Abstract
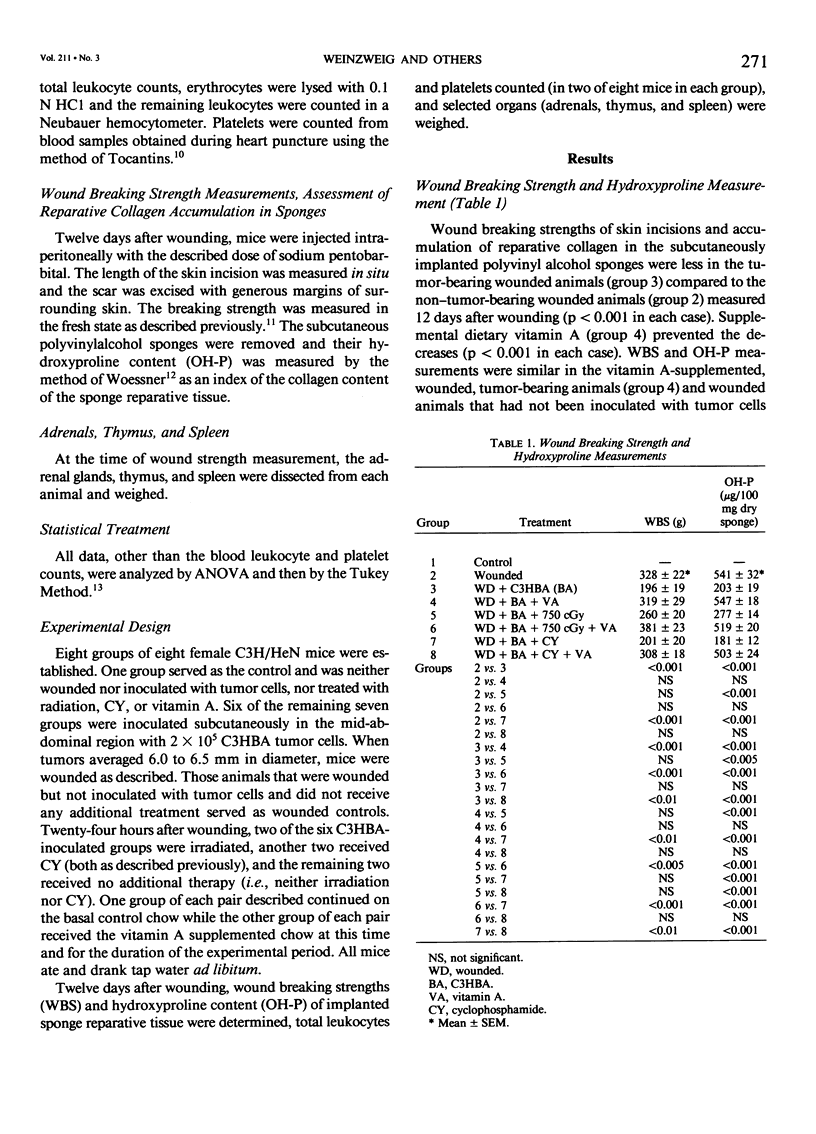
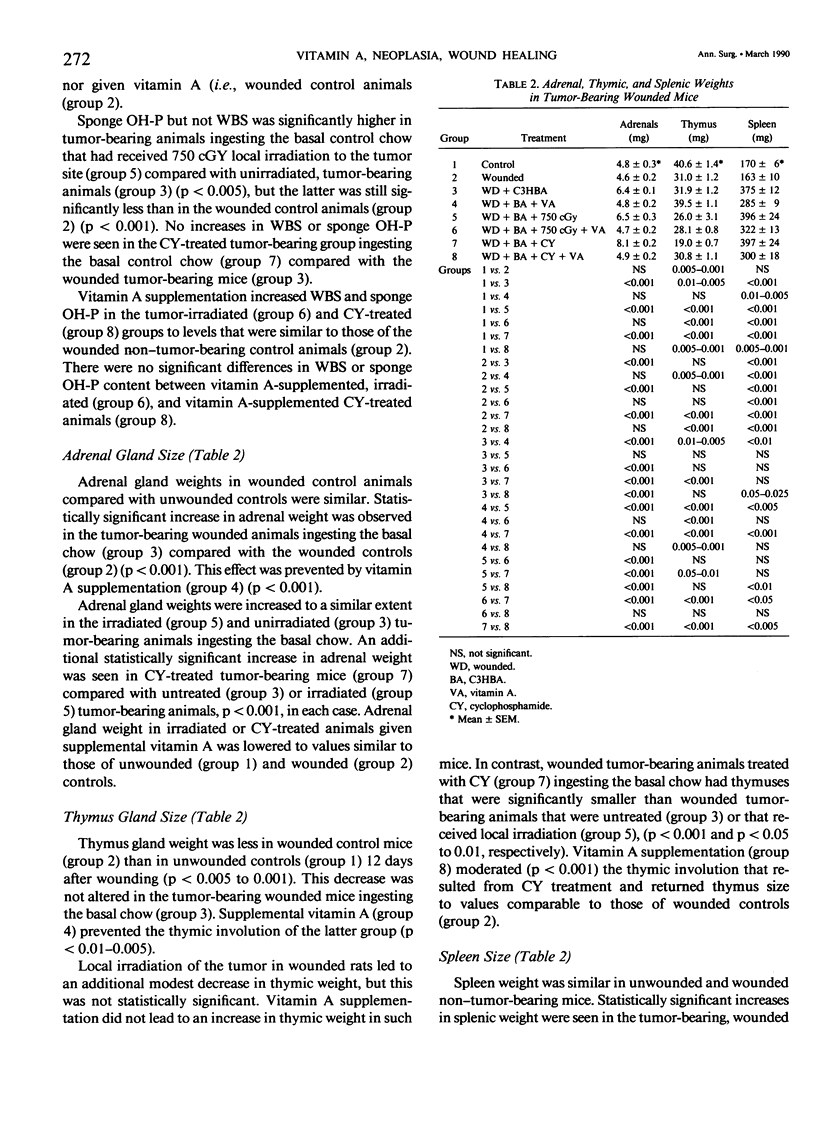
To test our hypothesis that supplemental vitamin A would mitigate the impaired healing that occurs in tumor-bearing animals, six groups of C3H mice, eight per group, eating a standard commercial mouse chow ad libitum that supports normal growth, reproduction, and longevity were innoculated with 200,000 C3HBA cells. When tumors measured approximately 6 mm in diameter, the mice were anesthesized and wounded (dorsal skin incisions and subcutaneous polyvinyl alcohol sponges). Twenty-four hours later, two groups (one continued on the chow and the other started on the chow supplemented with 150,000 IU vitamin A/kg chow) underwent local tumor irradiation; two groups, one ingesting the chow, the other the vitamin A supplemented chow, were started on cyclophosphamide therapy; two groups, one ingesting the chow, the other the vitamin A supplemented chow, received neither local tumor irradiation nor cyclophosphamide therapy. An additional two groups ingesting the chow, one group neither innoculated with tumor nor wounded, the other wounded by not innoculated, served as controls. Wound breaking strength and sponge reparative collagen accumulation (assessed by hydroxyproline proline measurement) were used as indicators of wound healing. The mice were killed 12 days after wounding. Tumor presence decreased wound breaking strength and sponge hydroxyproline content; these effects were largely negated by supplemental vitamin A. Local tumor irradiation diminished the adverse effect of tumor on sponge reparative collagen content but to a lesser extent than the supplemental vitamin A. Supplemental vitamin A added to the irradiation effect on healing but irradiation did not add to the vitamin A effect. Cyclophosphamide, a systemic radiomimetic anti-tumor agent, did not alter the impaired wound healing of the tumor-bearing mice. Supplemental vitamin A mitigated the impaired wound healing in the cyclophosphamide-treated tumor-bearing mice. Supplemental vitamin A also moderated the effects of wounding, tumor, and tumor therapies (local irradiation and cyclophosphamide) on the increase in adrenal size, leukopenia, thrombocytopenia, and thymic involution (except the last was not moderated in the cyclophosphamide-treated tumor-bearing rats). The splenic enlargement in the untreated tumor-bearing wounded rats and in those treated with cyclophosphamide was lessened by supplemental vitamin A. We hypothesize that these anti-stress effects of vitamin A underlie, in part, its action in mitigating the impaired wound healing of tumor-bearing mice, including those treated by local irradiation or cyclophosphamide. These findings have implications for the care of patients with malignant tumors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbul A., Rettura G., Levenson S. M., Seifter E. Arginine: a thymotropic and wound-healing promoting agent. Surg Forum. 1977;28:101–103. [PubMed] [Google Scholar]
- Barbul A., Sisto D., Rettura G., Levenson S. M., Seifter E., Efron G. Thymic inhibition of wound healing: abrogation by adult thymectomy. J Surg Res. 1982 Apr;32(4):338–342. doi: 10.1016/0022-4804(82)90110-x. [DOI] [PubMed] [Google Scholar]
- Barbul A., Thysen B., Rettura G., Levenson S. M., Seifter E. White cell involvement in the inflammatory, wound healing, and immune actions of vitamin A. JPEN J Parenter Enteral Nutr. 1978 May;2(2):129–138. doi: 10.1177/014860717800200208. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., McMillan R. M., Dayer J. M., Harris E. D., Jr Inhibition by retinoic acid of collagenase production in rheumatoid synovial cells. N Engl J Med. 1980 Aug 21;303(8):432–436. doi: 10.1056/NEJM198008213030805. [DOI] [PubMed] [Google Scholar]
- Cohen S. C., Gabelnick H. L., Johnson R. K., Goldin A. Effects of antineoplastic agents on wound healing in mice. Surgery. 1975 Aug;78(2):238–244. [PubMed] [Google Scholar]
- Demetriou A. A., Levenson S. M., Rettura G., Seifter E. Vitamin A and retinoic acid: induced fibroblast differentiation in vitro. Surgery. 1985 Nov;98(5):931–934. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux D. F., Kent H., Brennan M. F. Time dependent effects of adriamycin and x-ray therapy on wound healing in the rat. Cancer. 1980 Jun 1;45(11):2805–2810. doi: 10.1002/1097-0142(19800601)45:11<2805::aid-cncr2820451115>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Thistlethwaite P. A., Thibault L. E., Brennan M. F. Effects of tumor bearing and protein depletion on wound breaking strength in the rat. J Surg Res. 1979 Oct;27(4):233–238. doi: 10.1016/0022-4804(79)90135-5. [DOI] [PubMed] [Google Scholar]
- Devereux D. F., Triche T. J., Webber B. L., Thibault L. E., Brennan M. F. A study of adriamycin-reduced wound breaking strenght in rats. An evaluation by light and electron microscopy, induction of collagen maturation, and hydroxyproline content. Cancer. 1980 Jun 1;45(11):2811–2815. doi: 10.1002/1097-0142(19800601)45:11<2811::aid-cncr2820451116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Hunt T. K. Effects of cortisone and vitamin A on wound healing. Ann Surg. 1968 Mar;167(3):324–328. doi: 10.1097/00000658-196803000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B., Costantino J., Redmond C., Poisson R., Bowman D., Couture J., Dimitrov N. V., Wolmark N., Wickerham D. L., Fisher E. R. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Dimitrov N. V., Bowman D., Legault-Poisson S., Wickerham D. L., Wolmark N., Fisher E. R., Margolese R., Sutherland C. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989 Feb 23;320(8):473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- LEVENSON S. M., GEEVER E. F., CROWLEY L. V., OATES J. F., 3rd, BERARD C. W., ROSEN H. THE HEALING OF RAT SKIN WOUNDS. Ann Surg. 1965 Feb;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson S. M., Gruber C. A., Rettura G., Gruber D. K., Demetriou A. A., Seifter E. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984 Oct;200(4):494–512. doi: 10.1097/00000658-198410000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Edwards A. J., Hunt R., Palmer L., Medawar P. B. T-cell-mediated enhancement of host-versus-graft reactivity in mice fed a diet enriched in vitamin A acetate. Nature. 1983 Mar 24;302(5906):338–340. doi: 10.1038/302338a0. [DOI] [PubMed] [Google Scholar]
- Mansour E. G., Gray R., Shatila A. H., Osborne C. K., Tormey D. C., Gilchrist K. W., Cooper M. R., Falkson G. Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer. An intergroup study. N Engl J Med. 1989 Feb 23;320(8):485–490. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettura G., Stratford F., Levenson S. M., Seifter E. Prophylactic and therapeutic actions of supplemental beta-carotene in mice inoculated with C3HBA adenocarcinoma cells: lack of therapeutic action of supplemental ascorbic acid. J Natl Cancer Inst. 1982 Jul;69(1):73–77. [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Seifter E., Crowley L. V., Rettura G., Nakao K., Gruber C., Kan D., Levenson S. M. Influence of vitamin A on wound healing in rats with femoral fracture. Ann Surg. 1975 Jun;181(6):836–841. doi: 10.1097/00000658-197506000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifter E., Mendecki J., Holtzman S., Kanofsky J. D., Friedenthal E., Davis L., Weinzweig J. Role of vitamin A and beta carotene in radiation protection: relation to antioxidant properties. Pharmacol Ther. 1988;39(1-3):357–365. doi: 10.1016/0163-7258(88)90083-6. [DOI] [PubMed] [Google Scholar]
- Seifter E., Rettura G., Barbul A., Levenson S. M. Arginine: an essential amino acid for injured rats. Surgery. 1978 Aug;84(2):224–230. [PubMed] [Google Scholar]
- Seifter E., Rettura G., Padawer J., Stratford F., Goodwin P., Levenson S. M. Regression of C3HBA mouse tumor due to X-ray therapy combined with supplemental beta-carotene or vitamin A. J Natl Cancer Inst. 1983 Aug;71(2):409–417. [PubMed] [Google Scholar]
- Seifter E., Rettura G., Padawer J., Stratford F., Weinzweig J., Demetriou A. A., Levenson S. M. Morbidity and mortality reduction by supplemental vitamin A or beta-carotene in CBA mice given total-body gamma-radiation. J Natl Cancer Inst. 1984 Nov;73(5):1167–1177. [PubMed] [Google Scholar]
- Seifter E., Rettura G., Stratford F., Yee C., Weinzweig J., Jacobson N. L., Levenson S. M. Vitamin A inhibits some aspects of systemic disease due to local x-radiation. JPEN J Parenter Enteral Nutr. 1981 Jul-Aug;5(4):288–294. doi: 10.1177/0148607181005004288. [DOI] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. M., Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972 Aug;51(8):2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Youssef S. A., Geever E. F., Levenson S. M. Penicillamine effect on wound healing. Surg Forum. 1966;17:89–90. [PubMed] [Google Scholar]


