Abstract
Parvovirus B19 is the causative agent of erythema infectiosum. In addition, parvovirus B19 infection may be associated with other disease manifestations, namely, thrombocytopenia or granulocytopenia, spontaneous abortion or hydrops fetalis in pregnant women, acute and chronic arthritis, and systemic lupus erythematosus. Based on sequence homology data, a phospholipase A2 motif has been identified in the VP1 unique region of parvovirus B19. (Y. Li et al., J. Gen. Virol. 82:2821-2825, 2001; Z. Zadori et al., Dev. Cell 1:291-302, 2001). We have established a new in vitro assay based on electrospray ionization tandem mass spectroscopy to show that phospholipase A2 activity is present in the VP1 unique region produced in Escherichia coli and in virus-like particles consisting of combinations of VP1 and VP2 proteins expressed by recombinant baculovirus. The enzyme activity of the VP1 unique region showed typical Ca2+ dependency and could be inhibited by manoalide and 4-bromophenacylbromide, which bind covalently to lysine and histidine residues, respectively, as part of the active center of the enzyme. By using subfragments, we demonstrated an association between the phospholipase A2-like activity and the carboxy-terminal domain of the VP1 unique region.
Infection with the human parvovirus B19 usually results in a mild disease known as erythema infectiosum, or fifth disease (M. J. Anderson, S. E. Jones, H. S. Fisher, E. Lewis, S. M. Hall, C. L. Bartlett, B. J. Cohen, P. P. Mortimer, and M. S. Pereira, Letter, Lancet i:1378, 1983.) Frequently, the patients develop arthralgias, which may become long lasting and can develop into severe chronic arthritis (7, 10, 15, 19, 22, 23). B19 virus infection may further be associated with granulocytopenia or thrombocytopenia (18, 21). During pregnancy, the virus may be transmitted to the fetus and may cause hydrops fetalis and fetal loss or spontaneous abortion (5).
The icosahedral capsid of parvovirus B19 consists of two structural proteins, VP1 (83 kDa) and VP2 (58 kDa), which are identical except for 227 amino acids (aa) at the amino-terminal end of the VP1 protein (the VP1 unique region). The capsid protein VP1 has a variety of other functions that are important for the viral life cycle, because neutralizing antibodies interact preferentially with the amino-terminal part of the VP1 unique region (9) and globoside, a glycosphingolipid which has been identified as a cellular B19 virus receptor, binds to the region formed by aa 577 to 677, which are present in both the VP1 and VP2 capsid proteins (4). According to homology studies, a phospholipase A2 motif is present in the amino acid sequence of the VP1 unique region spanning positions 130 to 195 (Fig. 1) that was initially observed by Li et al. (13) and Zadou and coworkers (25). Amino acid residues at positions 153 (His), 157 (Tyr), 168 (Tyr), and 195 (Asp) are proposed to form the catalytic network for the enzymatic activity (1, 16). Residues 130 (Tyr), 132 (Gly), 134 (Gly), and 154 (Asp) are thought to be important for binding calcium ions, and residue 162 (Lys) is thought to be important for binding to phospholipid environments, e.g., cellular membranes or membrane compartments (2, 11, 24).
FIG. 1.
Sequence homology of the VP1 unique region with different phospholipases A2 (PLA2 IB, from bovine pancreas; PLA2 IIC, from a rat; PLA2, from bee venom) (1) and schematic representation of the location of functional activities in the sequence of the VP1 protein. The VP1 protein consists of the VP1 unique region (aa 1 to 227) and the VP2 protein (aa 227 to 780). The hypothesized phospholipase A2 motif ranges from aa 130 to 195 in the VP1 unique region and the VP1N-B fragment. A potential globoside binding site is in the region from aa 577 to 677. The amino acid sequence of the VP1 unique region from positions 130 to 195 shows the important catalytic residues that are observed (shaded gray), the residues important for binding of Ca2+ (arrows), the residues forming the catalytic network (circles), and the phospholipid binding site (squares).
Phospholipases A2 are found in many different organisms and organs as intracellular or secretory enzymes, e.g., bee venom or pancreatic phospholipase A2. Their functions can be varied even though their enzyme activity is restricted to the hydrolysis of phospholipid esters at the sn-2 position to fatty acids and lysophospholipids. One important function is the cleavage of phospholipids to arachidonic acid, which is used as an intermediate for the synthesis of the eicosanoids, prostaglandins, and leukotrienes that play an important role in inflammatory reactions.
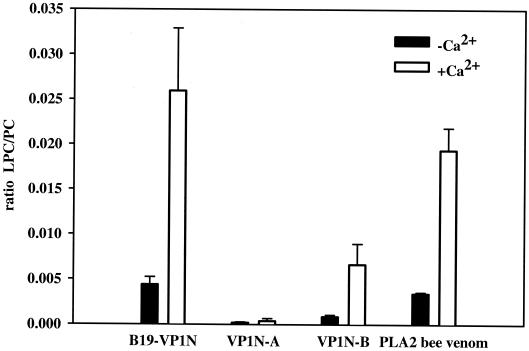
In order to show the association of the phospholipase A2 activity with the VP1 protein, we expressed the genome segment spanning the VP1 unique region in Escherichia coli and purified the resultant protein domain comprised of 227 aa by using affinity chromatography (8). The phospholipase A2 assay was based on the use of β-arachidonyl-γ-stearoyl-phosphatidylcholine (PC) liposomes (Sigma-Aldrich, Deisenhofen, Germany) as the substrate. Analysis by electrospray ionization tandem mass spectrometry revealed exclusive γ-stearoyl-lysophosphatidylcholine (LPC) as the cleavage product for both the VP1 unique region and the bee venom phospholipase A2 (Sigma-Aldrich) that was used for standardization and comparison. The intensity ratio of hydrolyzed products to substrate (LPC to PC) was used to determine the degree of phospholipase A2 activity. The activities for both enzymes were shown to be dependent upon the presence of Ca2+ (10 mM), as indicated by the increase of the LPC/PC ratio from 0.008 to 0.035 (Fig. 2). In order to obtain comparable activities for both enzymes, we had to use concentrations of the VP1 unique region (1 μM) that were higher than those of the bee venom phospholipase A2 (0.017 μM) (Fig. 2). The reaction terminated when about 10% of the substrate was hydrolyzed (10 min at 37°C).
FIG. 2.
Phospholipase A2 assay. Comparison of the phospholipase A2 activities of the VP1 unique region (B19-VP1N) and shortened subfragments of the protein domain with that of a control bee venom phospholipase A2. The VP1 unique region (B19-VP1N), subfragments VP1N-A and VP1N-B (1 μM), and the bee venom phospholipase A2 (0.017 μM) were incubated at 37°C for 10 min with or without 10 mM calcium chloride. The basic reaction mixture was comprised of 100 μl of phospholipid liposomes containing 25 or 50 nM PC in 10 mM Tris buffer (pH 8.5). The reaction was stopped by the addition of methanol-chloroform (2:1 [vol/vol]). Lipids were extracted according to the method of Bligh and Dyer (3). The dried lipid extracts were dissolved in methanol-chloroform (3:1 [vol/vol]) containing 10 mM ammonium formate and characterized by electrospray ionization tandem mass spectrometry. We used a Quattro LC triple-quadrupole mass spectrometer (Micromass, Manchester, United Kingdom) with the following settings: capillary, 3.5 kV; cone, 41 V; collision energy, 24 V; and collision gas pressure, 1.3 10−3 torr. Samples were injected at a constant flow rate of 75 μl/min with the Waters (Milford, Mass.) Alliance model 2790 filtration system and analyzed by a parent scan of m/z 184 specific for phosphocholine-containing lipids (6). The intensity of the ratio of hydrolyzed product (LPC) to substrate (PC) was used to determine the degree of phospholipase A2 activity. Standard deviations are indicated by bars.
To further characterize the phospholipase A2 activity of the VP1 unique region of parvovirus B19, we expressed and purified subfragments of the protein domain (VP1N-A, aa 1 to 121; VP1N-B, aa 91 to 227) (Fig. 1). Both subfragments were tested with respect to their phospholipase A2 activity (Fig. 2). The carboxy-terminal fragment VP1N-B displayed an enzyme activity that was lower than that of the full-length VP1 unique region. This indicates that fragment VP1N-B is sufficient to support the phospholipase A2 activity, since it contains all the important catalytic sites. The shortened fragment VP1N-B showed a dependence on Ca2+ similar to that of the full-length VP1 unique region (Fig. 2). The amino-terminal fragment VP1N-A showed no reactivity whatsoever. Since VP1N-A was produced and purified by the same method used for the VP1 unique region and VP1N-B, it is obvious that the enzyme activity observed was not caused by contaminating proteins or other factors. To exhibit maximal activity, the phospholipase domain may depend on the adjoining amino-terminal part of the protein, which may stabilize the enzyme or control the accessibility of the active center for the substrates. In this context, it may be important to mention that this amino-terminal protein domain is unique to the parvoviral enzyme here described and not part of secretory phospholipases A2 from eucaryotes (Fig. 1) (1). Its presence may be responsible for different substrate specificities and accessibilities.
Measurement of the enzyme kinetics at 25°C showed an elevated activity of the bee venom enzyme with respect to that of the VP1 unique region (Fig. 3). The phospholipase A2 activities of the VP1 unique region (1 μM) and the bee venom phospholipase A2 (0.017 μM), which was used for comparison, were inhibited in a time-dependent manner by two specific inhibitors, manoalide and 4-bromophenacylbromide (Biomol, Hamburg, Germany) (Fig. 4) (20). Manoalide covalently attaches to lysine residues, and 4-bromophenacylbromide covalently attaches to histidine residues that are part of the active center and the phospholipid binding site of the enzyme. In testing the bee venom phospholipase A2, we concluded that the enzyme activity is only partly inhibited by manoalide after 1 h of incubation (Fig. 4). Time-dependent inhibition by manoalide has also been shown for cobra venom phospholipase A2. Differences between the bee and cobra venom inhibition profiles have been linked to the different distributions of reactive lysine residues (29). The initial increase in activity after incubation of the VP1 unique region with 4-bromophenacylbromide, which could not be observed in tests of the bee venom enzyme, may be due to a delay in the substrate's access to the catalytic site of the enzyme. This may be caused by the additional amino-terminal protein domain present in the VP1 unique region. This region contains several histidine residues, which may intercept the inhibitor molecules, thus delaying the inhibitory effect. Alternatively, temporary protein stabilization of the catalytic site before inhibition occurs may have contributed to this effect.
FIG. 3.
Kinetics showing the reactivities of the VP1 unique region (2 μM) (B19-VP1N) and bee venom phospholipase A2 (0.034 μM) (PLA2 bee venom). The proteins were incubated at 25°C for 32 min in the presence of 10 mM calcium chloride. The reaction was stopped after 0, 0.5, 1, 2, 4, 8, 16, and 32 min, and the LPC/PC ratio was determined.
FIG. 4.
Inhibition of the phospholipase A2 activity with manoalide (MLD) and 4-bromophenacylbromide (BPB). The VP1 unique region (1 μM) (VP1N) and the bee venom phospholipase A2 (0.017 μM) (PLA2) were preincubated with 100 μM MLD and 1 mM BPB for 1, 2, 3, 4, and 5 h. The negative sample (neg) was incubated for 5 h without inhibitor. After incubation, the LPC/PC ratio was determined and calculated.
In further experiments, we tested virus-like particles consisting of either mixtures of VP1 and VP2 proteins or of VP2 proteins alone for their phospholipase A2 activity (Fig. 5). Particle production was performed with recombinant baculoviruses that were produced by the Bac-to-Bac system (Life Technologies, Karlsruhe, Germany) using pFast Bac DUAL vectors. The capsids were purified by cesium chloride centrifugation. The VP2 capsids showed no enzyme activity at all. The LPC/PC ratio produced by VP1-VP2 capsids (0.0035) was about 10 times lower than that produced by the VP1 unique region (0.035), even though equal protein concentrations (1 μM) were used. The lower activity of the virus-like particles might be due to the low proportion of the VP1 protein, which represents only 10 to 30% of the total protein content in VP1-VP2 capsids. Furthermore, the correct distribution of the VP1 protein in the particles may be important for the phospholipase A2 activity. The reason that parvovirus B19 has phospholipase A2 activity as part of the virion remains unexplained. It is not associated with any potential receptor-destroying activity, since the globoside incorporated into the liposomes used for the enzyme assay was not hydrolyzed by the phospholipase A2 (data not shown). The enzyme might be necessary for viral entry because it binds to a potential coreceptor. This hypothesis is supported by the fact that globoside alone cannot be responsible for the cell tropism of the B19 virus, since the glycosphingolipid is present not only on erythroid precursor cells but also on endothelial cells or megakaryocytes that are not permissive for productive B19 virus infection (18). Furthermore, phospholipase A2 activity might be necessary during the process of virus release that may be mediated by the secretory pathway for phospholipases A2. It has been shown that phospholipase A2 is secreted from cells after stimulation with interleukin 6 and tumor necrosis factor alpha (17). After release, the enzymes are targeted to cells by binding to a specific phospholipase A2 receptor, undergo endocytosis, and are transported to the nucleus (12). Parvovirus B19 could use the phospholipase A2 pathway for its own purpose by entering the cell, undergoing transport to the nucleus, and leaving the cell after interleukin 6 stimulation mediated by the viral NS1 protein (14).
FIG. 5.
Comparison of enzyme activities of the VP1 unique region (1 μM protein) (B19-VP1N), recombinant VP1-VP2 capsids (1 μM protein), and VP2 capsids (1 μM protein).
With respect to patients suffering from acute or persistent B19 virus infection, the presence of the viral phospholipase A2 may contribute to inflammatory and autoimmune processes and therefore might be associated with the pathogenesis of parvovirus B19.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft grant Mo620/5-3.
We thank Wolfgang Jilg (Institute for Medical Microbiology, Universität Regensburg) for extremely helpful discussions. In addition, we thank Sean Doyle, National University of Ireland, Dublin, Ireland, for critically reading the manuscript.
REFERENCES
- 1.Arni, R. K., and R. J. Ward. 1996. Phospholipase A2—a structural review. Toxicon 34:827-841. [DOI] [PubMed]
- 2.Bekkers, A. C., P. A. Franken, E. Toxopeus, H. M. Verheij, and G. H. de Haas. 1991. The importance of glycine-30 for enzymatic activity of phospholipase A2. Biochim. Biophys. Acta 1076:374-378. [DOI] [PubMed] [Google Scholar]
- 3.Bligh, E. G., and W. J. Dyer. A rapid method of total lipid extraction and purification. 1956. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 5.Brown, T., A. Anand, L. D. Ritchie, J. P. Clewley, and T. M. Reid. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033-1034. [DOI] [PubMed] [Google Scholar]
- 6.Brugger, B., G. Erben, R. Sandhoff, F. T. Wieland, and W. D. Lehmann. 1997. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 94:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassinotti, P., G. Siegl, B. A. Michel, and P. Bruhlmann. 1998. Presence and significance of human parvovirus B19 DNA in synovial membranes and bone marrow from patients with arthritis of unknown origin. J. Med. Virol. 56:199-204. [PubMed] [Google Scholar]
- 8.Dorsch, S., B. Kaufmann, U. Schaible, E. Prohaska, H. Wolf, and S. Modrow. 2001. The VP1-unique region of parvovirus B19: amino acid variability and antigenic stability. J. Gen. Virol. 82:191-199. [DOI] [PubMed] [Google Scholar]
- 9.Gigler, A., S. Dorsch, A. Hemauer, C. Williams, S. Kim, N. S. Young, S. Zolla-Pazner, H. Wolf, M. K. Gorny, and S. Modrow. 1999. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J. Virol. 73:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemauer, A., A. Gigler, K. Searle, K. Beckenlehner, U. Raab, K. Broliden, H. Wolf, G. Enders, and S. Modrow. 2000. Prevalence of NS1-specific antibodies in patients infected with parvovirus B19 and in pregnant women. J. Med. Virol. 60:48-55. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers, O. P., R. Dijkman, C. E. Pals, H. M. Verheij, and G. H. de Haas. 1989. Evidence for the involvement of tyrosine-69 in the control of stereospecificity of porcine pancreatic phospholipase A2. Protein Eng. 2:467-471. [DOI] [PubMed] [Google Scholar]
- 12.Lambeau, G., and M. Lazdunski. 1999. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 20:162-170. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y., Z. Zadori, H. Bando, R. Dubuc, G. Fediere, J. Szelei, and P. Tijssen. 2001. Genome organization of the densovirus from Bombyx mori (BmDNV-1) and enzyme activity of its capsid. J. Gen. Virol. 82:2821-2825. [DOI] [PubMed] [Google Scholar]
- 14.Moffatt, S., N. Tanaka, K. Tada, M. Nose, M. Nakamura, O. Muraoka, T. Hirano, and K. Sugamura. 1996. A cytotoxic nonstructural protein, NS1, of human parvovirus B19 induces activation of interleukin-6 gene expression. J. Virol. 70:8485-8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, T. L. 2000. Parvovirus-associated arthritis. Curr. Opin. Rheumatol. 12:289-294. [DOI] [PubMed] [Google Scholar]
- 16.Moore, T. L., R. Bandlamudi, S. M. Alam, and G. Nesher. 1999. Parvovirus infection mimicking systemic lupus erythematosus in a pediatric population. Semin. Arthritis Rheum. 28:314-318. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee, A. B., L. Miele, and N. Pattabiraman. 1994. Phospholipase A2 enzymes: regulation and physiological role. Biochem. Pharmacol. 48:1-10. [DOI] [PubMed] [Google Scholar]
- 18.Nagai, K., T. Morohoshi, T. Kudoh, Y. Yoto, N. Suzuki, and Y. Matsunaga. 1992. Transient erythroblastopenia of childhood with megakaryocytopenia associated with human parvovirus B19 infection. Br. J. Haematol. 80:131-132. [DOI] [PubMed] [Google Scholar]
- 19.Naides, S. J., L. L. Scharosch, F. Foto, and E. J. Howard. 1990. Rheumatologic manifestations of human parvovirus B19 infection in adults. Initial two-year clinical experience. Arthritis Rheum. 33:1297-1309. [DOI] [PubMed] [Google Scholar]
- 20.Nuhn, P., and K. Koch. 1993. Hemmer der Phospholipase A2. Pharmazie 48:494-508. [PubMed] [Google Scholar]
- 21.Scheurlen, W., K. Ramasubbu, O. Wachowski, A. Hemauer, and S. Modrow. 2001. Chronic autoimmune thrombopenia/neutropenia in a boy with persistent parvovirus B19 infection. J. Clin. Virol. 20:173-178. [DOI] [PubMed] [Google Scholar]
- 22.Stahl, H. D., B. Hubner, B. Seidl, U. G. Liebert, I. M. van der Heijden, B. Wilbrink, M. C. Kraan, F. Emmrich, and P. P. Tak. 2000. Detection of multiple viral DNA species in synovial tissue and fluid of patients with early arthritis. Ann. Rheum. Dis. 59:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi, Y., C. Murai, S. Shibata, Y. Munakata, T. Ishii, K. Ishii, T. Saitoh, T. Sawai, K. Sugamura, and T. Sasaki. 1998. Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 95:8227-8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Bergh, C. J., A. J. Slotboom, H. M. Verheij, and G. H. de Haas. 1989. The role of Asp-49 and other conserved amino acids in phospholipases A2 and their importance for enzymatic activity. J. Cell Biochem. 39:379-390. [DOI] [PubMed] [Google Scholar]
- 25.Zadori, Z., J. Szelei, M.-C. Lacoste, Y. Li, S. Gariepy, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]