surgery. 1989 Aug;106(2):195–202. [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- Shim W. K., Kasai M., Spence M. A. Racial influence on the incidence of biliary atresia. Prog Pediatr Surg. 1974;6:53–62. [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K., Chew C. Slow exponential decay of rate of superoxide production in phorbol ester-activated human neutrophils. Inflammation. 1985 Dec;9(4):407–417. doi: 10.1007/BF00916340. [DOI] [PubMed] [Google Scholar]







