Abstract
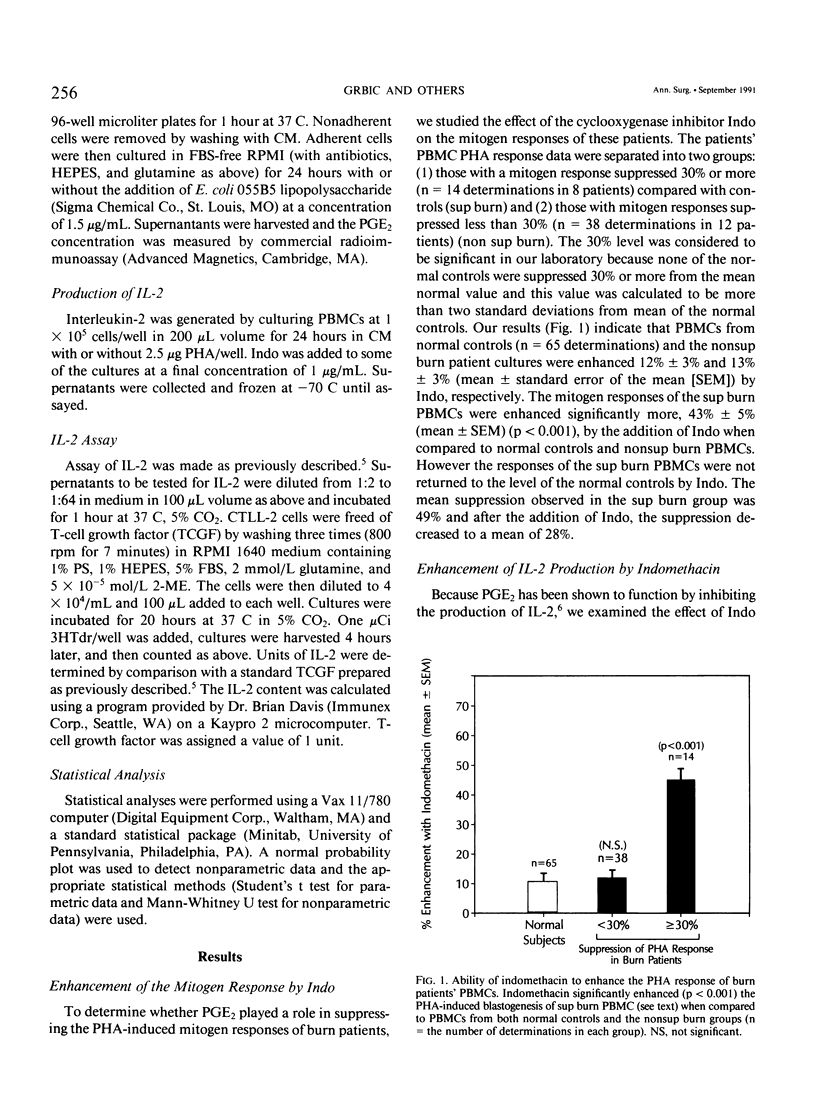
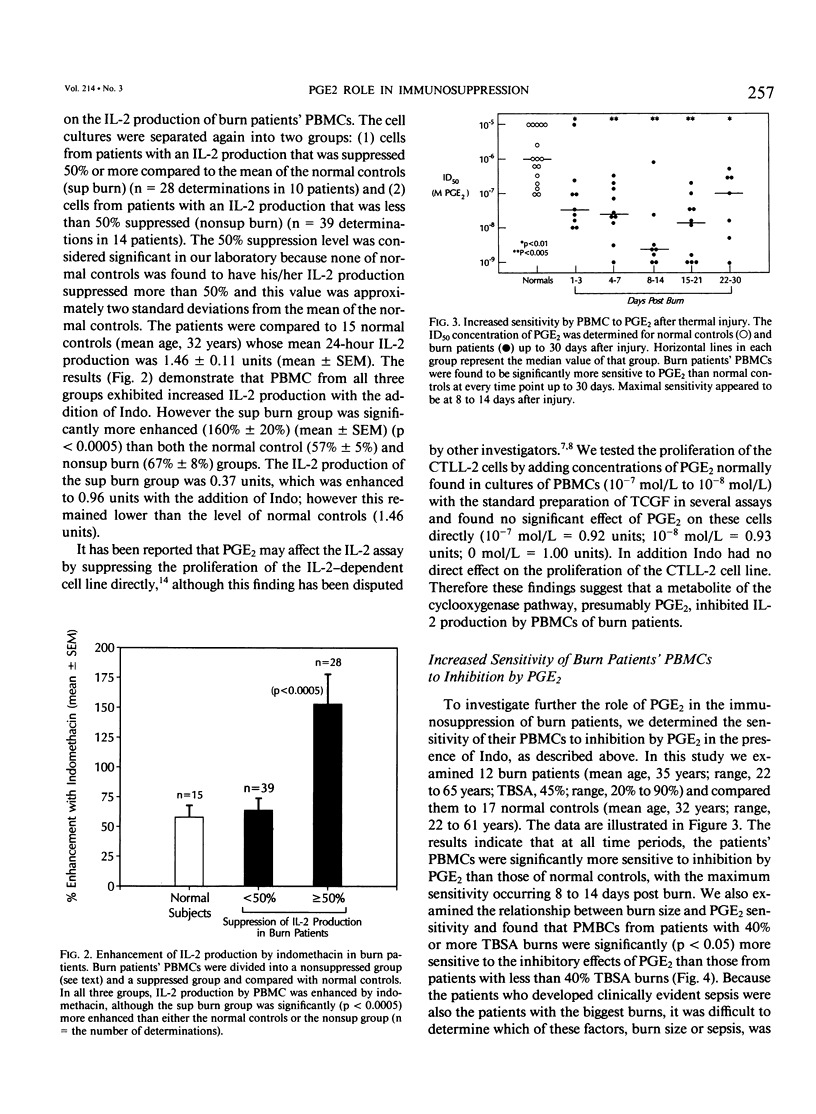
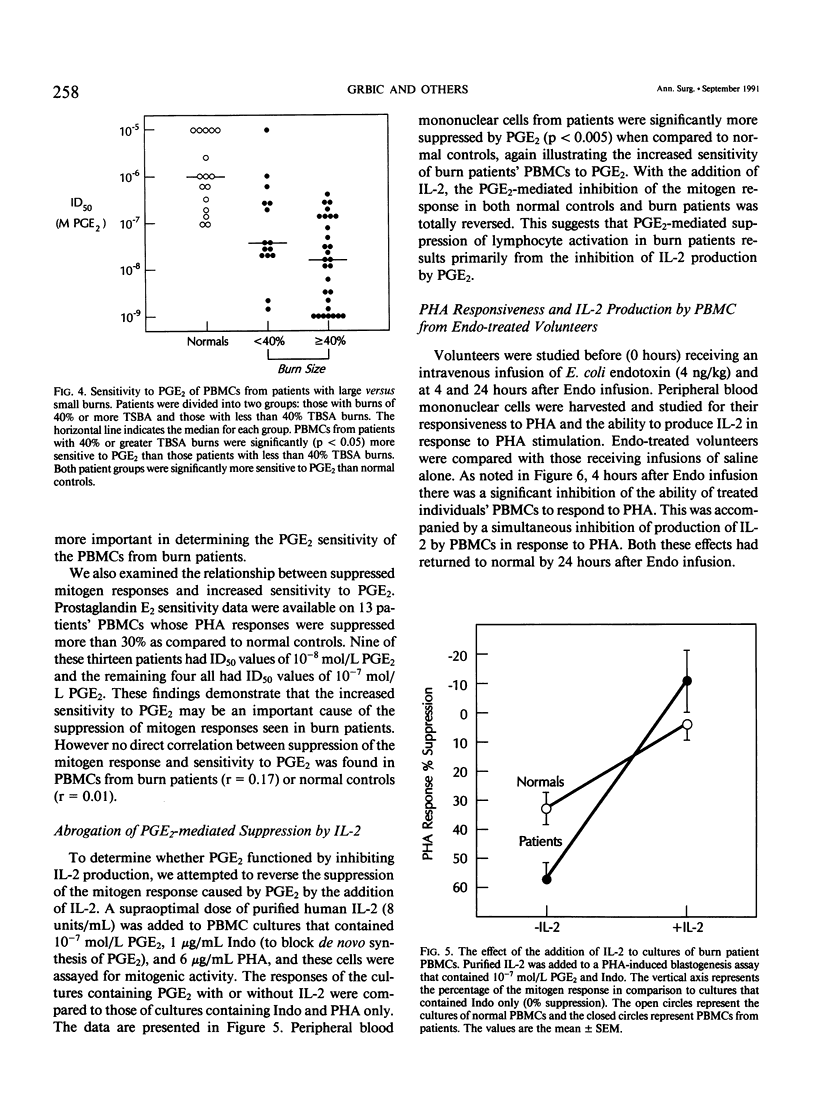
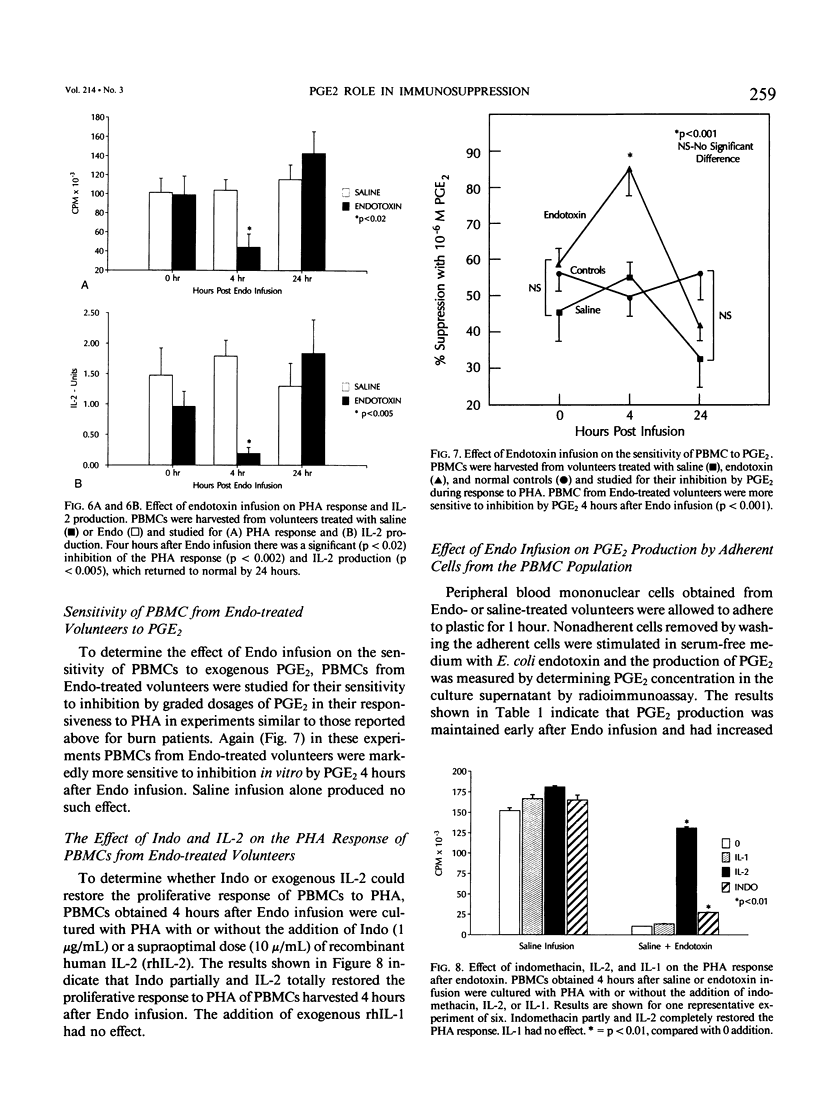
It has been thought for some time that prostaglandin E2 (PGE2) released from activated monocytes/macrophages may contribute to the suppression of immunity seen after burns and major injury because PGE2 inhibits the activation of T lymphocytes. To clarify this issue, we studied 15 patients with total body surface area burns of 20% to 90% (mean, 48%). Peripheral blood mononuclear cells (PBMC) were obtained from these patients one to two times each week for 1 month after burn and were stimulated with the T-cell mitogen phytohemagglutinin (PHA). On 14 occasions the PBMCs from eight patients were significantly suppressed (30% or more) in their response to PHA (suppressed [sup] burn) as compared with PBMCs from normal controls. In 38 instances PBMCs from 12 patients were not significantly suppressed in PHA (nonsuppressed [nonsup] burn). Sup burn PBMCs and control PBMCs were cultured with or without the addition of the cyclooxygenase (CO) inhibitor indomethacin (Indo, 1 microgram/mL) and studied for PHA response and the production of the stimulatory cytokine interleukin-2 (IL-2). Indo partially restored the PHA response of sup burn PBMCs to normal. Sup burn PBMCs also were deficient in production of IL-2. Indo increased IL-2 production by sup burn PBMCs significantly more (160% +/- 20%, p less than 0.005) than control (57% +/- 5%) and nonsup PBMCs (67% +/- 8%). Next inhibition of the PHA response of PBMCs from 12 burn patients and 17 controls was studied by exogenous PGE2. At all time periods after burn injury, patients' PBMCs were significantly more sensitive to inhibition by PGE2 (50% inhibition at 10(-8) mol/L [molar] PGE2) than PBMCs from normal controls (50% inhibition at 10(-6) mol/L PGE2) with maximum sensitivity occurring 8 to 14 days after injury. Peripheral blood mononuclear cells from patients with more than 40% burns were significantly (p less than 0.05) more sensitive to PGE2 than those from patients with lesser burns. Interleukin-2 was added to cultures of sup burn PBMC, nonsup burn PBMC, and controls containing 10(-7) mol/L PGE2. Interleukin-2 totally reversed PGE2 inhibition of the PHA response in PBMC from both controls and burn patients. Because endotoxin leak from the gut has been implicated as a trigger for a number of the metabolic and immunologic abnormalities following injury, the authors looked for the effect of a bolus infusion of Escherichia coli endotoxin (Endo, 4 ng/kg) in seven normal healthy volunteers on the response of PBMC to PHA and on the production of PGE2 and IL-2.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonacci A. C., Calvano S. E., Reaves L. E., Prajapati A., Bockman R., Welte K., Mertelsmann R., Gupta S., Good R. A., Shires G. T. Autologous and allogeneic mixed-lymphocyte responses following thermal injury in man: the immunomodulatory effects of interleukin 1, interleukin 2, and a prostaglandin inhibitor, WY-18251. Clin Immunol Immunopathol. 1984 Feb;30(2):304–320. doi: 10.1016/0090-1229(84)90064-3. [DOI] [PubMed] [Google Scholar]
- Antonacci A. C., Good R. A., Gupta S. T-cell subpopulations following thermal injury. Surg Gynecol Obstet. 1982 Jul;155(1):1–8. [PubMed] [Google Scholar]
- Berenbaum M. C., Cope W. A., Bundick R. V. Synergistic effect of cortisol and prostaglandin E2 on the PHA response. Relation to immunosuppression induced by trauma. Clin Exp Immunol. 1976 Dec;26(3):534–541. [PMC free article] [PubMed] [Google Scholar]
- Cahill J., Hopper K. E. Immunoregulation by macrophages. III. Prostaglandin E suppresses lymphocyte activation but not macrophage effector function during Salmonella enteritidis infection. Int J Immunopharmacol. 1984;6(1):9–17. doi: 10.1016/0192-0561(84)90029-8. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Cobb E. K., Lewis S. R., Larson D. L., Ritzmann S. E. Evaluation of lymphocyte reactivity studies in patients with thermal burns. J Trauma. 1971 Jul;11(7):595–601. doi: 10.1097/00005373-197107000-00011. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Ma W. J., Ma L., Berg R., Specian R. D. Endotoxin-induced bacterial translocation: a study of mechanisms. Surgery. 1989 Aug;106(2):292–300. [PubMed] [Google Scholar]
- Faist E., Ertel W., Cohnert T., Huber P., Inthorn D., Heberer G. Immunoprotective effects of cyclooxygenase inhibition in patients with major surgical trauma. J Trauma. 1990 Jan;30(1):8–18. doi: 10.1097/00005373-199001000-00002. [DOI] [PubMed] [Google Scholar]
- Faist E., Kupper T. S., Baker C. C., Chaudry I. H., Dwyer J., Baue A. E. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986 Sep;121(9):1000–1005. doi: 10.1001/archsurg.1986.01400090026004. [DOI] [PubMed] [Google Scholar]
- Fischer A., Le Deist F., Durandy A., Griscelli C. Separation of a population of human T lymphocytes that bind prostaglandin E2 and exert a suppressor activity. J Immunol. 1985 Feb;134(2):815–819. [PubMed] [Google Scholar]
- Galanaud P., Crevon M. C., Emilie D., Abella A. Effect of hydrocortisone on the in vitro human antibody response: interaction with monocytes and prostaglandins. Clin Immunol Immunopathol. 1983 Dec;29(3):403–414. doi: 10.1016/0090-1229(83)90043-0. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bromberg S., Staszak C., Kaszubowski P. A., Messner R. P., Neal J. F. Effect of physical stress on sensitivity of lymphocytes to inhibition by prostaglandin E2. J Immunol. 1981 Aug;127(2):518–522. [PubMed] [Google Scholar]
- Hansbrough J., Peterson V., Zapata-Sirvent R., Claman H. N. Postburn immunosuppression in an animal model. II. Restoration of cell-mediated immunity by immunomodulating drugs. Surgery. 1984 Mar;95(3):290–296. [PubMed] [Google Scholar]
- Horgan P. G., Mannick J. A., Dubravec D. B., Rodrick M. L. Effect of low dose recombinant interleukin 2 plus indomethacin on mortality after sepsis in a murine burn model. Br J Surg. 1990 Apr;77(4):401–404. doi: 10.1002/bjs.1800770415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Boone R. F. Enhanced effects of prostaglandin E1 and dibutyryl cyclic AMP upon human lymphocytes in the presence of cortisol. J Clin Invest. 1973 Sep;52(9):2129–2137. doi: 10.1172/JCI107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss N. M., Gough D. B., Jordan A. L., Grbic J. T., Wood J. J., Rodrick M. L., Mannick J. A. Temporal correlation of impaired immune response after thermal injury with susceptibility to infection in a murine model. Surgery. 1988 Nov;104(5):882–887. [PubMed] [Google Scholar]
- RAPAPORT F. T., CONVERSE J. M., HORN L., BALLANTYNE D. L., Jr, MULHOLLAND J. H. ALTERED REACTIVITY OF SKIN HOMOGRAFTS IN SEVERE THERMAL INJURY. Ann Surg. 1964 Mar;159:390–395. doi: 10.1097/00000658-196403000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revhaug A., Michie H. R., Manson J. M., Watters J. M., Dinarello C. A., Wolff S. M., Wilmore D. W. Inhibition of cyclo-oxygenase attenuates the metabolic response to endotoxin in humans. Arch Surg. 1988 Feb;123(2):162–170. doi: 10.1001/archsurg.1988.01400260042004. [DOI] [PubMed] [Google Scholar]
- Wolfe J. H., Wu A. V., O'Connor N. E., Saporoschetz I., Mannick J. A. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch Surg. 1982 Oct;117(10):1266–1271. doi: 10.1001/archsurg.1982.01380340002002. [DOI] [PubMed] [Google Scholar]
- Wood J. J., Grbic J. T., Rodrick M. L., Jordan A., Mannick J. A. Suppression of interleukin 2 production in an animal model of thermal injury is related to prostaglandin synthesis. Arch Surg. 1987 Feb;122(2):179–184. doi: 10.1001/archsurg.1987.01400140061007. [DOI] [PubMed] [Google Scholar]
- Wood J. J., Rodrick M. L., O'Mahony J. B., Palder S. B., Saporoschetz I., D'Eon P., Mannick J. A. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg. 1984 Sep;200(3):311–320. doi: 10.1097/00000658-198409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Sirvent R. L., Hansbrough J. F., Bender E. M., Bartle E. J., Mansour M. A., Carter W. H. Postburn immunosuppression in an animal model. IV. Improved resistance to septic challenge with immunomodulating drugs. Surgery. 1986 Jan;99(1):53–59. [PubMed] [Google Scholar]
- Zapata-Sirvent R. L., Hansbrough J. F. Postburn immunosuppression in an animal model. III. Maintenance of normal splenic helper and suppressor lymphocyte subpopulations by immunomodulating drugs. Surgery. 1985 Jun;97(6):721–727. [PubMed] [Google Scholar]


