Abstract
Wild-type (WT) influenza A/PR/8/34 virus and its variant lacking the NS1 gene (delNS1) have been compared for their ability to mediate apoptosis in cultured cells and chicken embryos. Cell morphology, fragmentation of chromatin DNA, and caspase-dependent cleavage of the viral NP protein have been used as markers for apoptosis. Another marker was caspase cleavage of the viral M2 protein, which was also found to occur in an apoptosis-specific manner. In interferon (IFN)-competent host systems, such as MDCK cells, chicken fibroblasts, and 7-day-old chicken embryos, delNS1 virus induced apoptosis more rapidly and more efficiently than WT virus. As a consequence, delNS1 virus was also more lethal for chicken embryos than WT virus. In IFN-deficient Vero cells, however, apoptosis was delayed and developed with similar intensity after infection with both viruses. Taken together, these data indicate that the IFN antagonistic NS1 protein of influenza A viruses has IFN-dependent antiapoptotic potential.
The influenza virus genome contains eight single-stranded RNA segments of negative polarity, coding for nine structural proteins and a nonstructural polypeptide designated NS1. NS1 is expressed together with the nucleocapsid protein NP soon after virus entry into the cell and acts at an early stage of infection (for a review, see reference 30). It has many regulatory functions, such as inhibition of host mRNA polyadenylation (40), inhibition of nuclear export of polyadenylated host mRNA (8), inhibition of mRNA splicing (14, 37, 56), stimulation of translation of viral RNA (2, 9, 13), and modulation of viral RNA transcription and replication (45). In addition, NS1 has the ability to bind double-stranded RNA (dsRNA) and thus to prevent intracellular dsRNA-activated protein kinase R (PKR) activation (20, 37). It was also shown that NS1 directly interacts with PKR, suppressing its function (52). Recently the important observation has been made that the NS1 gene is not absolutely necessary for virus replication. Influenza virus lacking this gene partly or completely was able to replicate efficiently in hosts defective in interferon (IFN) production, whereas replication of delNS1 virus (influenza A/PR/8/34 virus variant lacking the NS1 gene) in IFN-competent cells was significantly reduced (12, 17). In a similar manner delNS1 virus replicated effectively in host organisms lacking PKR (4, 7). This replication phenotype of delNS1 virus has been explained by its inability to shut off activation of the IFN-PKR system (7, 17, 51). On the basis of these data it has been concluded that the NS1 protein is an antagonist of the IFN system.
Influenza virus induces apoptosis in infected cells (24, 48). It has been observed recently that the caspase 8 pathway is involved in influenza virus-mediated apoptosis (3) and that the viral nucleocapsid protein NP is specifically cleaved by host cell caspases into the truncated form aNP (62). Another interesting observation was that PKR sensitizes cells to apoptosis induced by influenza virus (3). The observations that PKR is a common link in the signaling pathways leading to IFN induction and apoptosis, that NS1 interferes with the IFN-PKR system, and that it directly inhibits PKR raised the question whether NS1 may be involved in regulation of apoptosis in influenza virus-infected cells.
In order to test this hypothesis the wild type (WT) of influenza A/PR/8/34 virus and its variant lacking the NS1 gene (delNS1) were compared for the ability to mediate apoptosis in cultured cells and chicken embryos. We show here that delNS1 virus is a stronger inducer of apoptosis in IFN-competent MDCK and chicken fibroblasts (CF) than WT virus. Likewise, in 7-day-old chicken embryos that also contain a normal IFN system characterized only by its reduced inducibility to exogenous stimuli (39), delNS1 virus replicated effectively and caused more-intensive apoptosis and higher lethality than WT virus. However, when IFN-deficient Vero cells (10) were infected with these viruses, there was no significant difference in apoptosis. Taken together, these data indicate that NS1 has IFN-dependent antiapoptotic potential and down-regulates the apoptotic response in influenza virus-infected cells.
MATERIALS AND METHODS
Viruses and cells.
Influenza A/PR/8/34 (H1N1) WT virus and the mutant lacking the NS1 gene (12, 17) were propagated in 9- and 7-day-old embryonated chicken eggs, respectively. Eggs were infected with about 1,000 PFU and incubated for 50 h at 37°C. Appropriate dilutions of virus-containing allantoic fluid were used for the infection of cultured cells. MDCK cells, primary cultures of CF prepared from 10-day-old embryos (60), and Vero cells were grown as monolayers in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum (GIBCO-BRL, Karlsruhe, Germany). For infection, 2-day-old confluent cell monolayers were incubated with egg-grown influenza virus (multiplicity of infection [MOI], 1 to 4 PFU/cell) for 1 h at 37°C. After infection, cells were washed and incubated with Dulbecco's minimal essential medium without serum. Infected cells were incubated at 37°C for different periods of time and were then prepared either for light microscope examination, protein gel electrophoresis, or DNA fragmentation analysis.
Polyacrylamide gel electrophoresis (PAGE).
Polypeptide electrophoresis in polyacrylamide gels with Tris-glycine buffer containing sodium dodecyl sulfate (SDS) were described earlier (61). Analyzed samples were dissolved in buffer containing 10% glycerol, 1% SDS, and 10 mM dithiothreitol and boiled for 4 min at 96°C. Running gels (10% polyacrylamide) were used to separate NP and aNP. To distinguish between the 14- and the 12-kDa forms of M2, 14% polyacrylamide gels were used. Under these conditions, the NP and aNP bands overlapped when analyzed by Western blotting (WB). For protein gel staining with Coomassie blue R-350, the protocol recommended by Pharmacia (Freiburg, Germany) was applied.
WB analysis.
After SDS-PAGE, the polypeptides were transferred from the gel onto Protran-nitrocellulose membranes (pore size, 0.45 μm; Schleicher & Schuell, Dassel, Germany) by semidry electroblotting with a Tris-HCl ɛ-aminocaproic acid buffer system (pH, ∼9.8) (62). Membranes were then washed with 150 mM phosphate-buffered saline (PBS) and incubated overnight at 4°C in 10% dried milk in PBS. After washing with PBS, membranes were incubated for 2 h at room temperature in PBS containing 0.5% bovine serum albumin and either anti-NP monoclonal antibodies (clone A3; Centers for Disease Control and Prevention, Atlanta, Ga.), anti-M1 goat antibodies (Virostat), or anti-M2 goat antibody (G-74; donated by A. Hay, Mill Hill, United Kingdom). After that, membranes were washed five times with PBS and exposed to horseradish peroxidase (HRP)-conjugated secondary anti-species antibodies (Dako, Glostrup, Denmark), followed by visualization of positive bands with the Pierce (Rockford, Ill.) enhanced chemiluminescence (ECL) procedure by using Kodak BioMax film.
DNA fragmentation analysis.
For the isolation of low-molecular-weight cellular DNA, the SDS-high-salt extraction method has been used (25). Mock-infected and influenza virus-infected cells were removed with a rubber policeman and pelleted for 15 min at 1,500 × g. Cell pellets (∼106 cells) were suspended in 80 μl of PBS and gently mixed with 300 μl of buffer containing 10 mM Tris-HCl (pH 7.6), 10 mM EDTA, and 0.6% SDS. In the case of chicken eggs, 300 μl of allantoic fluid was mixed with EDTA and SDS at final concentrations of 10 mM and 0.6%, respectively (final volume, 350 μl). Then, cell and allantoic fluid lysates were mixed with 100 μl of 5 M NaCl and incubated overnight at 4°C. The mixtures were centrifuged at 14,000 rpm for 20 min at 4°C, and supernatants were treated sequentially with RNase A (1 mg/ml) and proteinase K (0.2 mg/ml) for 20 min at 37°C and then mixed with 2 volumes of ethanol and left overnight at −20°C. Precipitated DNA was resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 7.6), electrophoresed in 1.5% agarose prepared in Tris-borate-EDTA (TBE) buffer, and stained with ethidium bromide. MassRuler DNA Ladder mixes (MBI Fermentas) were used as molecular weight markers.
Sedimentation analysis.
Four hundred microliters of virus-containing allantoic fluid was clarified at 2,000 rpm for 20 min and loaded onto 400 μl of 40% glycerol prepared in PBS containing 10 mM Tris-HCl (pH 7.6) and centrifuged at 30,000 rpm (∼100,000 × g) in a TLS-55 rotor (Beckman TL-100). After ultracentrifugation, the combined supernatant (glycerol and allantoic fluid; 800 μl) was withdrawn, and the pellet was suspended in 200 μl of PBS. Equivalent amounts (10 μl of pellet and 40 μl of supernatant fractions) were loaded onto SDS-containing gels for PAGE analysis.
RESULTS
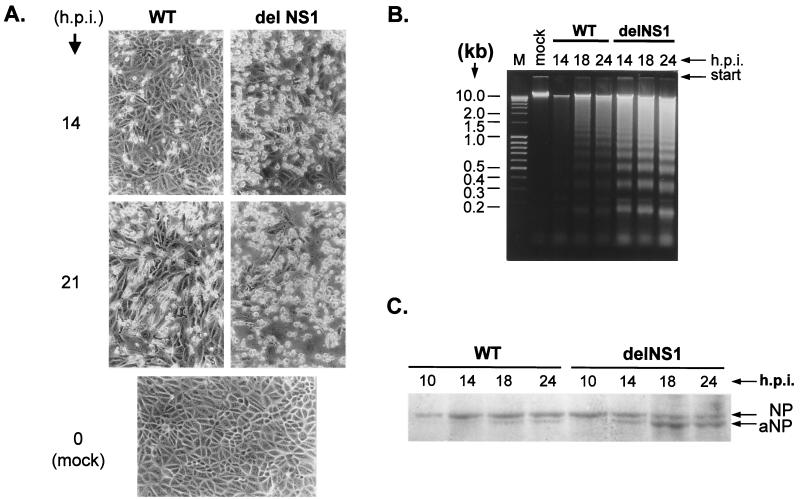
In this study, we first looked for the development of apoptosis in MDCK cells and CF. The cells are highly susceptible to infection with A/PR/8/34 WT virus but produce very little virus when infected with delNS1 (17). Apoptosis has been analyzed by monitoring shrinking and detachment of cells from support matrix as well as chromatin DNA fragmentation (DNA laddering). In addition, proteolysis of the nucleocapsid protein NP by host caspases has been used as a marker of apoptosis (62). Figure 1A shows the effects of virus infection on the morphology of MDCK cells monolayers. At 14 h after infection with WT virus, monolayers were still largely intact, whereas about 70% of the cells shrank and detached after 21 h. The same degree of apoptosis was seen already 14 h after infection with delNS1 virus, and only very few cells were still attached to the plastic dish after 21 h. Basically the same observations have been obtained with CF cells (not shown). Figure 1B shows the DNA fragmentation patterns obtained from infected MDCK cells. Low-molecular-weight host DNA was isolated at different times after infection from equal amounts of cells and analyzed by agarose electrophoresis. The typical ladder of 0.2- to 10-kb DNA fragments became visible 18 h after infection with WT virus and remained still relatively weak after 24 h. In contrast, after infection with delNS1 virus the ladder was already clearly seen after 14 h and later still increased in intensity. Similar differences were observed when the kinetics of the conversion of NP into aNP were compared in WT- and delNS1-infected cells (Fig. 1C). aNP appeared in cells infected with WT virus at 18 h postinfection (hpi) and was always present in smaller amounts than NP, whereas in delNS1-infected cells aNP was clearly visible already at 12 to 14 hpi and became the predominant form of the nucleocapsid protein at the late stages of infection (18 to 20 hpi). These data indicate that host caspases responsible for NP cleavage and formation of aNP are activated earlier in delNS1- than in WT-infected cells. Similar observations were made when cell morphology, DNA fragmentation, and aNP accumulation as depending on infection with WT and delNS1 virus were analyzed in CF cells (not shown). Taken together, these data clearly indicate that delNS1 virus causes earlier and more intensive apoptosis in MDCK and CF cells than WT virus, suggesting that the NS1 protein has antiapoptotic potential.
FIG. 1.
Apoptosis in MDCK cells infected with WT and delNS1 viruses (A) Shrinkage and death of MDCK cells after infection with WT and delNS1 influenza viruses. MDCK cells were infected with WT and delNS1 influenza A/PR/8/34 (H1N1) viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, cell cultures were examined by light microscopy. (B) DNA laddering in MDCK cells infected with WT and delNS1 influenza viruses. MDCK cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, host cell chromatin DNA was isolated by SDS-high-salt extraction. Equal amounts of DNA were analyzed by electrophoresis on 1.6% agarose gels followed by ethidium bromide staining. (C) Conversion of NP into its proteolytic cleavage product aNP. MDCK cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, cellular polypeptides were analyzed by electrophoresis in 10% polyacrylamide gels followed by gel staining with Coomassie blue R-350. For better detection of the NP bands, the delNS1 samples contained twice as much material as the WT samples.
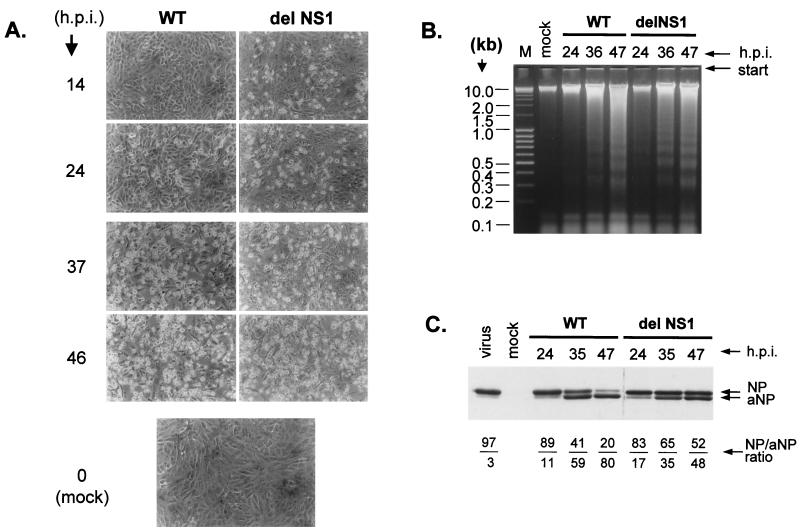
It was then of interest to analyze apoptosis in Vero cells, since these cells have deleted alpha and beta IFN1 genes (10) and therefore allow replication of WT and delNS1 virus to similarly high titers (17). Unlike MDCK and CF cells, Vero cells did not show large differences in the extent of apoptosis after infection with both viruses. This was quite evident when cell shrinking, rounding, and detachment (Fig. 2A) and DNA laddering (Fig. 2B) were compared. Figures 2A and B show also that apoptosis developed in Vero cells at a significantly lower rate than in MDCK and CF cells. Whereas delNS1 virus caused prominent apoptosis in MDCK cells already at 12 to 20 h after infection (hpi), intensive cell rounding and detachment, marked DNA laddering, and NP cleavage developed at 35 to 40 hpi in Vero cells. There were some differences between delNS1 and WT virus, when cleavage of NP was analyzed in Vero cells (Fig. 2C). First, the level of NP accumulation in Vero cells was comparable to the NP level in MDCK cells, indicating similar rates of virus replication in both host systems. Second, as indicated by the ratios of NP to aNP, cleavage rates were reduced at late stages of infection with delNS1 virus. The possible significance of this observation will be discussed below. Taken together, however, the data shown in Fig. 2 clearly indicate that the rapid and intensive apoptosis mediated by NS1 in IFN-competent MDCK cells and CF does not occur in IFN-deficient Vero cells. Thus, it is possible that the antiapoptotic effect of NS1 is IFN dependent.
FIG. 2.
Apoptosis in Vero cells infected with WT and delNS1 viruses. (A) Shrinkage and death of Vero cells after infection with WT and delNS1 viruses. Vero cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, cell cultures were examined by light microscopy. (B) DNA laddering in Vero cells infected with WT and delNS1 viruses. Vero cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, host cell chromatin DNA was isolated by SDS-high-salt extraction. Equivalent amounts of DNA were analyzed by electrophoresis on 1.6% agarose gels followed by ethidium bromide staining. (C) Conversion of NP to aNP. Vero cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, cellular proteins were analyzed by PAGE-WB. For better detection of the NP bands, the delNS1 samples contained twice as much material as the WT samples. NP and aNP polypeptides were identified with an anti-NP mouse monoclonal antibody (clone A3) and an anti-mouse HRP conjugate developed by ECL. WBs were scanned, and the NP/aNP ratios were calculated by the TINA program (version 2.09; Isotopenmessgeraete GmbH) (below the gel).
We then compared synthesis of viral proteins in MDCK and Vero cells infected with WT and delNS1 viruses using PAGE. As expected NS1 was detected after staining with Coomassie blue only in WT-, but not in delNS1- infected cells (not shown). NP, M1, and M2 were monitored by WB analysis using specific monoclonal antibodies (Fig. 3). NP was reduced in delNS1-infected cells by about 50% when compared to WT-infected cells. The lower amounts of NP in delNS1-infected MDCK cells can be explained by the observation that translation factors eIF2 and 4G1 are not activated when the IFN machinery is not inhibited by NS1 (2). A dramatic reduction was observed with M1 and M2. delNS1-infected MDCK and Vero cells contained less than 5% of the amounts of M1 and M2 found in cells infected with WT virus (Fig. 3A and B). These data support earlier observations stressing the necessity of NS1 for efficient expression of late viral genes (28, 55). The observation that M1 and M2 synthesis was impeded in MDCK as well as in Vero cells indicates that NS1 promotes virus growth not only as an IFN antagonist but also by up-regulating viral protein synthesis in an IFN-independent manner. As shown in Fig. 3A and B, M2 was detected as a 14- and a 12-kDa protein. The 14-kDa protein showed some heterogeneity, presumably reflecting differences in acylation (47). The 14- and 12-kDa forms of M2 have also been observed before with the WSN strain of influenza A virus (58). The 12-kDa band appeared only late in infection and then became the predominant one in delNS1-infected MDCK cells at later stages, suggesting that it was the apoptotic form of M2. It was therefore called aM2. The appearance of aM2 at the late apoptotic stage of infection prompted us to test the idea that it may be derived from M2 by host caspase cleavage, as is the case with NP and aNP.
FIG. 3.
Accumulation of NP, M1, M2, and aM2 in MDCK and Vero cells infected with WT and delNS1 viruses. MDCK cells (A) and Vero cells (B) were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At different times postinfection, cellular proteins were analyzed by PAGE-WB (14% polyacrylamide gels). The membrane was cut into three pieces, and the strips containing NP, M1, and M2 were incubated separately with mouse monoclonal anti-NP antibody (clone A3), monospecific goat anti-M1 antibody (Virostat), and monospecific goat anti-M2 antibody (G-74), respectively, as first antibodies and anti-mouse HRP (Dako) and anti-goat HRP (Sigma) conjugates developed as secondary antibodies. (C) Treatment with caspase inhibitor. MDCK cells were infected with WT and delNS1 viruses at an MOI of 2 to 4 PFU per cell. At 10 hpi, caspase 2 inhibitor (VDVAD-CMK) was added to the culture medium, and cells were incubated up to 20 hpi. Cellular polypeptides were analyzed by PAGE-WB and developed with goat anti-M2 antibody (G-74) and anti-goat HRP (Sigma) conjugate by ECL. (D) Potential caspase cleavage sites in M2 and CM2. The 97-aa sequence of the influenza A virus M2 (31) and the 139-aa sequence of influenza C virus CM2 (26) are shown. The predicted caspase proteolytic sites are shown by arrows.
Inspection of the primary structure of M2 revealed the sequence VDVDD↓G, a consensus motif of a caspase 2 cleavage site (49), at positions 84 to 90 of this 97-amino-acid (-aa) protein (31). Cleavage at this site (indicated by the arrow) would be in agreement with the rise of a 12-kDa protein. To test this hypothesis we have studied the effects of the caspase 2-specific inhibitor VDVAD-FMK on aM2 formation. Under this treatment only M2 was observed, whereas aM2 did not appear (Fig. 3C). Also, cleavage of NP into aNP did not occur under these conditions (not shown). These observations suggest that M2 and NP are cleaved by caspase 2 activated in apoptotic cells. Since caspase 2 shows some cross-reactivity for VDVAD-FMK with caspases 3 and 7 (49), we cannot exclude the possibility that the latter enzymes are also involved. It is interesting that CM2, the M2 analog of influenza C virus, has three potential caspase cleavage sites at the C terminus, while the analogous protein NB of influenza B viruses does not have such motifs (Fig. 3D).
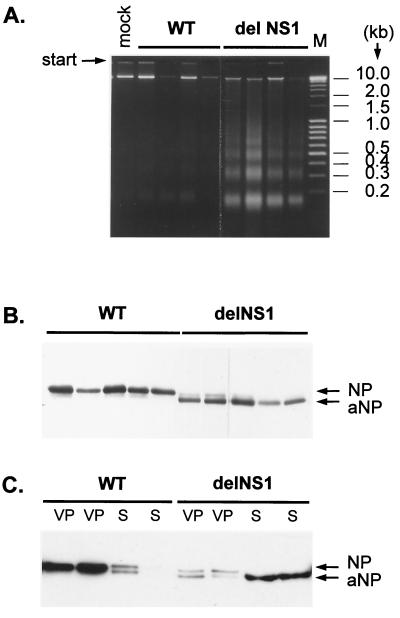
In the last set of experiments we have analyzed apoptosis in chicken embryos infected with WT and delNS1 viruses. Seven-day-old chicken eggs were used that were permissive for both viruses. At this stage of development, chicken embryos already show IFN response (39), but the IFN titers are not high enough to prevent growth of delNS1 virus as is the case in older embryos (17, 51). At 48 h after infection with different virus doses, hemagglutinin titers, NP cleavage, and DNA laddering were analyzed in allantoic fluid. Survival of embryos has also been analyzed. The data summarized in Fig. 4 show that WT virus grows to titers about 10 times higher in the chorioallantoic membrane, although it is about 100 times less lethal for the embryo than delNS1 virus (Fig. 4). As indicated by DNA fragmentation, cells present in the allantoic fluid of eggs infected with WT virus showed little apoptosis, whereas it was distinct in delNS1-infected eggs (Fig. 5A). Accordingly, NP prevailed in the allantoic cavity of eggs infected with WT, whereas predominantly apoptotic aNP accumulated after infection with delNS1 virus (Fig. 5B). To determine whether aNP is present in free or particulate form, allantoic fluid has been layered on a cushion of 40% glycerol and centrifuged at 100,000 × g for 2.5 h. Under these conditions NP obtained from WT-infected eggs was almost completely sedimentable, indicating that it is present in virions (Fig. 5C). In contrast, in delNS1-infected eggs only a minor portion of NP and aNP sedimented into the virion pellet, whereas the predominant part of aNP (more than 90%) did not pellet, suggesting that aNP accumulated either as nonsedimentable RNP or in a free state and is derived from infected cells that are destroyed by apoptosis. These results are consistent with earlier data indicating that aNP is not included into virions (59).
FIG. 4.
Virus titers in the allantoic cavity and lethality for chicken embryos. Seven-day-old chicken embryos were infected with different doses of WT and delNS1 viruses and incubated at 37°C for 48 h. Dead and living embryos were identified by the movement activity of embryos and retained blood vessel network. Eggs were cooled at 4°C, and hemagglutinin (HA) titers of allantoic fluids were determined.
FIG. 5.
DNA laddering and NP and aNP patterns in chicken eggs. Seven-day-old chicken embryos were infected in the allantoic cavity with WT and delNS1 viruses at an MOI of 20 to 100 PFU per egg. (A) At 48 hpi, samples of allantoic fluid were taken from four eggs separately, and DNA was isolated by SDS-high-salt extraction of 150 μl of allantoic fluid from each egg and analyzed by electrophoresis on a 1.6% agarose gel. Lane M, 0.4 μg of MassRuler DNA ladder mix (MBI Fermentas). (B) Samples of allantoic fluid were taken from five eggs separately and NP and aNP patterns were analyzed by PAGE-WB. (C) Allantoic fluid samples from two separate eggs were sedimented through a 40% glycerol cushion at 100,000 × g for 2.5 h. Equivalent amounts of virus pellet (VP) and supernatant (S) were analyzed by PAGE-WB. NP and aNP were identified by ECL with an anti-NP mouse antibody (clone A3) and anti-mouse HRP conjugate.
DISCUSSION
The data presented here show that the NS1 gene of influenza A virus down-regulates apoptosis of infected cells in an IFN-dependent manner. Programmed cell death has, therefore, to be included into the list of IFN-regulated restriction mechanisms of influenza virus replication, such as eIF2-dependent inhibition of protein synthesis, oligoadenylate synthetase-dependent RNA hydrolysis, and Mx-dependent virus suppression.
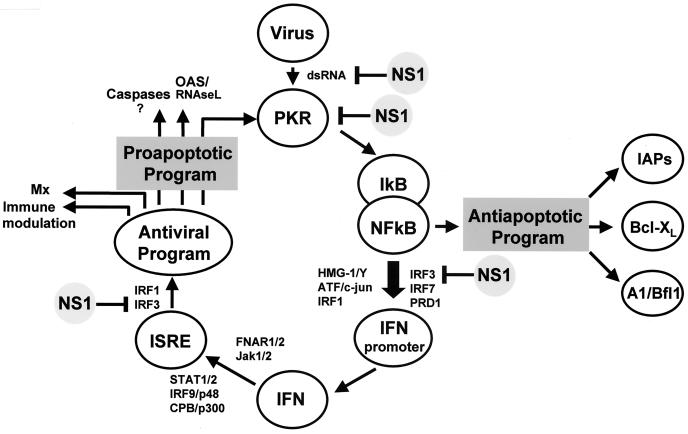
Our data suggest that the IFN machinery and the apoptosis program are coupled (Fig. 6). In the absence of a functional IFN cascade, apoptosis is delayed and its down-regulation by NS1 in infected cells is not effective. That IFN may be an essential mediator for apoptosis of virus-infected cells has also been suggested in other studies (53). However, the downstream targets in the proapoptotic program that are controlled by IFN are not yet known. It is therefore difficult to explain how NS1 down-regulates apoptosis. We can only assume that specific targets of NS1 in the IFN-cascade, such as PKR, IRF3, and NF-κB, are also related to apoptosis regulation. PKR and IRF3 are believed to promote apoptosis by activation of the so-called death-induced signaling complex (3, 23). The observations that NS1 suppresses activation of NF-κB (54) through the inhibition of PKR (37, 52) and IRF3 (50) might therefore explain that NS1 elevates the apoptotic threshold and delays onset of programmed cell death in infected cultures.
FIG. 6.
IFN-related pro- and antiapoptotic pathways down-regulated by NS1. There are two NF-κB-regulated apoptotic pathways. The first one is proapoptotic and IFN dependent. In this pathway viral dsRNA intermediates activate PKR, which initiates phosphorylation and ubiquitination of IκB, an inhibitor of NF-κB. Activated NF-κB, a transcription factor, forms intranuclear enhanceosome complexes containing HMG-1/Y, ATF (also known as c-jun), and PRD-1 as well as IRF 1, 3, and 7 to promote IFN expression. IFN triggers antiviral genes through FNAR-STAT pathways and the IFN-stimulated responsive element. Certain elements in this antiviral program, such as PKR, oligoadenylate synthetase (or RNase L,) are known to initiate apoptosis and induce caspases 1, 3, and 8 (for a review, see reference 19). This is a major cascade in normal IFN-competent cells, such as MDCK and CF cells, and its down-regulation by NS1 delays apoptosis in WT-infected cells. The second pathway is antiapoptotic. It also involves PKR-dependent activation of NF-κB that becomes available for apoptosis inhibitors (IAPs, Bcl-XL, A1/Bfl1, etc.) in IFN-deficient systems, such as Vero cells. Under these conditions, inhibition of PKR by NS1 may promote apoptosis. As a result, apoptosis develops in WT-infected cells more intensively than in delNS1-infected ones. The targets of NS1 interference are indicated.
There is evidence that NS1 may down-regulate programmed cell death in cooperation with another viral protein(s), such as the matrix protein M1 that also appears to have antiapoptotic potential (O. P. Zhirnov, T. E. Konakova, S. G. Krichevets, S. N. Iordansky, and H.-D. Klenk, Abstr. 11th Int. Conf. Neg. Strand Viruses, p. 102, 2000). Cooperation of NS1 and M1 seems to involve different antiapoptotic targets. NS1 may reduce the IFN-promoted sensitivity of cells to apoptosis (3), while M1 may interfere with host caspases (Zhirinov et al., Abstr. 11th Int. Conf. Neg. Strand Viruses), key apoptotic enzymes. A similar interaction between two viral proteins may exist with the paramyxovirus simian virus 5 (SV5). Protein V of SV5 has been found be an IFN antagonist (11, 57), whereas protein SH down-regulates apoptosis (22). It should also be mentioned here that NS1 stimulates, rather than suppresses, apoptosis when it is expressed in the absence of other viral proteins and dsRNA-inducing PKR. NS1 has been shown to be highly toxic (43) and may induce apoptosis in a stress-dependent fashion under these conditions.
Apoptosis in IFN-deficient Vero cells differed from apoptosis in MDCK and CF cells in two important aspects. First, it developed more slowly in Vero cells. Second, the intensity of apoptosis in Vero cells was generally similar with WT and delNS1 viruses. These observations support the concept that apoptosis is induced in Vero cells by alternative IFN-independent pathways not used in MDCK cells and CF. Moreover, NF-κB may trigger an antiapoptotic program in IFN-deficient Vero cells by activating BcL-XL, IAPs, and A1/Bfl1 (Fig. 6). In WT-infected Vero cells this antiapoptotic program may be restrained by the NS1-directed down-regulation of NF-κB (54). Triggering of this program may also explain why Vero cells infected with delNS1 virus showed less caspase activation at late stages of infection than did Vero cells infected with WT virus.
Apoptotic activation of caspases results in the cleavage of the NP protein (62), and we have found now that the M2 protein is also cleaved by these enzymes. M2 is a virus-specific ion channel. It is an integral membrane protein with an external N terminus and a large C-terminal cytoplasmic tail (32). Sequence and location of the putative cleavage signal as well as sensitivity to a specific inhibitor suggest that M2 cleavage involves removal of the cytoplasmic tail by caspase 2. This concept is consistent with the observation that caspase 2 is a cytoplasmic enzyme and that it is partly membrane bound (38). The role of apoptotic cleavage of NP and M2 in virus replication is not yet clear. It will be interesting to find out, for instance, if the caspase-generated peptides are involved in the development of antiviral CTL response (1) and pathogenesis of autoimmune disease (41). It has been reported that numerous autoantigens observed in autoimmune diseases are specifically cleaved by caspases during apoptosis (41). If so, this apoptosis mechanism is pertinent for influenza A and C virus ion channel proteins possessing caspase proteolytic sites at the cytoplasmic C terminus.
It was found here that delNS1 virus induces intensive apoptosis in 7-day-old chicken embryos. Furthermore, there was a high lethality after infection with this virus. In contrast, apoptosis and lethality were low when embryos were infected with WT virus, although virus titers were significantly higher under these conditions. These observations demonstrate that apoptosis, rather than virus load, may play a predominant role in determining pathogenicity.
Recently a rational approach for the generation of live vaccines containing altered NS1 has been proposed to attenuate virus virulence while retaining immunogenicity (51). The data presented here on the enhanced apoptotic potential of such viruses add a note of caution to this approach. The possibility that such vaccine viruses may have side effects due to intensive apoptotic cell injury at the primary locus of infection cannot be excluded. Thus, the respiratory epithelium may be damaged in the case of intranasal vaccine application, with a special risk for infants and aged persons having a compromised IFN status. On the other hand, it is also possible that delNS1-mediated apoptosis may play a beneficial role in vaccination, providing enhanced virus clearance by phagocytosis of infected cells (15).
delNS1 virus may also be of therapeutic value for tumor treatment. Compared to normal human fibroblasts, fibroblasts with a neoplastic phenotype and a high tumorigenic potential in vivo are more susceptible to influenza virus infection and, as indicated by extensive NP cleavage, to virus-induced apoptosis (34). It is therefore tempting to speculate that delNS1 virus may be selective in its apoptotic capacity for neoplastic cells. Better knowledge on the molecular details of the interaction between NS1 and its viral and cellular partners may also allow the design of new drugs for the selective killing of cancer cells.
Finally, it should be pointed out that NS1 of influenza A virus is not the only viral IFN antagonist. Proteins with similar function have been found in many other viruses. They include VP35 of Ebola virus (6), protein C of Sendai virus (18, 29), protein V of paramyxovirus SV5 (11), protein sigma3 of reovirus (27), protein E3L of vaccinia virus (44, 46), the NSP3 gene product of porcine rotavirus (33), proteins NS1 and NS2 of bovine respiratory viruses (42), protein E7 of human papillomavirus type 16 (5), protein E1A of adenovirus (36), protein NS5A of hepatitis C virus (16), protein IcP34.5 of herpes simplex virus (21, 35), and a number of other virus proteins. It will be interesting to see if any of these proteins down-regulates apoptosis in infected cells as does NS1.
Acknowledgments
We thank Ralf Wagner for helpful discussions and Marcus Moll and Rüttger Ebendt for help with the gel scanning and figure work. A. Egorov, Vienna, Austria, and Alan Hay are acknowledged for giving us delNS1 virus and anti-M2 antibodies, respectively.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 286), German-Russian (DFG-RFBR) grant 436 RUS 113/587, Howard Hughes Medical Institute (United States) grant 75195546302, NATO linkage grant HT-974619, Russian Basic Research Foundation grant 04-48442 and 04-04000, and ASGL-Research Laboratories grant 11-D/1/CHT-99.
REFERENCES
- 1.Albert, M. L., B. Saufer, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Aragon, T., S. de la Luna, I. Novoa, L. Carrasco, J. Ortin, and A. Nieto. 2001. Eucaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol. 20:6259-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 74:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, S., P. C. Roberts, L. E. Brown, I. I. Troung, A. K. Pattnalk, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent of protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 5.Barnard, P., and N. A. McMillian. 1999. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-α. Virology 259:305-313. [DOI] [PubMed] [Google Scholar]
- 6.Basler, C. F., X. Wang, E. Mühlberger, V. Volchkov, J. Paragas, H.-D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I INF antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Z., Y. Li, and R. M. Krug. 1999. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 18:2273-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De la Luna, S., P. Fortes, A. Beloso, and J. Ortin. 1995. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J. Virol. 69:2427-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz, M. O., S. Zeimin, M. M. Le Beau, P. Pitha, S. D. Smith, R. R. Chilcote, and J. D. Rowley. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. USA 85:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 2000. The V protein of simian virus 5 inhibits interferon signaling by targeting STAT 1 for proteosome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egorov, A., S. Brandt, S. Sereinig, J. Romaniva, B. Ferko, D. Katinger, A. Grassaur, G. Alexandrova, H. Katinger, and T. Muster. 1998. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol 72:6437-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enami, K., T. A. Sato, S. Nakada, and M. Enami. 1994. Influenza virus NS1 protein stimulates translation of the M1 protein. J. Virol. 68:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortes, P., A. Beloso, and J. Ortín. 1994. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 13:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto, I., J. Pan, T. Takizawa, and Y. Nakanishi. 2000. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J. Virol. 74:3399-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, E. D. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 18.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 73:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 20.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 73:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He, B., M. Gross, and B. Roizman. 1997. The y1-34.5 protein of herpes simplex virus I complexes with protein phosphatase 1a to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He, B., G. Y. Lin, J. E. Durbin, R. L. Durbin, and R. A. Lamb. 2001. The SH integral membrane protein of the paramyxovirus simian virus 5 is required to block apoptosis in MDBK cells. J. Virol. 75:4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heylbroeck, C., S. Balachandran, M. J. Servant, C. DeLuca, G. N. Barber, R. Lin, and J. Hiscott. 2000. The IRF-3 transcription factor mediates Sendai virus-induced apoptosis. J. Virol. 74:3781-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinshaw, V. G., C. W. Olsen, N. Dybdahi-Sissoko, and D. Evans. 1994. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol. 68:3667-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 26.Hongo, S., K. Sugawara, H. Nishimura, Y. Muraki, F. Kitame, and K. Nakamura. 1994. Identification of a second protein encoded by influenza C virus RNA segment 6. J. Gen. Virol. 75:3503-3510. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, B. L., and J. O. Langland. 1998. Reovirus sigma 3 protein: dsRNA binding and inhibition of RNA-activated protein kinase. Curr. Top. Microbiol. Immunol. 233:185-196. [DOI] [PubMed] [Google Scholar]
- 28.Koennecke, I., C. B. Boschek, and C. Scholtissek. 1981. Isolation and properties of a temperature-sensitive mutant (ts 412) of an influenza A virus recombinant with a ts lesion in the gene coding for the nonstructural protein. Virology 110:16-25. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu, T., K. Takeuchi, J. Yokoo, Y. Tanaka, and B. Gotoh. 2000. Sendai virus blocks alpha interferon signaling to signal transducers and activators of transfection. J. Virol. 74:2477-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug, R. M. 1998. Unique functions of the NS1 protein, p. 82-92. In K. G. Nicholson, R. G. Webster, and A. J. Hay (ed.), Textbook of influenza. Blackwell Science Ltd., Oxford, United Kingdom.
- 31.Lamb, R. A., C. J. Lai, and P. W. Choppin. 1981. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: collinear and interrupted mRNAs code for overlapping proteins. Proc. Natl. Acad. Sci. USA 78:4170-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb, R. A., L. J. Holsinger, and L. H. Pinto. 1994. The influenza A virus M2 ion channel protein and its role in the influenza virus life cycle, p. 303-321. In E. Wimmer (ed.), Receptor-mediated virus entry into cells. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 33.Langland, J. O., S. Pettiford, B. Jiang, and B. L. Jacobs. 1994. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J. Virol. 68:3821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leavitt, J. 1983. Tumorigenic potential of human fibroblasts as a function of ability to express a novel form of influenza A nucleocapsid protein. Carcinogenesis 4:1229-1237. [DOI] [PubMed] [Google Scholar]
- 35.Leib, D. A., M. A. Machalek, B. R. G. Williams, R. H. Silverman, and H. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard, G. T., and G. C. Sen. 1996. Effects of adenovirus E1A protein on interferon-signaling. Virology 234:25-33. [DOI] [PubMed] [Google Scholar]
- 37.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1994. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the e1F-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 38.Manchini, M., C. E. Machemer, S. Roy, D. W. Nicholson, L. A. Thornberry, L. A. Casciola-Rosen, and A. Rosen. 2000. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 3:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morahan, P. S., and S. E. Grossberg. 1970. Age-related resistance of the chicken embryo to viral infections. I. Interferon and natural resistance to myxoviruses and vesicular stomatitis. J. Infect. Dis. 121:615-623. [DOI] [PubMed] [Google Scholar]
- 40.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 41.Rosen, A., and L. Casciola-Rosen. 1999. Autoantigens as substrate for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 6:6-12. [DOI] [PubMed] [Google Scholar]
- 42.Schlender, J., B. Bossert, U. Buchholtz, and K. K. Conzelman. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. R. Hinshaw. 2001. Influenza virus NS1 protein induces apoptosis in cultured cells. J. Virol. 75:7875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250:302-315. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu, K., H. Handa, S. Nakada, and K. Nagata. 1994. Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res. 22:5047-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shors, T., K. V. Kibler, K. B. Perkins, R. Seidler-Wulff, M. P. Banaszak, and B. L. Jacobs. 1997. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology 239:269-276. [DOI] [PubMed] [Google Scholar]
- 47.Sugrue, R. J., R. B. Belshe, and A. J. Hay. 1990. Palmitoylation of the influenza A virus M2 protein. Virology 179:51-56. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 74:2347-2355. [DOI] [PubMed] [Google Scholar]
- 49.Talanian, R. V., C. Quinlan, S. Trautz, M. C. Hackett, J. A. Mankovich, D. Banach, T. Ghayur, K. D. Brady, and W. W. Wong. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272:9677.. [DOI] [PubMed] [Google Scholar]
- 50.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talon, J., M. Salvatore, R. E. O'Neill, Y. Nakaya, H. Zheng, T. Muster, A. Garcia-Sastre, and P. Palese. 2000. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. USA 97:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan, S. L., and M. G. Katze. 1998. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res. 18:757-766. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka, N., M. Sato, M. S. Lamphier, H. Nozawa, E. Oda, S. Noguchi, R. D. Schreiber, Y. Tsujimotot, and T. Taniguchi. 1998. Type 1 interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells 3:29-37. [DOI] [PubMed] [Google Scholar]
- 54.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolstenholme, A. J., T. Barrett, S. T. Nichol, and B. W. J. Mahy. 1980. Influenza virus-specific RNA and protein synthesis in cells infected with temperature-sensitive mutants defective in the genome segment encoding nonstructural proteins. J. Virol. 35:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolff, T., R. E. O'Neill, and P. Palese. 1998. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J. Virol. 72:7170-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young, D. F., L. Didcock, S. Goodbourn, and R. F. Randall. 2000. Paramyxoviridae use distinct virus-specific mechanisms to circumstance the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]
- 58.Zebedee, S. L., and R. A. Lamb. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 62:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhirnov, O. P., and A. G. Bukrinskaya. 1981. Two forms of influenza virus nucleocapsid protein in infected cells and virions. Virology 109:174-179. [DOI] [PubMed] [Google Scholar]
- 60.Zhirnov, O. P., A. V. Ovcharenko, and A. C. Bukrinskaya. 1982. A modified plaque assay method for accurate analysis of infectivity of influenza viruses with uncleaved hemagglutinin. Arch. Virol. 71:177-183. [DOI] [PubMed] [Google Scholar]
- 61.Zhirnov, O. P. 1990. Solubilization of matrix protein M1/M from virions occurs at different pH for orthomyxo- and paramyxoviruses. Virology 176:274-279. [DOI] [PubMed]
- 62.Zhirnov, O. P., T. E. Konakova, W. Garten, and H.-D. Klenk. 1999. Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J. Virol. 73:10158-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]