Abstract
Pichinde virus is an arenavirus that infects guinea pigs and serves as an animal model for human Lassa fever. An attenuated Pichinde virus variant (P2) and a virulent variant (P18) are being used to delineate pathogenic mechanisms that culminate in shock. In guinea pigs, the infection has been shown to begin in peritoneal macrophages following intraperitoneal inoculation and then spreads to the spleen and other reticuloendothelial organs. We show here that infection of the murine monocytic cell line P388D1 with either Pichinde virus variant resulted in the induction of inflammatory cytokines and effectors, including interleukin-6 and tumor necrosis factor alpha. Since these genes are regulated in part by the cellular transcription factors NF-κB and RBP-Jκ, we compared the activities of NF-κB and RBP-Jκ in P388D1 cells following infection with Pichinde virus. The attenuated P2 virus inhibited NF-κB activation and caused a shift in the size of the RBP-Jκ complex. The virulent P18 virus showed less inhibition of NF-κB and failed to alter the size of the RBP-Jκ complex. Peritoneal cells from P2-infected guinea pigs showed induction of NF-κB RelA/p50 heterodimer and p50/p50 homodimer and manifested an increase in the size of RBP-Jκ. By contrast, P18 induced large amounts of the NF-κB p50/p50 dimer but failed to induce RelA/p50 or to cause an increase in the RBP-Jκ size. Taken together, these changes suggest that the attenuated viral strain induces an “activation” of macrophages, while the virulent form of the virus does not.
The arenaviruses include the viruses responsible for Argentine hemorrhagic fever (Junin virus), Venezuela hemorrhagic fever (Guanarito virus), Bolivian hemorrhagic fever (Machupo virus) and Lassa fever (Lassa virus). Pichinde virus is an arenavirus that infects guinea pigs and can be used to study arenavirus infection outside of the high-containment facilities required for working with the human hemorrhagic fever viruses such as Lassa fever virus. Perhaps the best-studied member of the group is the generally nonvirulent lymphocytic choriomeningitis virus, which has been used to establish the importance of cytotoxic T cells and major histocompatibility complex (MHC) restriction in the immune response to viral infections. By contrast, little is known about pathogenic mechanisms for the arenaviruses causing hemorrhagic fever.
Pichinde virus infection in animals results in fever after several days, progressive weight loss, and death after approximately 2 weeks (10). In both human Lassa fever and guinea pig Pichinde infection, the disease is characterized by minimal direct cellular damage. The terminal events in the fatal disease include hypotension, circulatory shock, and respiratory distress with pulmonary edema (9, 38). Immune mediators are believed to play a major role in the pathogenesis of the disease, and increased levels of tumor necrosis factor alpha (TNF-α) have been detected in the serum of infected guinea pigs (3). Following intraperitoneal inoculation in guinea pigs, the infection begins in macrophages and then spreads to the spleen and other reticuloendothelial organs (2). Only later in the lethal infection does the virus expand into other cell types.
This macrophage tropism of Pichinde virus is a characteristic of many hemorrhagic fever viruses (reviewed in reference 39) and may be important in both the virus’s evasion of the immune system and the eventual fatal shock syndrome. Macrophages are important in the immune response to viruses. They serve as professional antigen-presenting cells, act as phagocytic cells, and produce a variety of cytokines to stimulate both cytotoxic T cells and antibody-producing B cells. While bacterial lipopolysaccharide (LPS) is known to activate monocytes, it is not clear what all the effects of virus infection are on the host macrophage, but viruses have evolved a variety of mechanisms to deal with the immune system (reviewed in reference 41).
It is known that human cytomegalovirus infects monocyte/macrophages, where it acts on the antigen-presenting machinery in its effort to evade the immune system. It inhibits cytosolic proteolysis and the generation of peptide fragments for use in antigen presentation via class I genes. It also sequesters the class I molecules in the endoplasmic reticulum and destroys two components of the MHC class II antigen presentation pathway, HLA-DR-α and DM-α (42). Epstein-Barr virus, another herpesvirus, encodes EBNA-1, which interferes with proteosomal proteolysis, and encodes a protein homologous to interleukin-10 (IL-10), which inhibits the immune response. Vesicular stomatitis virus and encephalomyocarditis virus are known to induce macrophage production of alpha/beta interferon, which can then act to induce an antiviral state (6). Measles virus, which infects macrophages, binds to the cell via CD46, cross-linking it and inhibiting IL-12 production by monocytes. Like the measles virus, Pichinde virus targets macrophages as its primary target. It is therefore possible that it interferes directly in the immune response of the host.
In response to LPS activation, macrophages induce a variety of cytokines and cell surface receptors. LPS is known to induce TNF-α, IL-1β, IL-6, and IL-8 as well as other cytokines. Many of these cytokines and much of the antigen presentation machinery are induced by activation of the transcription factor NF-κB as well as other transcription factors. The NF-κB family of transcription factors bind DNA as dimers (16). The classic transactivating form of NF-κB is composed of RelA/p50 heterodimers. The p50/p50 homodimer is also very widespread, but is generally a repressor of transcription (40). It is, however, possible for the p50/p50 homodimer to interact with Bcl3 to activate transcription (8).
Normally, NF-κB dimers are kept sequestered in the cytoplasm by interaction with the IκB proteins. Upon stimulation, the IκB is phosphorylated, ubiquitinated, and degraded, and the active dimer moves to the nucleus (26). Since IκB synthesis is upregulated by NF-κB, new IκB is synthesized and accumulates to serve as feedback inhibition of the NF-κB signal.
RBP-Jκ (RBP) is another DNA-binding protein which can participate in the control of both cytokines and NF-κB proteins (20, 24, 29, 36). RBP is a ubiquitously expressed protein whose deletion is lethal during embryogenesis (18, 35). Though it is a DNA-binding protein, it does not have any of the recognizable structures (zinc finger, etc) found in many transcription factors other than NF-κB. It is highly conserved, with 75% homology between the mouse protein and the Drosophila homolog, Suppressor of Hairless (SuH) (1, 15). In Drosophila melanogaster, it is known to act in the signaling pathway of Notch (13). It complexes with a cleaved Notch receptor and provides the DNA-binding activity to the transactivating transcription factor complex. In mammals (mice and humans), it has been shown to act, at least in some instances, as a repressor whose binding interferes with transcription activation. This has been shown for IL-6 (24) and NF-κB2 (36). The inhibitory complex includes RBP as well as histone deacetylase and three auxiliary proteins, SMRT (25), SKIP (46), and CIR (21). Transactivation by RBP has been studied in mammalian cells in conjunction with Epstein-Barr virus infection of B cells. The Epstein-Barr virus protein EBNA2 binds to RBP in a manner analogous to the binding of Notch. The EBNA2 protein displaces the SMRT protein from the repressor complex and instead forms an activating complex with RBP and SKIP (45).
There are several aspects to potential interactions between the NF-κB proteins and RBP. One of the genes which RBP has been shown to repress is the NF-κB2 gene, which codes for the NF-κB p52 protein (36). In addition, an examination of the promoter region preceding the RBP gene shows at least one NF-κB site. There is also a possible interplay between NF-κB and RBP when binding in promoter regions. The consensus sequence for RBP is TGGGAA (43), and for NF-κB it is GGGRNNT(Y)CC (where R is a purine, Y is a pyrimidine, and N is any base) (34); therefore, there are some NF-κB sites which overlap RBP sites. Examples of overlapping NF-κB and RBP binding sites can be found in the promoters of a variety of genes, including IL-6, MIP, and B7-1.
We have examined the effect of Pichinde virus infection on macrophages both in guinea pigs and in cell culture, where the virus infects murine macrophage/monocyte cell lines. Comparison of the effects of two strains of Pichinde virus helps to distinguish an effective immune response from a lethal response. Both the virulent, P18 passage of virus and the attenuated, P2 passage infect macrophages, but the P18 virus is lethal, while the animal mounts an effective immune response to the P2 virus and recovers (22). We have determined that both passages of Pichinde virus can induce TNF-α and IL-6 in monocytes in cell culture, though P2 induces higher levels of these cytokines. Since cytokine gene expression is regulated, in part, by NF-κB and RBP, we examined the effects of virus infection on these factors. In both the cell culture system and in vivo in the guinea pig, the attenuated P2 strain of virus, to which the animal mounts an effective immune response, causes changes in these factors. The virulent strain of virus, P18, fails to alter RBP and causes increased synthesis of the repressive p50/p50 NF-κB homodimer. In addition, both UV-inactivated P2 virus and conditioned medium from virus-infected cells fails to alter the cells, suggesting that active virus growth is required for this alteration of the macrophages.
MATERIALS AND METHODS
Antibodies.
Antiserum to p50 and RelA were kindly provided by Nancy Rice (Frederick Cancer Research Facility, Frederick, Md.). Monoclonal antibodies to RBP (K0043 and T6417) were kindly provided by H. Kurooka (Kyoto University) (19).
Oligonucleotides.
Single-stranded oligonucleotides were synthesized to order by Bio-Synthesis Inc. (Lewisville, Tex.). The Igκ oligonucleotide (AGTTGAGGGGACTTTCCCAGGC) is also available from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.) as an NF-κB gel shift oligonucleotide and from Promega Corp. (Madison, Wis.) as the NF-κB oligonucleotide. Duplex oligonucleotides were annealed at 1.75 μM in 10 mM Tris-HCl (pH 7.6)−2 mM MgCl2−50 mM NaCl−1 mM EDTA by heating briefly at 95°C and allowing them to cool slowly. Oligonucleotides were radiolabeled with T4 polynucleotide kinase (Promega Corp., Madison, Wis.) and [γ-32P]ATP (DuPont NEN, Boston, Mass.) under standard reaction conditions.
Cell lines and culture conditions.
70Z/3 (murine pre-B lymphocyte) cells were maintained in RPMI medium supplemented with 5% fetal bovine serum, 1% β-mercaptoethanol, and 2 mM glutamine. They were treated with 10 μg of Salmonella enterica serovar Typhosa LPS (W0901; Difco, Detroit, Mich.) per ml for 6 h prior to extraction. RAW264.7 (murine monocyte-like) and P388D1 (murine monocyte-like) cells were maintained in RPMI medium supplemented with 5% fetal bovine serum (FBS) and 2 mM glutamine. When indicated, they were treated with 0.1 μg of LPS per ml prior to processing for extracts.
Virus stocks.
Virus was quantitated in a standard plaque assay on Vero cells as described previously (2). The two Pichinde virus strains were originally developed by Jahrling and others (22) from serial passage in guinea pigs and harvested from guinea pig spleens. The virulent P18 strain has now been passaged 18 times in guinea pigs, and the attenuated strain, P2, has been passaged twice (44). Each strain was passaged only once in P388D1 cells before use in these experiments. Virus was completely inactivated by irradiation for 30 min under a short-wave UV lamp. Inactivation was confirmed by failure to form plaques on Vero cells.
Animal protocols.
Male outbred Harley strain guinea pigs, 400 to 500 g (Harlan Sprague-Dawley), were inoculated intraperitoneally with 1 ml of phosphate-buffered saline (PBS) containing 1,000 PFU of Pichinde virus, either P2 or P18. Weights and rectal temperatures were recorded daily. Animals were sacrificed 6 days postinfection. Peritoneal cells were harvested by aseptic lavage with 100 ml of unlabeled, sterile PBS (Ca- and Mg-free) immediately following death. Following centrifugation, the cells were washed in 10 ml of PBS. Cells were counted, and an aliquot was removed for use in infectious center assays and differential cell counts. The remainder of the cells was used to make nuclear extract following the procedure used for cultured cells. For the differential cell count, 105 peritoneal cells in a 1-ml volume of PBS were applied to a slide by cytospin. The cells were fixed in methanol and stained using the Diff-Quik stain set (Dade Behring Inc., Newark, Del.) for the differential cell count
Infectious center assay.
Viral infectious center assays were performed as previously described (2). Briefly, peritoneal cells were plated onto monolayers of Vero cells in six-well plates in RPMI medium with 2% FBS, 2 mM glutamine, penicillin-streptomycin (100 U/ml), and 0.5% methylcellulose. After 5 days of incubation at 37°C, the medium was removed, and the monolayers were stained with neutral red (44).
Nuclear extract preparation.
Nuclear extracts were prepared following standard procedures. Pelleted cells were resuspended in buffer I with sucrose (0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris [pH 8.0], 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). Nuclei were pelleted, washed in buffer I without sucrose, and repelleted. Nuclei were then resuspended in a low-salt buffer (20 mM HEPES [pH 7.9], 25% glycerol, 1.5 mM MgCl2, 20 mM KCl, 0.2 mM EDTA, 0.5 mM PMSF), an equal volume of high-salt buffer (20 mM HEPES [pH 7.9], 25% glycerol, 1.5 mM MgCl2, 0.8 M KCl, 0.2 mM EDTA, 0.5 mM PMSF) was added, and the nuclei were incubated on ice for 20 min. They were then diluted by the addition of 2.5 volumes of dilution buffer (25 mM HEPES [pH 7.6], 25% glycerol, 0.1 mM EDTA, 0.5 mM PMSF), and debris was pelleted. The supernatant was removed as the nuclear lysate and stored at −80°C.
EMSA.
For electrophoretic mobility shift assay (EMSA) reactions, 1 to 5 μg of nuclear extract was incubated with 0.1 pmol of radiolabeled oligonucleotide in a 15-μl volume under standard reaction conditions (20 mM HEPES [pH 7.5], 50 mM KCl, 2.5 mM MgCl2, 20 mM dithiothreitol, 10% glycerol, plus 50 μg of poly[dI-C] and 0.1 mg of bovine serum albumin [BSA] per ml). For competition experiments, a 35-fold excess of unlabeled oligonucleotide was also added. After 15 min, the reaction was loaded onto a standard 6% nondenaturing polyacrylamide gel in 0.25× TBE. Following electrophoresis, the gel was dried, and radioactivity was quantitated using a Packard InstantImager. Images for figures were derived from exposure to XAR-5 film. For supershift experiments, nuclear extracts were incubated with antibody overnight at 4°C in a 15-μl volume under standard reaction conditions prior to the addition of the radiolabeled oligonucleotide.
Immunoblots.
Samples (10 to 20 μg) were electrophoresed on standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (8%) and transferred to Immobilon-P using a semidry transfer apparatus. Filters were blocked in 5% nonfat dry milk-Tris-buffered saline (TBS) overnight and incubated with a 1:5,000 dilution of primary antibody in milk-TBS plus BSA (100 μg/ml) for 1 h. Following a series of TBS-Tween (0.5%) washes, the filters were incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated secondary antibody in milk-TBS-BSA for 1 h. Following another series of washes, the filters were soaked in the Pierce SuperSignal chemiluminescent substrate for 5 min and exposed to Kodak X-Omat AR film.
Cytokine assays.
Murine IL-6 and TNF-α were measured using enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, Calif.) following the instructions of the manufacturer.
RESULTS
Pichinde virus induces cytokine changes.
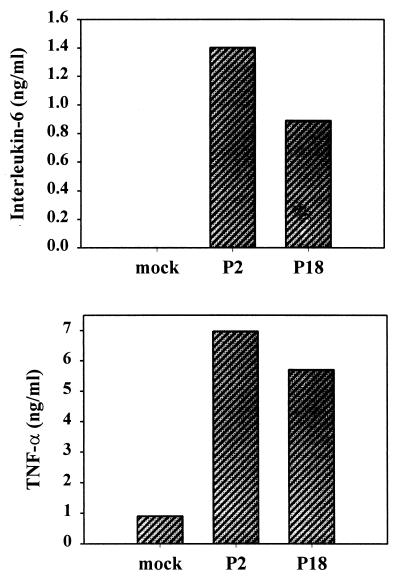
Pichinde virus infects and reproduces well in the murine monocytic cell line P388D1, though it generally does not cause death of the cells and the cells continue to grow and divide, at least for several days. We infected P388D1 cells at a multiplicity of infection (MOI) of 1 and allowed the virus to replicate for 3 days. Duplicate cultures were infected with either the P2 or the P18 strain of the virus. These virus strains display minimal differences in culture, but in the guinea pig, P18 is lethal, while P2 is not. We then examined the medium for the production of cytokines. Virus infection induced the synthesis of 5 to 7 ng of TNF-α per ml (Fig. 1) and, variably, of small amounts (800 to 1,500 pg/ml) of IL-6. There was no significant difference between the amounts induced by the two viruses.
FIG. 1.
Induction of TNF-α and IL-6 by Pichinde virus infection. The murine monocytic cell line P388D1 was infected with either the P2 or P18 strain of Pichinde virus at an MOI of 1. Medium samples were removed at 3 days postinfection and assayed for cytokines using a commercial ELISA kit. Data are the averages of two samples from a representative experiment. The experiment was repeated three times.
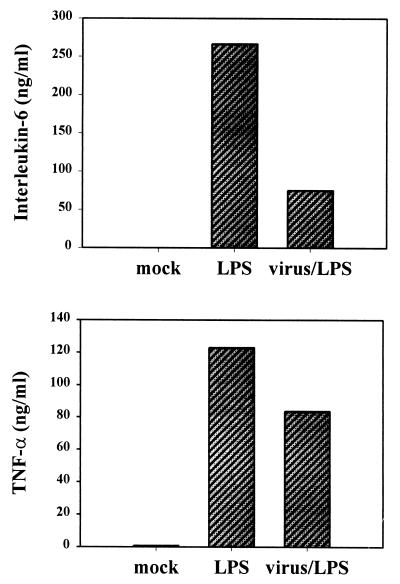
It was possible that the alteration of macrophage function would be more clearly evident in the modulation of the cell response to LPS, a standard stimulus of macrophages. This is the case with the measles virus alteration of IL-12. Measles virus does not alter the basal level of IL-12 production in macrophages but inhibits the induction of IL-12 by LPS (27). It is also true in certain nonviral cases. For example, vasoactive intestinal peptide inhibits the induction of IL-6 by LPS but increases the basal level of production (33). Therefore, we infected P388D1 cells with the Pichinde virus (P18) and allowed the virus to grow in the cells for 3 days before using LPS to stimulate cytokine production. LPS induced greater quantities of IL-6 (250 ng/ml) and TNF-α (120 ng/ml) than did virus infection alone. However, induction of virus-infected cells resulted in a 70% decline in IL-6 levels and a 30% decline in TNF-α levels following LPS stimulation (Fig. 2).
FIG. 2.
Inhibition of the LPS induction of cytokines by virus infection. The murine monocytic cell line P388D1 was infected with the P18 strain of Pichinde virus at an MOI of 1. At 3 days postinfection, LPS (10 ng/ml) was added to selected flasks. Medium samples were removed 19 h later and analyzed for cytokines by ELISA. Data are the averages of two samples from a representative experiment. The experiment was repeated three times.
Virus infection causes changes in NF-κB and RBP-Jκ.
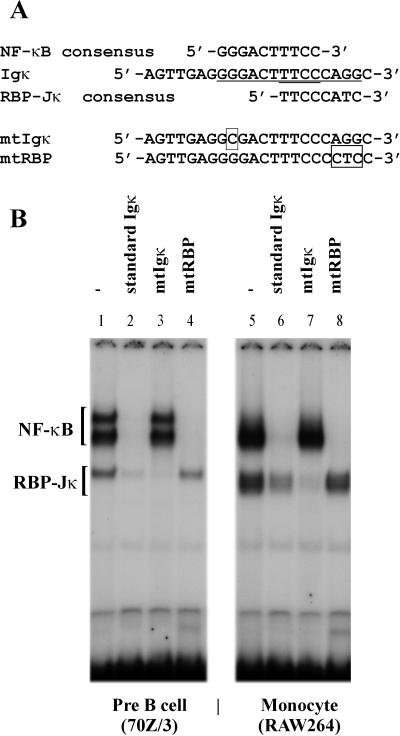
To determine if the alteration of cytokines which took place with virus infection may be mediated by changes in transcription factors, we examined the nuclear levels of NF-κB, a key transcription factor in the control of many cytokines, including TNF-α, and IL-6. EMSAs were performed using the Igκ oligonucleotide to detect binding of NF-κB proteins (see Fig. 3A for sequences of oligonucleotides used), with the well-studied 70Z/3 pre-B cells (17) serving as a standard. Preliminary analysis of uninfected and unstimulated cells showed that the P388D1 monocyte cell line as well as an additional murine monocytic cell line, RAW 264.7, contained Igκ binding complexes which were not comigrating with the known NF-κB complexes present in the 70Z/3 cell line. These were “specific” bands that were successfully competed away by a 35-fold excess of unlabeled oligonucleotide (Fig. 3B). When the consensus NF-κB site was mutated (mtIgκ), the oligonucleotide failed to compete for binding with the NF-κB complexes, but still bound and competed away the faster-migrating complexes (Fig. 3B, lanes 3 and 7).
FIG. 3.
Effect of base substitutions on competition for binding by the standard Igκ oligonucleotide. (A) Nucleotide sequences of oligonucleotides used for EMSAs. The NF-κB and RBP-Jκ consensus sequences are shown, underlined in the standard Igκ sequence. Bases that have been altered in the various oligonucleotides are shown boxed. (B) EMSA of nuclear extracts (2 μg) from LPS-treated 70Z/3 (lanes 1 to 5) or RAW264.7 (lanes 6 to 10) cells. The radiolabeled oligonucleotide was the standard Igκ oligonucleotide. A 35-fold excess of unlabeled oligonucleotide was added in competition: Igκ (lanes 2 and 6), mtIgκ (lanes 3 and 7), and mtRBP (lanes 4 and 8).
An examination of the sequence of the oligonucleotide revealed a potential binding site for a second transcription factor, RBP. The RBP consensus sequence is TGGGAA (43), which overlaps the NF-κB binding site in this oligonucleotide, and is not mutated in the mtIg oligonucleotide (Fig. 3A). To determine if this was the binding site for the additional complex(es), we mutated the RBP binding site (mtRBP) and used it to compete with binding by the Igκ oligonucleotide. The mtRBP oligonucleotide successfully competed for NF-κB binding, but no longer bound and competed away the faster-migrating RBP complexes (Fig. 3B, lanes 4 and 8). Therefore, the binding site for the additional complexes overlaps the RBP consensus site, suggesting that the additional bands seen are due to RBP binding to the Igκ oligonucleotide.
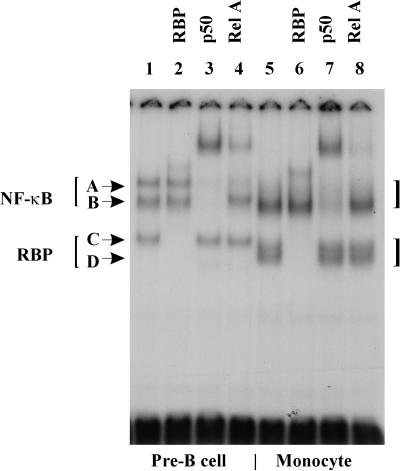
To confirm our identification of the complexes, we performed supershift assays, in which antibody is used to shift the size of specific complexes. This has previously been done to assign the NF-κB bands of the 70Z/3 cell line (17). The anti-p50 antibody shifted or abolished the majority of the NF-κB band (B in Fig. 4) present in the RAW264.7 and P388D1 monocytic cells, as well as the corresponding band in the 70Z/3 cells (Fig. 4, lanes 3 and 7). The anti-RelA antibody altered the binding to the upper band (A in Fig. 4) of the 70Z/3 cells, consistent with its identification as the RelA/p50 heterodimer (Fig. 4, lane 4). There is little to no detectable band corresponding to the RelA/p50 band in the monocytic cell lines. Therefore, uninfected monocytic cells contain large amounts of the NF-κB p50/p50 homodimer but very little of the RelA/p50 heterodimer. This predominance of the p50/p50 homodimer in monocytic cells has been seen by others (14).
FIG. 4.
Identification of NF-κB and RBP-Jκ proteins using supershift with antibodies. EMSA of nuclear extracts (5 μg) of 70Z/3 (lanes 1 to 3), RAW264.7 (lanes 4 to 6) and P388D1 (lanes 7 to 9) cells with radiolabeled standard Igκ oligonucleotide. 70Z/3 extracts are from cells treated with LPS for 6 h. Monoclonal antibody to RBP-Jκ (lanes 2, 5, and 8), or to p50 (lanes 1, 4, and 7) was added to the reaction 15 min before the addition of the oligonucleotide. Complexes A to D are indicated by arrows.
The preliminary identification of the faster-migrating complexes (C and D in Fig. 4) as RBP was confirmed with an anti-RBP monoclonal antibody (Fig. 4, lanes 2 and 6). In the 70Z/3 cell line there was only one such complex present, but in the monocytic cell lines there were usually two visible complexes, though they were difficult to distinguish if the samples were electrophoresed for a shorter period of time. Both of these bands were identified as RBP proteins based on their binding to the anti-RBP monoclonal antibody. A review of the literature confirmed that others might have observed a band corresponding to the RBP complex of 70Z/3 cells, though they did not identify it as an RBP complex.
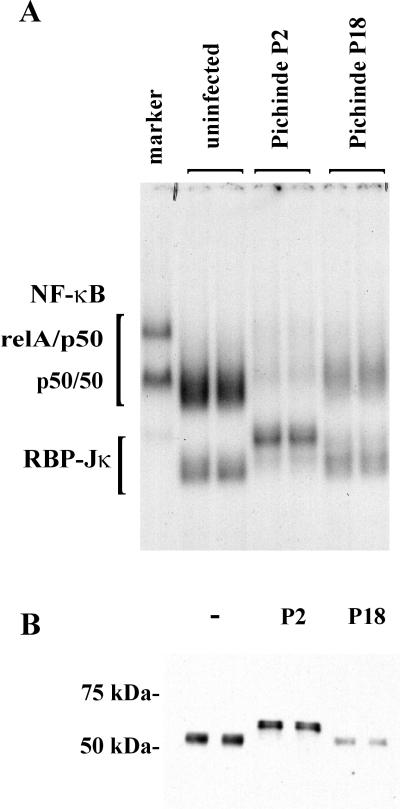
With the complexes identified, we were able to interpret EMSA gels using lysates from virus-infected P388D1 cells. Nuclear extracts were prepared at 3 days postinfection, when the medium samples were taken for the cytokine assays (Fig. 1). Virus infection led to changes not only in NF-κB levels, but also in the size of the RBP complex (Fig. 5A). In all cultures, the p50/p50 homodimer was the most abundant NF-κB complex. Very little, if any, RelA/p50 heterodimer was present. These cells had large amounts of the p50/p50 homodimer present in the uninfected control cells. While the NF-κB levels in the P18-infected cells are twice as high as in the P2-infected cells, the uninfected cells had five times the level.
FIG. 5.
Pichinde virus infection induces changes in NF-κB and RBP-Jκ in P388D1 cells. Virulent (P18) and attenuated (P2) Pichinde viruses were used to infect P388D1 cells at an MOI of 1. Nuclear lysates were made at 3 days postinfection. DNA-binding proteins were analyzed by EMSA (A) using the Igκ standard oligonucleotide and by immunoblot (B) using anti-RBP antibody. The marker (lane 1) is nuclear extract from LPS-treated 70Z/3 cells. Duplicate cultures were used for each treatment and analyzed in separate lanes of the gels: mock infected, lanes 2 and 3; P2 virus, lanes 4 and 5; and P18 virus, lanes 6 and 7.
The alterations in the RBP bands were qualitative rather than quantitative. Though the levels of RBP did not change significantly, the complex formed from the P2-infected cells migrated more slowly. The appearance of two RBP bands was examined by immunoblot to determine if the RBP protein was present in two different sizes or if the EMSA size change was due to binding with other proteins or factors (Fig. 5B). Samples of the nuclear extracts were separated on SDS-PAGE gels, transferred to a membrane, and probed with an anti-RBP antibody. The RBP protein size varied in agreement with the migration in the EMSA (Fig. 5B). The RBP protein is approximately 55 kDa in both the mock- and P18-infected cells and 62 kDa in the P2-infected cells. Multiple RBP protein sizes have not been reported previously, though alternative initiation and splicing of the RBP mRNA has been reported to occur in both murine and human cells (1), suggesting that multiple sizes of the RBP protein may exist.
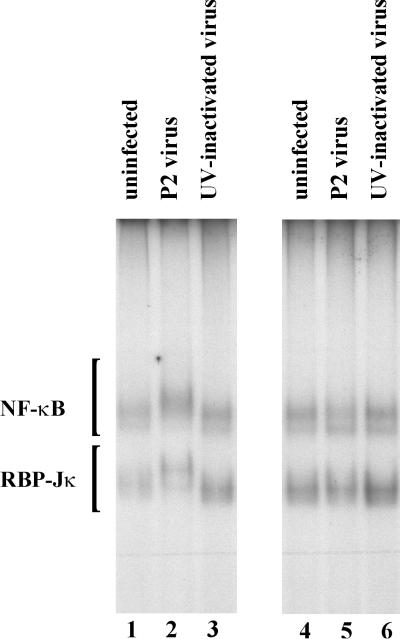
To help establish whether the alteration of the macrophages was dependent on virus replication within the cell or could be the result of viral receptor binding, UV-inactivated virus was used to “infect” the cells. UV-inactivated virus had no effect on the transcription factors (Fig. 6). In addition, the transfer of conditioned medium from the virus-infected cells to fresh cells failed to induce the transcription factor change, suggesting that it is not a rapid response to one of the cytokines produced in response to the virus infection.
FIG. 6.
UV-inactivated P2 virus fails to alter the transcription factor binding. Pichinde P2 virus (lane 2) or UV-inactivated P2 virus (lane 3) was used to infect P388D1 cells at an MOI of 1. At 3 days postinfection, the conditioned medium was removed from the cells, and nuclear lysates were made (lanes 1 to 3). The medium was transferred to new P388D1 cells that were used for nuclear lysates 2 h later (lanes 4 to 6). DNA-binding proteins were analyzed by EMSA using the Igκ standard oligonucleotide
Virus infection of guinea pig peritoneal cells results in similar transcription factor alterations.
We next examined the effect of in vivo infection of guinea pigs on the NF-κB and RBP transcription factors of the peritoneal macrophages. Guinea pigs were injected intraperitoneally with either the P2 or P18 variant of Pichinde virus. On day 6 postinfection, the animals were sacrificed, and peritoneal macrophages were harvested. A portion of the cells was used to determine infectious centers and for making slides for differential cell counts, and the remainder was used to make nuclear lysates. It should be noted that the peritoneal macrophages were not plated or cultured before the nuclear lysates were made. Virus infection did not cause a major change in the number or type of cells used to make the nuclear lysates, and the number of cells harvested from the guinea pigs did not vary significantly with virus infection (Table 1). In addition, the differential cell count showed that the majority of the cells were monocytic cells in all animals, though uninfected animals tended to have more granulocytes than the virus-infected animals.
TABLE 1.
Peritoneal cells harvested from Pichinde virus-infected guinea pigs
| Virus | No. of cells har-vested | No. of infectious centers/1,000 cells | Differential cell counts
|
||
|---|---|---|---|---|---|
| Monocytes | Lymphocytes | Granulocytes | |||
| None | 2.2 × 107 | 60 | 9 | 31 | |
| 2.0 × 107 | 62 | 1 | 37 | ||
| P2 | 1.8 × 107 | 3.9 | 67 | 4 | 29 |
| 2.1 × 107 | 2.4 | 76 | 12 | 12 | |
| P18 | 2.2 × 107 | 13.2 | 81 | 8 | 11 |
| 1.0 × 107 | 16.8 | 86 | 14 | 0 | |
The cells from the P18 virus-infected animals exhibited a higher level of virus infection. The infectious center assay measures the number of cells capable of transmitting the virus and forming plaques on Vero cells. According to this assay (Table 1), the P18-infected animals had approximately five times as many virus-infected cells as the P2-infected animals. The P18-infected animals also had a higher fever and weighed less than either the control or P2-infected animals (data not shown). This is the standard pattern seen with Pichinde virus infection, and if the infection is allowed to proceed, we would expect the P2-infected animals to recover, with their temperature decreasing and their weight increasing by day 12 postinfection, while the P18-infected animals would continue to have an elevated temperature and lose weight until death, usually at day 16 (on average) (44).
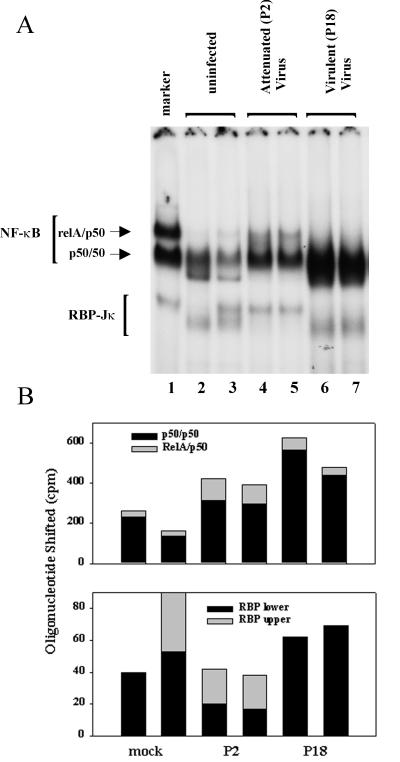
The peritoneal macrophages were used to make nuclear lysates, and NF-κB and RBP levels were examined by EMSA. The NF-κB and RBP patterns both changed with viral infection (Fig. 7). The most common transcriptionally active form of NF-κB is believed to be the RelA/50 heterodimer. Only very low levels of RelA/50 were detectable in the resident peritoneal cells of uninfected animals, but this level rose threefold in the P2-infected animals. Little RelA/50 complex was detectable in the peritoneal cells of the P18-infected animals, but the quantitation is difficult due to the large amount of p50/p50 present in the same samples.
FIG. 7.
In vivo Pichinde virus infection induces changes in NF-κB and RBP-Jκ in guinea pig peritoneal cells. (A) EMSA gel using standard Igκ oligonucleotide and nuclear lysates from peritoneal cells of Pichinde virus-infected guinea pigs. Guinea pigs were injected intraperitoneally with 1,000 PFU of Pichinde virus (P2 or P18). Animals were sacrificed, and peritoneal cells were harvested 6 days postinfection. The marker (lane 1) is nuclear extract from LPS-treated 70Z/3 cells. (B) Radioactivity was counted with a Packard InstantImager. Two animals were used for each treatment and are analyzed in separate lanes of the gels: mock infected, lanes 2 and 3; P2 virus, lanes 4 and 5; and P18 virus, lanes 6 and 7.
The NF-κB p50/p50 homodimer is thought to act as a repressor in most studies, though it can also act as an activator if associated with other proteins. The uninfected cells showed easily detectable levels of this complex, consistent with previous reports that resident peritoneal macrophages have constitutive nuclear NF-κB p50 protein (14). This level increased approximately twofold with P2 virus infection and even more dramatically (threefold) with P18 virus infection. Therefore, attenuated P2 Pichinde virus infection leads to an increase in the activating RelA/p50 heterodimer as well as the more “repressive” p50/p50 homodimer. The virulent P18 virus fails to induce the active RelA/p50 heterodimer and instead causes a larger increase in the amount of the “repressor” p50/p50 homodimer.
Pichinde virus infection caused qualitative rather than quantitative changes in the RBP complexes. The resident peritoneal cells of the uninfected animals contained the smaller, faster-migrating RBP complex or a combination of the smaller and the larger forms. The peritoneal cells from the P18-infected animals contained primarily the smaller form of RBP, as did those from the uninfected animals. However, the cells from the attenuated P2-infected animals contained the larger form of RBP. The changes in the RBP sizes correspond to the equivalent sizes seen with Pichinde virus P2 and P18 infection in the P388D1 cell line. Therefore, virus infection can induce a shift in the size of the RBP protein both in cell culture and in the animal.
DISCUSSION
The viral infection of macrophages presents an opportunity for a virus to evade the immune system by modulating the immune response of the infected cell. Some examples of this already exist (41). Perhaps the most studied, though not fully understood, is the human immunodeficiency virus (HIV) infection of both T cells and macrophages and the resulting immunodeficiency. This is not the only example. Measles virus infects macrophages and induces an immunodeficiency which lasts well beyond the period of the actual viral illness. At least in part, this has been attributed to measles virus inhibition of macrophage production of IL-12 (27). Epstein-Barr virus, which infects B cells, both produces an IL-10 analog of its own and interferes with the same RBP-Jκ transcription factor that we describe here.
Our studies present an additional insight into how viral infection can alter macrophage function. Pichinde virus infection of guinea pigs and Lassa fever virus infection of humans cause a syndrome that suggests immune system malfunction. Compared to P2 infection, the virulent P18 infection of guinea pigs is characterized by dysregulated proinflammatory cytokine production (5) with profound terminal shock and immunosuppression (T. R. Jerrells and V. K. Brown, unpublished data). In previous studies, macrophages explanted from Pichinde virus-infected guinea pigs and stimulated ex vivo with LPS showed some suppression of TNF induction (2). The contribution of direct virus-induced alterations in macrophage function to these phenomena is not known.
Because of the peculiarities of the arenavirus system and our own interest in NF-κB and other transcription factors, we have examined the NF-κB response to the viral infection despite minimal data available concerning expression of cytokines. Studies on the cytokines and immune factors involved in the developing illness are hampered by the fact that the human Lassa virus requires biosafety level 4 containment and the Pichinde virus animal model of arenavirus infection is the guinea pig, for which few immunological reagents are available. However, since transcription factors are generally measured by their ability to bind to specific conserved DNA sequences, the guinea pig system is amenable to such investigation. In addition, the RBP-Jκ and NF-κB proteins are highly conserved among species, and many of the antibodies to the human or mouse protein cross-react with the homologous guinea pig protein. Also, peritoneal lavage of the guinea pig yields sufficient cells for preparation of nuclear lysates from the resident, infected cells without any need for cell culture steps that may introduce additional complications.
Our examination of the NF-κB transcription factors yielded some unexpected and novel findings relevant to the transcription factors active in macrophages and how they are altered by virus infection. The resident peritoneal cells contain predominantly the p50/p50 homodimer form of NF-κB, which is associated with repression of transcription. With virulent arenavirus infection, the amount of p50/p50 is greatly increased, though the transactivating RelA/p50 form fails to accumulate. This is similar to LPS “tolerance,” in which a small preliminary dose of LPS causes an initial and transient activation of the RelA/p50 complex, followed by an increase in the amount of p50/p50 homodimer present (4, 7). The cells then become tolerant to LPS and fail to activate properly when challenged with a second dose of LPS. This may account for the viral inhibition of LPS-induced IL-6 production (Fig. 2) and for Lassa virus inhibition of IL-8 production (31).
It is possible that the nonlethal, attenuated P2 virus activates macrophages with some increase in both RelA/p50 and p50/p50 but that the lethal, virulent P18 virus induces only p50/p50, leading to nonresponsive, tolerant cells. By inhibiting the activation of macrophages, the virus could perhaps alter the subsequent cytotoxic T-cell response and evade the immune system. There have been similar suggestions concerning septic shock induced by LPS. For at least a subset of patients, macrophages show decreased HLA levels and nonresponsiveness. Treatment with gamma interferon can restore the responsiveness of the macrophages and diminish the illness (11). This approach may be effective in virally induced shock. This does not imply that the subsequent shock syndrome is not dependent on some aspect of the immune response, merely that one aspect needed for virus clearance is not induced.
The alterations in RBP in response to the nonlethal virus infection are more unusual and without precedent. RBP has been known to have alternative initiation and splicing variants, since the initial cDNA cloning identified several different variants in both mice and humans (1, 28). However, there has been no publication of clearly distinct RBP proteins associated with a particular cell or cellular response. The RBP band seen when performing EMSAs with the Igκ oligonucleotide has seldom been identified as RBP. The absence of reports on the altered size of the RBP band in macrophages is perhaps due to the fact that most studies do not make lysates directly from resident peritoneal macrophages.
Our preliminary results suggest that elicited macrophages contain only the most often observed, higher-molecular-weight form of RBP (unpublished data). In addition, many protocols involve culturing the peritoneal macrophages before use, and the conditions of cell culture may induce an alteration in RBP. The change in size of RBP following P2 virus infection is similar to the change elicited by LPS (unpublished data). Therefore, for both NF-κB and RBP, the nonlethal infection is associated with the activated forms of transcription factors present in the macrophages, while the lethal infection is associated with the “nonactivated” state of the transcription factors and the failure to mount an effective immune response.
It is unknown at this point what causes these alterations in NF-κB and RBP. Epstein-Barr virus codes for an IL-10 analog capable of downregulating the immune response of macrophages and T cells. However, the arenaviruses are small RNA viruses and code for only four known proteins, none of which shows any obvious homology to cellular proteins. Viruses have also been shown to affect cells through binding to a cellular receptor via the viral glycoprotein with no need for viral replication. Measles virus inhibition of cellular activity is not dependent on virus replication. Other viruses require virus replication for the effect on the host cell; Rous sarcoma virus (12) and Dengue virus (32) have been shown to induce NF-κB during virus replication. The mere expression of the influenza virus hemagglutinin protein, without virus infection, can induce NF-κB in a redox-sensitive manner (37). The Lassa virus inhibition of IL-8 has been reported to require replicating virus (31), in agreement with our finding that the Pichinde virus alteration of NF-κB and RBP does not take place if the virus has been inactivated.
The consequences of the NF-κB and RBP alterations are also presently unknown. From studies with the p50/p50 homodimer, it is probable that it is acting as a repressor. In cell culture, where other signals are limited, there is merely a modest increase in cytokine levels. Perhaps in the animal, with signals from T cells and other cells involved, a critical function is being inhibited. This would be somewhat similar to the inhibition by measles virus of the induction of IL-12 by LPS (27).
There is little basis for speculation about the consequences of the change in RBP. In this case, however, the more lethal virus fails to cause the change in size that seems associated with activation of the macrophage. There is precedence for viral interaction with the RBP protein in the immune system. The Epstein-Barr virus EBNA2 protein interacts directly with the RBP protein to form a transactivating complex. RBP, when complexed with a repressor complex containing SMRT, Sin3a, HDAC, SAP30, and CIR, acts as a repressor of transcription. The EBNA2 protein displaces this repressor complex, resulting in the activation of transcription (45). A variety of cellular genes that are activated by EBNA2 have RBP binding sites in their promoters, including IL-1β, beta interferon, and CD23 (23, 29, 30)
While the association of EBNA2 with RBP is known to alter the activity from that of a repressor to an activator of transcription, the consequences of the alteration in size of the RBP protein are unknown. One possibility is that the protein-protein interactions are altered. RBP is known to interact not only with the repressor complex but also with an additional protein, SKIP (46), and EBNA2. Any of these interactions could be disrupted, or additional interactions induced. Presumably, the change in size that we observed in macrophages is associated with some change in function, but it is yet to be determined if the two forms have different DNA-binding specificities or protein interactions. It is known that RBP and NF-κB sites overlap in the promoters of a variety of immune function genes, including IL-6, MHC class I, MIP, and B7-1. It is possible that a subset of these sites are affected, resulting in an alteration of cytokine production or antigen presentation and T-cell activation.
Acknowledgments
We thank Amy Shurtleff and Kat Marriott for contributions of time and technical assistance with the animal experiments and Barry Elsom for technical assistance with all other aspects of the experiments. We are grateful to R. Shope for his role in directing the research efforts supported by this grant.
This work was supported by DARPA grant 9624-107 FP from the Department of Defense.
REFERENCES
- 1.Amakawa, R., W. Jing, K. Ozawa, N. Matsunami, Y. Hamaguchi, F. Matsuda, M. Kawaichi, and T. Honjo. 1993. Human Jk recombination signal binding protein gene (IGKJRB):comparison with its mouse homologue. Genomics 17:306–315. [DOI] [PubMed] [Google Scholar]
- 2.Aronson, J. F., N. K. Herzog, and T. R. Jerrells. 1994. Pathological and virological features of arenavirus disease in guinea pigs. Comparison of two Pichinde virus strains. Am. J. Pathol. 145:228–235. [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson, J. F., N. K. Herzog, and T. R. Jerrells. 1995. Tumor necrosis factor and the pathogenesis of Pichinde virus infection in guinea pigs. Am. J. Trop. Med. Hyg. 52:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141–179. [DOI] [PubMed] [Google Scholar]
- 5.Bartholdy, C., A. Nansen, J. E. Christensen, O. Marker, and A. R. Thomsen. 1999. Inducible nitric-oxide synthase plays a minimal role in lymphocytic choriomeningitis virus-induced, T cell-mediated protective immunity and immunopathology. J. Gen. Virol. 80:2997–3005. [DOI] [PubMed] [Google Scholar]
- 6.Belardelli, F., F. Vignaux, E. Proietti, and I. Gresser. 1984. Injection of mice with antibody to interferon renders peritoneal macrophages permissive for vesicular stomatitis virus and encephalomyocarditis virus. Proc. Natl. Acad. Sci. USA 81:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohuslav, J., V. V. Kravchenko, G. C. Parry, J. H. Erlich, S. Gerondakis, N. Mackman, and R. J. Ulevitch. 1998. Regulation of an essential innate immune response by the p50 subunit of NF-κB. J. Clin. Investig. 102:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bours, V., G. Franzoso, V. Azarenko, S. Park, T. Kanno, K. Brown, and U. Siebenlist. 1993. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72:729–739. [DOI] [PubMed] [Google Scholar]
- 9.Compans, R. W., and D. H. Bishop. 1985. Biochemistry of arenaviruses. Curr. Top. Microbiol. Immunol. 114:153–175. [DOI] [PubMed] [Google Scholar]
- 10.Connolly, B. M., A. B. Jenson, C. J. Peters, S. J. Geyer, J. F. Barth, and R. A. McPherson. 1993. Pathogenesis of Pichinde virus infection in strain 13 guinea pigs: an immunocytochemical, virologic, and clinical chemistry study. Am. J. Trop. Med. Hyg. 49:10–24. [DOI] [PubMed] [Google Scholar]
- 11.Docke, W. D., F. Randow, U. Syrbe, D. Krausch, K. Asadullah, P. Reinke, H. D. Volk, and W. Kox. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678–681. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler, M. A., K. Wernke-Dollries, and J. M. Stark. 1996. Inhibition of viral replication reverses respiratory syncytial virus-induced NF-κB activation and interleukin-8 gene expression in A549 cells. J. Virol. 70:9079–9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortini, M. E., and S. Artavanis-Tsakonas. 1994. The Suppressor of Hairless protein participates in Notch receptor signaling. Cell 79:273–282. [DOI] [PubMed] [Google Scholar]
- 14.Frankenberger, M., A. Pforte, T. Sternsdorf, B. Passlick, P. A. Baeuerle, and H. W. Ziegler-Heitbrock. 1994. Constitutive nuclear NF-κB in cells of the monocyte lineage. Biochem. J. 304:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa, T., M. Kawaichi, N. Matsunami, H. Ryo, Y. Nishida, and T. Honjo. 1991. The Drosophila RBP-Jκ gene encodes the binding protein for the immunoglobulin Jκ recombination signal sequence. J. Biol. Chem. 266:23334–23340. [PubMed] [Google Scholar]
- 16.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune response. Annu. Rev. Immunol. 16:225–260. [DOI] [PubMed] [Google Scholar]
- 17.Grumont, R. J., and S. Gerondakis. 1994. The subunit composition of NF-κB complexes changes during B-cell development. Cell Growth Differ. 5:1321–1331. [PubMed] [Google Scholar]
- 18.Hamaguichi, Y., Y. Yamamoto, H. Iwanari, S. Maruyama, T. Furukawa, N. Matsunami, and T. Honjo. 1992. Biochemical and immunological characterization of the DNA binding protein (RBP-Jκ) to mouse Jκ recombination signal sequence. J. Biochem. 112:314–320. [DOI] [PubMed] [Google Scholar]
- 19.Hassett, D. E., J. Zhang, and J. L. Whitton. 1997. Neonatal DNA immunization with a plasmid encoding an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J. Virol. 71:7881–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honjo, T. 1996. The shortest path from the surface to the nucleus: RBP-Jk/Su(H) transcription factor. Genes Cells 1:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh, J. J., S. Zhou, L. Chen, D. B. Young, and S. D. Hayward. 1999. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc. Natl. Acad. Sci. USA 96:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahrling, P. B., R. A. Hesse, J. B. Rhoderick, M. A. Elwell, and J. B. Moe. 1981. Pathogenesis of a Pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect. Immun. 32:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda, K., B. Kempkes, G. W. Bornkamm, A. von Gabain, and T. Decker. 1999. The Epstein-Barr virus nuclear antigen 2 (EBNA2), a protein required for B lymphocyte immortalization, induces the synthesis of type I interferon in Burkitt’s lymphoma cell lines. Biol. Chem. 380:213–221. [DOI] [PubMed] [Google Scholar]
- 24.Kannabiran, C., X. Zeng, and L. D. Vales. 1997. The mammalian transcriptional repressor RBP (CBF1) regulates interleukin-6 gene expression. Mol. Cell. Biol. 17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao, H.-Y., P. Ordentlich, N. Koyano-Nakagawa, Z. Tang, M. Downes, C. R. Kintner, R. M. Evans, and T. Kadesh. 1998. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 12:2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karin, M., and Y. Ben Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 27.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228–231. [DOI] [PubMed] [Google Scholar]
- 28.Kawaichi, M., C. Oka, S. Shibayama, A. E. Koromilas, N. Matsunami, Y. Hamaguchi, and T. Honjo. 1992. Genomic organization of mouse Jκ recombination signal binding protein (RBP-Jκ) gene. J. Biol. Chem. 267:4016–4022. [PubMed] [Google Scholar]
- 29.Krauer, K. G., D. K. Belzer, D. Liaskou, M. Buck, S. Cross, T. Honjo, and T. Sculley. 1998. Regulation of Interleukin-1β transcription by Epstein-Barr virus involves a number of latent proteins via their interaction with RBP. Virology 252:418–430. [DOI] [PubMed] [Google Scholar]
- 30.Ling, P. D., J. J.-D. Hsieh, I. K. Ruf, D. R. Rawlins, and D. Hayward. 1994. EBNA2 up regulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF-1. J. Virol. 68:5375–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukashevich, I. S., R. Maryankova, A. S. Vladyko, N. Nashkevich, S. Koleda, M. Djavani, D. Horejsh, N. N. Voitenok, and M. S. Salvato. 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J. Med. Virol. 59:552–560. [PMC free article] [PubMed] [Google Scholar]
- 32.Marianneau, P., A. Cardona, L. Edelman, V. Deubel, and P. Despres. 1997. Dengue virus replication in human hepatoma cells activates NF-κB, which in turn induces apoptotic cell death. J. Virol. 71:3244–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez, C., M. Delgado, D. Pozo, J. Leceta, J. R. Calvo, D. Ganea, and R. P. Gomariz. 1998. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J. Leukoc. Biol. 63:591–601. [DOI] [PubMed] [Google Scholar]
- 34.May, M. J., and S. Shosh. 1997. Rel/NF-κB and IκB proteins: an overview. Semin. Cancer Biol. 8:63–73. [DOI] [PubMed] [Google Scholar]
- 35.Oka, C., T. Nakano, A. Wakeham, C. Mori, T. Sakai, S. Okazaki, M. Kawaichi, K. Shiota, T. Mak, and T. Honjo. 1995. Disruption of the mouse RBP-Jκ gene results in early embryonic death. Development 121:3291–3301. [DOI] [PubMed] [Google Scholar]
- 36.Oswald, F., S. Liptay, G. Adler, and R. M. Schmid. 1998. NF-κB2 is a putative target gene of activated Notch-1 via RBP-Jκ. Mol. Cell. Biol. 18:2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pahl, H. L., and P. A. Baeuerle. 1995. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Virol. 69:1480–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters, C. J., P. B. Jahrling, C. T. Liu, R. H. Kenyon, K. T. McKee, Jr., and J. G. Barrera Oro. 1987. Experimental studies of arenaviral hemorrhagic fevers. Curr. Top. Microbiol. Immunol. 134:5–68. [DOI] [PubMed] [Google Scholar]
- 39.Peters, C. J., C. T. Liu, G. W. Anderson, Jr., J. C. Morrill, and P. B. Jahrling. 1989. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev. Infect. Dis. 11(Suppl. 4):S743–S749. [DOI] [PubMed] [Google Scholar]
- 40.Plaksin, D., P. A. Baeuerle, and L. Eisenbach. 1993. KBF1 (p50 NF-κB homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J. Exp. Med. 177:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248–253. [DOI] [PubMed] [Google Scholar]
- 42.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Altschuler, Y., T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039–1043. [DOI] [PubMed] [Google Scholar]
- 43.Tun, T., Y. Hamaguchi, N. Matsunami, T. Furukawa, T. Honjo, and M. Kawaichi. 1994. Recognition sequence of a highly conserved DNA binding protein RBP-Jκ. Nucleic Acids Res. 22:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, L., K. Marriott, and J. F. Aronson. 1999. Sequence analysis of the small RNA segment of guinea pig-passaged Pichinde virus variants. Am. J. Trop. Med. Hyg. 61:220–225. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, and S. D. Hayward. 2000. A role for SKIP in EBNA2 activation of CBF1-repressed promoters. J. Virol. 74:1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, A. Miyamoto, G. Weinmaster, and S. D. Hayward. 2000. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC To facilitate NotchIC function. Mol. Cell. Biol. 20:2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]