Abstract
Plum pox virus (PPV) is a member of the Potyvirus genus that, in nature, infects trees of the Prunus genus. Although PPV infects systemically several species of the Nicotiana genus, such as N. clevelandii and N. benthamiana, and replicates in the inoculated leaves of N. tabacum, it is unable to infect systemically the last host. The long-distance movement defect of PPV was corrected in transgenic tobacco plants expressing the 5"-terminal region of the genome of tobacco etch virus (TEV), a potyvirus that infects systemically tobacco. The fact that PPV was unable to move to upper noninoculated leaves in tobacco plants transformed with the same TEV transgene, but with a mutation in the HC protein (HC-Pro)-coding sequences, identifies the multifunctional HC-Pro as the complementing factor, and strongly suggests that a defect in an HC-Pro activity is responsible for the long-distance movement defect of PPV in tobacco. Whereas PPV HC-Pro strongly intensifies the symptoms caused by potato virus X (PVX) in the PPV systemic hosts N. clevelandii and N. benthamiana, it has no apparent effect on PVX pathogenicity in tobacco, supporting the hypothesis that long-distance movement and pathogenicity enhancement are related activities of the potyviral HC proteins. The movement defect of PPV in tobacco could also be complemented by cucumber mosaic virus in a mixed infection, demonstrating that at least some components of the long-distance machinery of the potyviruses are not strictly virus specific. A general conclusion of this work is that the HC-Pro might be a relevant factor for controlling the host range of the potyviruses.
Plant viruses must accomplish three main steps to complete their infection cycle: (i) replication inside the cell, (ii) cell-to-cell movement through plasmodesmata, and (iii) long-distance movement through the vascular tissue. It is the exception rather than the rule that viruses can successfully infect plants, and it is thought that, in most cases, host range limitations are the result of restrictions to virus movement rather than to the inability to replicate within cells (26). Viral proteins involved in cell-to-cell movement have been well characterized, specially the movement protein (MP) of tobacco mosaic virus (TMV). Abundant experimental evidence suggests that TMV MP, which is a nucleic acid binding protein (12), may facilitate intracellular and intercellular trafficking of the viral RNA through its interactions with the cytoskeleton and with plasmodesmata (30, 43, 63). Although several groups of viruses have MPs similar to that of TMV, many others have MPs of other types, such as those encoded by the triple gene block or the tubule-forming MPs (for a review, see references 10 and 19). Much less is known about the functions of viral proteins involved in long-distance movement. Usually, the coat protein (CP) is essential for efficient movement of plant viruses outside the inoculated leaves, although this is not a general rule. Moreover, there is genetic evidence indicating that MPs may perform specific long-distance movement functions. Proteins other than the CPs and the MPs, such as the potyviral HC protein (HC-Pro), the genome-linked protein (VPg), the cucumoviral 2b, or the tombusviral p19, are also involved in long-distance movement (for a review, see references 10, 13, 26, and 58).
The major contribution of movement defects to host range restrictions suggests that specific host factors could play key roles in virus movement. Both cell-to-cell and long-distance movements have been shown to depend on interactions between virus proteins and species-specific plant factors. However, in spite of the extensive divergence among different virus groups of the movement mechanisms derived from these interactions, they appear to be able to facilitate the transport of a wide range of heterologous viruses. Thus, many viruses belonging to different taxonomic groups have been shown to be able to complement one another's movement functions in nonhost plants (28, 29, 42, 62). Moreover, several studies have shown that different viral proteins, expressed from transgenes (for examples, see references 14, 25, and 32), heterologous sequences cloned in defective genomes (for examples, see references 18, 22, 44, 55, and 61), or cotransfected plasmids (for examples, see references 1 and 21), can functionally replace nonhomologous proteins from other viruses, sometimes resulting in an extension of their virus host ranges.
Plum pox virus (PPV) is a member of the Potyvirus genus (39). The potyvirus genome consists of a messenger-polarity RNA molecule of about 10 kb, which is translated into a polyprotein that is proteolytically processed by three virus-encoded proteinases in up to 10 final protein products (51, 52). At least four potyvirus proteins, CP, HC-Pro, the cylindrical inclusion protein, and the genome-linked protein (VPg), as well as the small 6K2 peptide, are involved in potyvirus movement (reviewed in reference 51). Moreover, both 6K2 and VPg are host-specific determinants for potyvirus movement (46, 48, 57).
In nature, PPV infects trees of the Prunus genus (66). However, the experimental host range of PPV is quite broad, including herbaceous species of several families (65). Namely, PPV infects and systemically replicates to high titers in several species of the Nicotiana genus, such as N. clevelandii and N. benthamiana. In contrast, PPV is not able to establish a systemic infection in N. tabacum. In this paper, the molecular basis of this deficiency is studied to gain insight in virus factors involved in host-specific interactions required for potyvirus movement. We show that PPV is able to cause a local infection in N. tabacum without inducing a general systemic resistance in noninoculated leaves and that the PPV deficiency for long-distance movement in this host can be complemented by HC-Pro from a tobacco-infecting potyvirus and by still-undetermined factors from a nonrelated virus.
MATERIALS AND METHODS
Viruses.
Virus progeny derived from pGPPV (strain D) (53) was used for most of the experiments. PPV-SwC (strain C) (16) and different variants of the PPV-PS isolate (strain M) (56) were also used to assess the susceptibility to PPV infection of Nicotiana tabacum. Tobacco etch virus (TEV) and the Fny strain of cucumber mosaic virus (CMV) were provided by E. Rodriguez-Cerezo, (Centro Nacional de Biotecnología, Madrid, Spain) and F. García-Arenal (Universidad Politécnica, Madrid, Spain), respectively. Infections with potato virus X (PVX) and PVX/PPV chimeric viruses were achieved by inoculation with capped RNA obtained by in vitro transcription of pP2C2S (a derivative of pGC3 provided by D. Baulcombe [11]) and of pPVX-HCK109G232 and pPVX-HCE109S232 (56), respectively. pP2C2S contains the full-length cDNA sequence of the wild-type PVX genome. pPVX-HCK109G232 and pPVX-HCE109S232 are pP2C2S derivatives with insertions in the PVX sequence of HC-Pro-coding sequences from two PPV-PS variants that differ in symptom severity; these foreign sequences are placed under the control of a duplicate of the PVX CP subgenomic promoter.
Plants.
The tobacco transgenic line U6-B transformed with a cDNA fragment from the 5" end of the TEV genome (9) and six independent tobacco transgenic lines transformed with the same genomic fragment but with different mutagenic insertions in the P1- or HC-Pro-coding sequences (59) have been described previously. Tobacco transgenic lines transformed with the TEV CP-coding sequence (FL3.3 [38]) and with a cDNA fragment from the 5" end of the tobacco vein mottling virus genome (B8, provided by Emilio Rodríguez-Cerezo [45]) have also been described.
All transgenic plants were grown from seeds germinated in the presence of kanamycin at a concentration of 100 μg/ml.
Inoculation and sampling.
Young N. tabacum (four-leaf stage), N. benthamiana (five-leaf stage), and N. clevelandii (four-leaf stage) plants were inoculated by rubbing inocula onto three leaves dusted with Carborundum. Crude sap from N. clevelandii plants infected with PPV (1 g in 2 ml of 5 mM sodium phosphate, pH 7.2) or N. tabacum plants infected with CMV or TEV (1 g in 4 ml of 5 mM sodium phosphate, pH 7.2) was used as the source of inoculum for these viruses. For PVX and PVX/PPV recombinant viruses, plants were infected either with transcripts obtained from SpeI-linearized plasmids, as previously described (56), or, in coinoculation experiments, with extracts of transcript-infected plants.
In the coinoculation experiments, the two inocula were mixed just before being applied to the Carborundum-dusted leaves. For sequential inoculation, the first virus was inoculated as described above and, 3 weeks later, the second virus was inoculated by the same procedure in three leaves with symptoms placed immediately above those first inoculated.
Plants were maintained in the greenhouse, and virus accumulation was assessed at different days postinoculation (d.p.i.) by Western blot analysis and by double antibody sandwich indirect-enzyme-linked immunosorbent assay (ELISA) with the REALISA kit (Durviz). Samples consisted in two to four discs of 7-mm diameter collected from symptomatic areas of the leaf, or randomly when no symptoms were visible.
Expression of HC-Pro was assessed by Western blot analysis using the serum of a rabbit immunized with His-tagged PPV HC-Pro produced in Escherichia coli (unpublished results). Anti-PPV-HC-Pro sera provided by Michel Ravelonandro (INRA, Villenave D'Ornon, France) and E. Maiss (University of Hannover, Germany) were used in preliminary experiments.
RESULTS
PPV causes a local infection in N. tabacum.
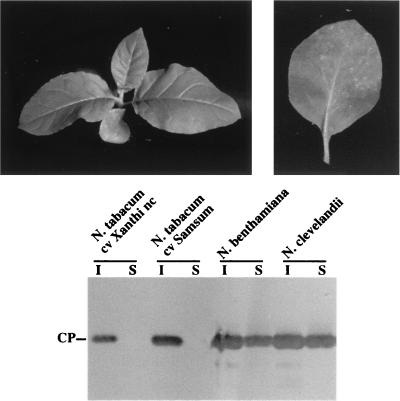
PPV replicates, spreads systemically, and accumulates to high titers in several species of the genus Nicotiana, especially N. clevelandii, which has been used as an indicator plant for the virus. However, PPV induces symptoms only in inoculated leaves of N. tabacum (35, 65). To investigate whether the absence of systemic symptoms is the result of a long-distance movement failure of PPV in this host, N. tabacum cv. Xanthi plants were inoculated with sap from N. clevelandii plants infected with virus progeny of the PPV cDNA clone pGPPV. The inoculated leaves displayed quite mild symptoms, consisting of small rings and spots that tended to necrotize (Fig. 1). No systemic symptoms could be observed in any case. ELISA tests (not shown) and Western blot analysis (Fig. 1) confirmed that PPV accumulated in the inoculated leaves but, in contrast with N. clevelandii and N. benthamiana infections, was undetectable in upper noninoculated leaves.
FIG. 1.
Symptoms displayed by N. tabacum (cv. Xanthi nc) inoculated with PPV. A whole plant and a detached inoculated leaf are shown in the left and right panels, respectively. A Western blot assessing virus accumulation in inoculated (I) and upper noninoculated (S) leaves of different Nicotiana plants infected with PPV is shown at the bottom of the figure.
To assess the specificity of the defect of PPV in long-distance movement in tobacco, different cultivars of this plant species (Samsum, Kentucky 14, Burley 21, and Havana 425) were infected with several isolates of the PPV strains D, C, and M. The results were similar in all cases, and the virus remained localized in the inoculated leaf, where it caused the same kind of symptoms (data not shown).
A nonrelated virus can complement the systemic movement defect of PPV in N. tabacum.
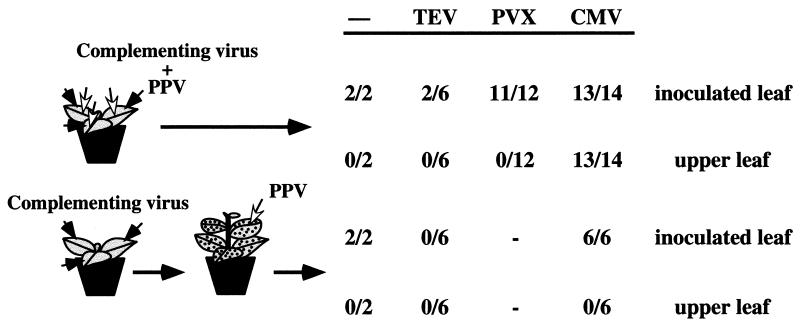
In some occasions, viruses that are able to infect a given plant could act as helpers to facilitate the movements of other viruses unable to move by themselves systemically in that plant (3). Thus, whether or not other viruses that were able to systemically infect tobacco could facilitate the long-distance movement of PPV in this host was tested. Both coinoculations and sequential inoculations were assayed (Fig. 2). No systemic movement of PPV was detected when it was coinoculated with a potyvirus, TEV, or with a helical virus from another family, PVX. PVX had no major effect on the capacity of PPV to infect the inoculated leaves of N. tabacum (92% of the inoculated plants were infected). However, coinoculation with TEV appeared to reduce both the infectivity (only 33% of the inoculated plants were infected; Fig. 2) and the levels of PPV accumulation in the inoculated leaves, estimated by ELISA (data not shown).
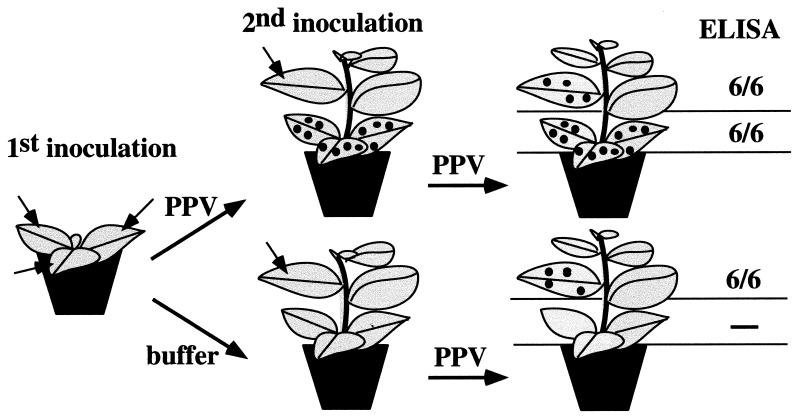
FIG. 2.
Complementation of the movement defect of PPV in tobacco in mixed infections. The figure shows the number of plants infected with PPV, assessed by ELISA, in relation to the total number of N. tabacum (cv. Xanthi nc) plants inoculated with PPV and the helper virus indicated above each column. Dashes indicate nonassayed virus combinations. Coinoculation and sequential inoculation experiments are represented in the upper and the lower parts of the figure, respectively. A schematic representation of the inoculation procedures is depicted at the left of the figure. Filled and empty arrowheads represent inoculations with the complementing virus and with PPV, respectively.
In contrast, PPV was able to infect leaves above the inoculated ones when it was inoculated together with the icosahedral virus CMV. Although almost all the coinoculated plants became systemically infected with PPV, it displayed an erratic distribution on the upper noninoculated leaves. The highest leaf in which we detected PPV was the third above the last one inoculated, at 13 d.p.i. Gal-On et al. (23) have reported that, in tobacco, many CMV strains cause cycles of acute disease followed by no symptoms. This was also the pattern of CMV infection that we observed in our experiments. Interestingly, extinction of PPV in coinfected plants correlated with the first vanishing of CMV symptoms, suggesting that CMV levels in the symptomless leaves are not sufficient to supply the movement function required for PPV progression.
No movement complementation was observed in any of the sequential inoculations (Fig. 2). In these experiments, N. tabacum plants were inoculated first with putative helper viruses and 20 d.p.i. with PPV in symptomatic systemically infected leaves. PPV was detected exclusively in inoculated leaves of plants infected with CMV. In the TEV-infected plants, PPV was incapable of infecting even the inoculated leaves, in agreement with the decrease in PPV infectivity observed in the PPV/TEV coinoculation experiments.
Transgenic expression of the 5"-terminal region of the TEV genome complements the movement defect of PPV in N. tabacum.
The interference of PPV by TEV in tobacco could be obscuring a putative TEV activity that is able to complement the movement defect of PPV. We assessed the possibility that stable transgenic expression of proteins from potyviruses that are able to infect tobacco could facilitate systemic movement of PPV.
Plants of N. tabacum cv. Xanthi transformed with the 5"-terminal region of the TEV genome including the coding sequences for P1 (with an insertion of nine nucleotides at genome position 973 in the P1-coding sequence), HC-Pro, and part of P3 (line 1031-1XB) (59), N. tabacum cv. Burley 49 transformed with the CP-coding sequence of TEV (line FL 3.3) (38), and N. tabacum cv. Burley 21 transformed with the 5"-terminal region of the tobacco vein mottling virus (TVMV) genome, including the coding regions for P1, HC-Pro, P3, and part of 6K1 (line B8) (45), were used in the initial experiments and inoculated with PPV (Fig. 3). Some upper noninoculated leaves from plants expressing the 5"-terminal region of the TEV genome (line 1031-1XB) showed small chlorotic spots that were not observed in any of the other plants (Fig. 4 and data not shown). ELISAs demonstrated the presence of PPV in the inoculated leaves and in the upper leaves of the 1031-1XB plants with symptoms, but not in upper leaves of FL3.3 and B8 plants, which had virus only in the inoculated leaves.
FIG. 3.
Susceptibility to PPV of tobacco transgenic lines transformed with different potyvirus sequences. The figure shows the number of plants accumulating virus in inoculated or upper noninoculated (systemic infection) leaves relative to the total number of plants of the transgenic line inoculated with PPV. A schematic representation of the different transgenes is depicted at the left of the figure.
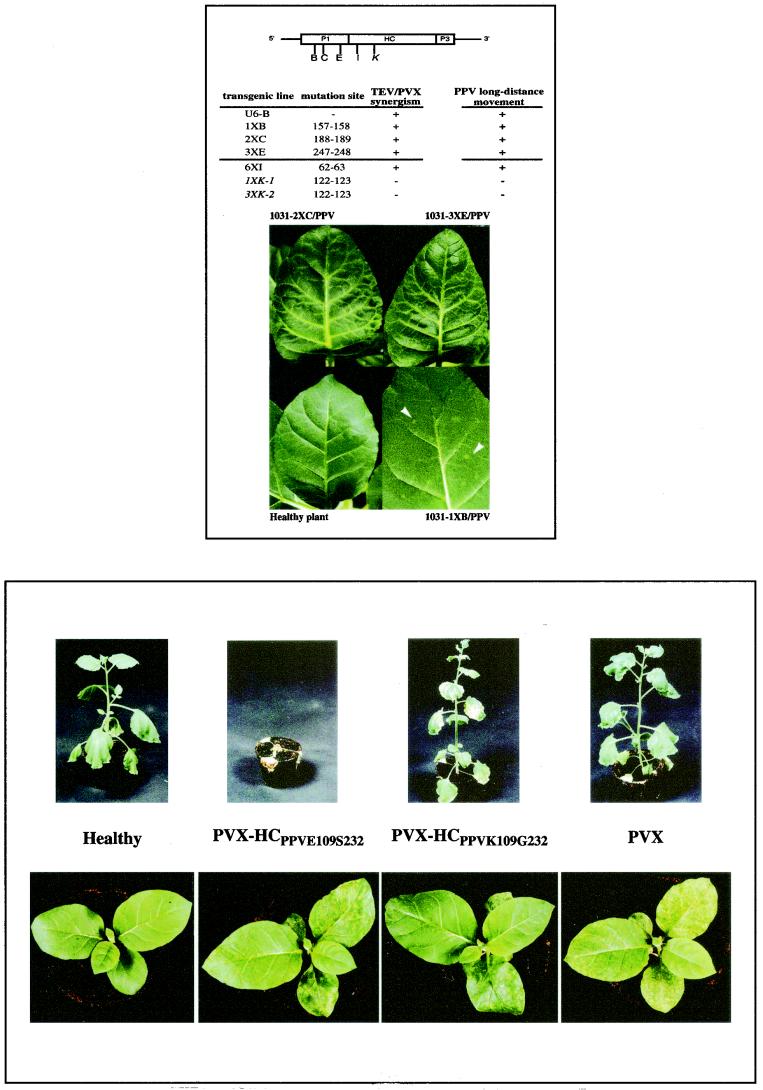
FIG.4 (Top).
Susceptibility to PPV of tobacco transgenic lines expressing the 5"-terminal region of the TEV genome with insertions within the P1- and HC-Pro-coding sequences. A diagram of the transgene showing the location of the mutations is shown at the top of the figure. The positions of the amino acids in TEV P1 and HC-Pro flanking the different insertions, as well as the abilities of the mutant transgenes to enhance PVX pathogenicity (taken from reference 59) and to facilitate the long-distance movement of PPV, are indicated below the diagram. Mutants disturbing these two activities are in italics. The lower part of the figure shows the symptoms displayed by upper noninoculated leaves of plants of some of the transgenic lines.
HC-Pro is the protein responsible for complementation of the movement defect of PPV in transgenic N. tabacum plants expressing the 5"-terminal region of the TEV genome.
Six transgenic lines of N. tabacum cv. Xanthi nc contained the TEV 5" genome region with nine-nucleotide insertions, resulting in three-amino-acid insertions in the P1 or HC proteins. Lines 1031-1XB, 1031-2XC, and 1031-3XE contained insertions affecting the P1 protein after residues 157, 188, and 247, respectively. Line 1210-6XI contained an insertion affecting HC-Pro after residue 62. Lines 1115-IXK-1 and 1115-3XK-2 contained independent insertions affecting HC-Pro after residue 122. Transgenic line U-6B contained the wild-type TEV 5" sequence in N. tabacum cv. Havana 425 (Fig. 4 and Table 1).
TABLE 1.
Detection of PPV in upper noninoculated leaves of transgenic tobacco plants at different days postinoculation
| Transgenic line | 15 d.p.i.
|
30 d.p.i.
|
45 d.p.i.
|
60 d.p.i.
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of plants with PPV/no. of inoculated plantsa | Position of highest leaf with virusb | Plant sizec | No. of plants with PPV/no. of inoculated plants | Position of highest leaf with virus | Plant size | No. of plants with PPV/no. of inoculated plants | Position of highest leaf with virus | Plant size | No. of plants with PPV/no. of inoculated plants | Position of highest leaf with virus | Plant size | |
| U6B | 3-4 | 2/8 | 5 | 8 | 4/8 | 7 | 12 | 1/8 | 7 | 16 | ||
| 1031-1XB | 6/8 | 6 | 10-11 | 7/8 | 6 | 14-15 | 24/26 | 36 | ||||
| 1031-2XC | 7/8 | 6 | 5-6 | 8/8 | 9 | 9-12 | 6/8 | 11 | 16/17 | 6/8 | 14 | 20-26 |
| 1031-3XE | 8/8 | 8 | 8-10 | 8/8 | 12-13 | 14-15 | 8/8 | 15 | 24/26 | 7/8 | 20 | 34 |
| 1210-6XI | 6 | 11 | 7/8 | NDd | ND | 2/8 | ND | ND | ||||
| 1115-1XK-1 | 10-11 | 16-20 | 22/31 | 39 | ||||||||
| 1115-3XK-2 | 10-11 | 17-19 | 26/33 | 39 | ||||||||
| Vector trans. | 7-8 | 17-20 | 28/30 | 28-30 | ||||||||
| Xanthi nc | 7-8 | 12-14 | 25/29 | 35 | ||||||||
As shown by ELISA.
Position above the three inoculated ones of the highest leaf showing PPV.
Total number of leaves above the three inoculated ones.
ND, not determined; upper leaves budded as bunches, preventing a confident assessment of leaf positions.
Plants of these transgenic lines were inoculated with PPV, and the infection was monitored by visual observation of the symptoms and measurement of virus accumulation by ELISA. All plants, except those of U6-B and 1210-6XI lines, showed local lesions resembling those of the nontransgenic plants (not shown). Upper noninoculated leaves of plants with transgenes that had an insertion in the P1-coding sequence (1031-1XB, 1031-2XC, and 1031-3XE) exhibited symptoms (Fig. 4). Chlorotic spots similar to those observed in the 1031-1XB plants were detected in the 1031-2XC and 1031-3XE plants. However, the most striking symptom associated with PPV infection in the 1031-2XC and 1031-3XE plants was a strong vein yellowing, which could be observed, but was much milder, in leaves close to the inoculated leaves of 1031-1XB plants. Systemic symptoms appeared in leaves near the inoculated leaves in plants of these three lines at 7 d.p.i. (data not shown). Infection spread to new leaves, although the uppermost leaves were always symptomless. Progression of the infection stopped earlier in 1031-1XB plants, with no symptoms detected above the seventh leaf.
No systemic symptoms associated with PPV infection were detected in transgenic plants of lines 1115-1XK-1 and 1115-3XK-2, which have mutagenic insertions in the central region of the HC-coding sequence of the TEV transgene.
ELISA data revealed a positive correlation between PPV accumulation and the appearance of symptoms, except for lines U6-B and 1210-6XI (both asymptomatic) (Table 1). In agreement with the symptom screening, ELISA data showed that virus progression halted earlier in 1031-1XB plants (between 30 and 45 d.p.i.) and only reached five or six leaves above the inoculated leaves. In plants of the 1031-2XC and 1031-3XE lines, movement of PPV occurred over a longer period (more than 60 d.p.i.), although, as suggested by symptoms, no virus was detected in the topmost leaves of the infected plants. Plants of the line 1210-6XI had severe developmental abnormalities. They showed a marked dwarfism and a progressively decreasing internode length, making it difficult to collect samples from individual leaves at late development times. In spite of the fact that no phenotypic differences between healthy and PPV-inoculated 1210-6XI plants could be observed, ELISAs demonstrated that PPV infected the inoculated leaves and was able to move systemically in these plants. PPV appeared to move very slowly in the 1210-6XI plants, but the movement continued for a very long time (75 d.p.i. [data not shown]). ELISA data also demonstrated PPV movement in the asymptomatic U6-B plants. In contrast with the rest of the plants used in this work, U6-B plants, which are transformed with a wild-type TEV transgene, derive from the cv. Havana 425 and grew very slowly. PPV was not detected in the inoculated leaves or in upper leaves at 15 d.p.i. However, at later times, it accumulated up to the seventh leaf above the inoculated leaves, although PPV movement appeared to cease between 45 and 60 d.p.i.
No accumulation of PPV was detected by ELISA in upper noninoculated leaves of 1115-1XK-1 and 1115-3XK-2 plants, although symptom development and virus accumulation in the inoculated leaves of these plants were similar to those observed for plants of the lines that supported PPV systemic movement. These results strongly suggest that HC-Pro is the limiting factor for the systemic movement of PPV in tobacco plants.
PPV HC-Pro does not exacerbate the PVX symptoms in N. tabacum.
Interaction between PVX and potyviruses, including TEV, leads to a synergistic increase of symptom severity compared with single infections. It was shown that HC-Pro is sufficient to induce this enhancement of PVX pathogenicity (47). The same enhancement effect was demonstrated for PPV HC-Pro when it was expressed from sequences cloned in the PVX genome in N. clevelandii and N. benthamiana, two systemic hosts for PPV (56) (Fig. 5). Since PPV HC-Pro appears to lack the activity required for virus movement in tobacco, PVX enhancement activity in N. tabacum was also tested. The same PVX chimeras harboring different variants of PPV HC-Pro that were shown to cause an exacerbated disease in PPV systemic hosts were inoculated in tobacco. Although Western blot analysis demonstrated that PPV HC-Pro accumulated in the systemically infected leaves (data not shown), neither HCK109G232 nor HCE109S232 (derived from a mild and a severe PPV isolate, respectively [56]), enhanced PVX symptoms in tobacco (Fig. 5).
FIG. 5 (Bottom).
Symptoms displayed by N. tabacum (cv. Xanthi nc) (lower panels) and N. benthamiana (upper panels) infected with PVX or PVX recombinant viruses expressing PPV HC protein.
Local infection of PPV in N. tabacum plants does not induce a systemic resistance in the whole plant.
It was proposed that the long-distance function of the potyvirus HC-Pro could result from the counteraction of an induced defensive response of the plant that prevents the development of the infection in new leaves (34). The discovery of HC-Pro as a suppressor of posttranscriptional gene silencing (2, 7, 33) is consistent with this hypothesis.
To test if the blockage of long-distance movement of PPV in tobacco could be due to an anti-PPV response triggered by local infection in the inoculated leaves, tobacco plants previously infected with PPV were challenge inoculated in upper leaves at 10 d.p.i. Virus accumulation was assayed by ELISA at 20 d.p.i. (Fig. 6). Both leaves from plants previously infected with PPV and mock-inoculated leaves were infected after the challenge inoculation. In all cases, the second infection also remained local, with no virus detected outside the inoculated leaves. Similar results were obtained when the first PPV inoculation was done in a half or a quarter of a leaf, and challenge inoculation was done in the noninoculated part of the same leaf. Symptoms were not detected in the noninoculated area of the leaf, but the leaf remained susceptible to PPV reinoculation (data not shown).
FIG. 6.
Susceptibility of tobacco to PPV reinoculation after a first local infection. The figure shows a schematic representation of the procedure used in the experiment. PPV was inoculated on upper healthy leaves of plants that had been previously infected with PPV (top row) or mock inoculated (bottom row). The column on the right shows the number of plants accumulating virus, assessed by ELISA, in the leaves inoculated in each of the challenges relative to the total number of inoculated plants.
DISCUSSION
Different PPV isolates cause in the inoculated leaves of N. tabacum irregular spots that tend to necrotize (Fig. 1). Similarly, some PPV isolates also produce necrotic lesions in the inoculated leaves of N. clevelandii and Nicotiana occidentalis that are usually more regular and conspicuous than those of N. tabacum. However, whereas PPV infection remains confined to the inoculated leaves of N. tabacum, it invades systemically both N. clevelandii and N. occidentalis, as well as other Nicotiana species such as N. benthamiana (56). These facts suggest that PPV factor(s) are able to induce a hypersensitive response (HR)-like response in plants of the Nicotiana genus, which, at least in some species, is not able to block the systemic spread of the virus. Usually, the hypersensitive reaction is associated with disease resistance. However, there are some reports showing that formation of local lesions and blockade of pathogen spread are separable events in some circumstances (for examples, see references 5, 31, and 37). Since HR induction appears to be a dominant character, the fact that the movement defect of PPV in tobacco can be complemented by virus factors supplied by virus coinoculation (Fig. 2) or by transgene expression (Fig. 3) strongly suggests that the HR-like response of N. tabacum, like those of N. clevelandii and N. occidentalis, is not relevant for PPV localization. The development of unaltered local symptoms of PPV in the complementing transgenic plants supports this assumption. Thus, either other plant defense mechanisms or host-specific defects of the movement machinery of PPV must be responsible for its local confinement in N. tabacum.
We took different approaches to identify the PPV factors involved in restriction of the movement of this virus in tobacco. Five potyviral protein products have been shown to participate in virus movement (reviewed in reference 51). The CP is involved both in cell-to-cell and in long-distance movement. However, transgenic expression of CP from TEV could not correct the movement defect of PPV in tobacco (Fig. 3). We have not analyzed whether host-specific deficiencies of three other movement proteins, cylindrical inclusion protein, 6K2, and VPg, are relevant for the localization of PPV in tobacco. Interestingly, transgenic expression of the 5"-terminal region of the TEV genome (including the P1 and HC-Pro coding sequences) facilitated the movement of PPV to upper noninoculated leaves of tobacco (Fig. 3). The fact that a mutation in the HC-Pro-coding sequence, but not in the P1-coding region, disturbed the complementing activity (Fig. 4) strongly suggests that PPV HC-Pro is the deficient factor in tobacco infection, a factor whose function is provided by HC-Pro from a potyvirus able to spread systemically in this plant.
Although PPV was able to move to upper noninoculated leaves in all the transgenic lines expressing the 5"-terminal fragment of the TEV genome without insertions in the central region of the HC-Pro-coding sequence, the pattern of infection was different in each of them. The possibility exists that the TEV P1 protein collaborates with HC-Pro in promoting PPV long-distance movement and that mutations affecting P1 modify the capacity of the transgenic plants to support movement of PPV. Although P1 is not essential for potyvirus movement (68), a collaborative action of P1 and HC-Pro was reported, namely on the stimulation of PVX minus-strand RNA synthesis in TEV/PVX mixed infections (47). Thus, we cannot rule out the possibility that small changes in PPV replication in the transgenic lines expressing different forms of the P1 protein could affect the ability of the virus to spread throughout the whole plant. However, we think that it is more likely that changes in the expression levels of HC-Pro could be the cause of the different movement patterns of PPV in the transgenic lines. In this regard, it has been reported that the level of HC-Pro accumulation in the line 1031-1XB was lower than in the rest of the transgenic lines (59), and among the lines that are able to support long-distance movement of PPV, this is the one that appears to spread to upper leaves with the least efficiency (Table 1). Similarly, 1031-3XE, which was reported to have the highest level of HC accumulation (59), is the line that appears to propagate PPV best (Table 1). We have not compared the levels of accumulation of HC-Pro in the TEV transgenic plants with that of TVMV HC-Pro in the B8 plants, although the analysis of published data may indicate that the amount of transgenic protein in B8 plants is even lower than that of 1031-1XB (45, 59). This low accumulation level of the transgenic HC-Pro could account for the failure of the B8 plants to support long-distance movement of PPV, although biological activity of TVMV HC expressed in the B8 plants, either to facilitate aphid transmission (6) or to enhance PVX symptomatology (67), has been reported. Alternatively, it is also possible that the HC-Pro of TEV and that of TVMV differ in their capacity to stimulate long-distance movement of PPV in tobacco.
Surprisingly, TEV is not able to act as a helper virus to facilitate long-distance movement of PPV in tobacco, in spite of the fact that active HC-Pro is produced in the mixed infections (Fig. 2). There are precedents of proteins that complemented virus defects when they were expressed from transgenic plants or from chimeric virus genomes but were unable to do so in mixed infections (18, 25). TEV also appears to interfere with PPV local infection in tobacco, especially after sequential inoculation (Fig. 2). This interference is host- and virus-specific, since it was not observed with N. clevelandii, a common host for PPV and TEV (data not shown), or, in tobacco, in mixed infections of PPV with PVX or CMV (Fig. 2). A possible explanation for this is that, in tobacco, HC-Pro of TEV could compete with a partially dysfunctional PPV HC-Pro for viral and/or host factors required to support PPV local and systemic infection.
Even in the best-complementing transgenic lines, systemic infection by PPV appears to be relatively inefficient. Progression of the infection is slow, and although the virus reached at least 20 leaves above the inoculated leaves, it was detected only when the leaf was in an advanced developmental stage (Table 1). This could be due to the following factors: (i) the levels of transgenic TEV HC-Pro may be suboptimal for PPV infection, (ii) TEV HC-Pro may not be completely compatible with the movement machinery of PPV, (iii) the movement deficiency of PPV in tobacco could result from the additive effects of several PPV-encoded factors in addition to HC-Pro, and (iv) the partially inactive HC-Pro from PPV could interfere with the complementing TEV HC-Pro (dominant negative effect). The data currently available do not enable us to favor any of these possibilities.
HC-Pro is a multifunctional protein (41) with proteolytic activity (8), which is able to bind RNA in vitro (40) and to interact with itself (64). In addition to its function in aphid transmission (6), HC-Pro has been implicated in maintenance of genome amplification (36, 38), cell-to-cell and long-distance movements (17, 36, 54, 57), symptom expression (4, 24, 56), and enhancement of pathogenicity (47, 59, 67) and of long-distance movement (60) of other viruses in mixed infections. The fact that mutations that caused defects in long-distance movement and in synergistic enhancement of PVX pathogenicity map to the same central region of HC-Pro and appear to be associated with a premature shutoff of virus RNA synthesis in infected protoplasts prompted the suggestion that the roles of HC in genome amplification, long-distance movement, and pathogenicity enhancement might be related and associated with a function of HC-Pro in interference with an induced host defense system (34, 47). The more recent discovery of the role of HC-Pro as a suppressor of posttranscriptional gene silencing (PTGS) supports this hypothesis (2, 7, 33). We have observed that PPV HC-Pro has a strong enhancement effect on the pathogenicity of PVX in N. clevelandii and N. benthamiana (Fig. 5) (56), but it does not intensify the symptoms of this virus in tobacco, in which PPV cannot move out of the inoculated leaf without the help of the HC-Pro of TEV (Fig. 5). These data support the hypothesis that the functions of the potyviral HC-Pro in pathogenicity enhancement and in long-distance movement have an overlapping molecular basis and suggest that the same defect limits both functions of the PPV HC-Pro in tobacco. In agreement with this assumption, mutations that have been shown to eliminate the capacity of TEV HC-Pro to enhance PVX pathogenicity (59) impede complementation of the movement defect of PPV in tobacco (Fig. 4).
It was shown that the upper leaves of plants infected with some viruses are symptom free, contain very reduced levels of virus, and are protected against a secondary virus infection by a PTGS-like mechanism induced by the primary infection (15, 49, 50). It was proposed that this systemic resistance is established when the infecting virus lacks a strong PTGS suppressor. No evidence for this type of induced resistance was observed with tobacco plants locally infected with PPV, as they remain susceptible to further local infection by a secondary PPV inoculation (Fig. 6). This result might suggest that the defect of HC-Pro in the PPV-infected tobacco affects a positive function directly involved in virus movement. However, we cannot rule out the possibility that PPV confinement in the inoculated leaf could be the consequence of the triggering of a localized PTGS-like response. For instance, silencing restricting PPV movement may be triggered specifically in or around vascular cells, and this may not have been detected in the challenge inoculation experiments.
It is not unusual that movement defects of a virus in a plant could be complemented by a helper virus able to spread in this plant (3). We have shown that PPV can move systemically in tobacco when it is coinoculated with CMV (Fig. 2). Interestingly, CMV facilitates long-distance movement of another potyvirus, pepper mottle virus, in the resistant cultivar Avelar of Capsicum annuum (27), although, in contrast with PPV in tobacco, pepper mottle virus appears to reach some old noninoculated leaves at very late times postinoculation in the single infections. A likely candidate to be the CMV factor complementing the PPV movement defect in tobacco was the 2b protein. This protein is known to be involved in CMV long-distance movement (20) and, like potyvirus HC-Pro, is a suppressor of PTGS (7). However, a chimeric virus that expressed the 2b protein of CMV as part of the PPV polyprotein infected systemically N. clevelandii and N. benthamiana very efficiently but caused the same local infection as wild-type PPV in tobacco (results not shown). The significance of this result is limited, however, by the observation that a PPV chimeric virus expressing TEV HC-Pro was also unable to move to upper noninoculated leaves in tobacco (results not shown). Thus, further research is still needed to know which is/are the factor(s) of CMV that stimulate(s) PPV systemic spread in tobacco.
In summary, this work demonstrates that the movement deficiency of PPV in tobacco can be compensated by factors of related or unrelated viruses and that the function in long-distance movement of the silencing suppressor HC-Pro might be a very relevant factor for controlling the host range of potyviruses.
Acknowledgments
We thank David Baulcombe for the pP2C2S clone, Emilio Rodríguez-Cerezo for TEV and the B8 plants, F. García-Arenal for CMV, Edgar Maiss and Michel Ravelonandro for anti-HC-Pro sera, Elena González for help in transgenic plant germination, and Elvira Domínguez for technical assistance.
This work was supported by grants BIO98-0769 and BIO2001-1434 from CICYT and QLK2-1999-00739 from the European Union.
REFERENCES
- 1.Agranovsky, A. A., A. S. Folimonov, S. Y. Folimonova, S. Y. Morozov, J. Schiemann, D. Lesemann, and J. G. Atabekov. 1998. Beet yellows closterovirus HSP70-like protein mediates the cell-to-cell movement of a potexvirus transport-deficient mutant and a hordeivirus-based chimeric virus. J. Gen. Virol. 79:889-895. [DOI] [PubMed] [Google Scholar]
- 2.Anandalakshmi, R., G. J. Pruss, X. Ge, R. Marathe, A. C. Mallory, T. H. Smith, and V. B. Vance. 1998. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 95:13079-13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atabekov, J. G., and M. E. Taliansky. 1990. Expression of a plant virus-coded transport function by different viral genomes. Adv. Virus Res. 38:201-248. [DOI] [PubMed] [Google Scholar]
- 4.Atreya, C. D., P. L. Atreya, D. W. Thornbury, and T. P. Pirone. 1992. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology 191:106-111. [DOI] [PubMed] [Google Scholar]
- 5.Bendahmane, A., K. Kanyuka, and D. C. Baulcombe. 1999. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, P. H., A. G. Hunt, G. M. Domier, G. M. Hellman, Y. Stram, D. W. Thornbury, and T. P. Pirone. 1989. Expression in transgenic plants of a viral gene product that mediates insect transmission of potyviruses. Proc. Natl. Acad. Sci. USA 86:8402-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigneti, G., O. Voinnet, W. X. Li, L. H. Ji, S. W. Ding, and D. C. Baulcombe. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 17:6739-6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Carrington, J. C., S. M. Cary, T. D. Parks, and W. G. Dougherty. 1989. A second proteinase encoded by a plant potyvirus genome. EMBO J. 8:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrington, J. C., D. D. Freed, and C.-S. Oh. 1990. Expression of potyviral polyproteins in transgenic plants reveals three proteolytic activities required for complete processing. EMBO J. 9:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrington, J. C., K. D. Kasschau, S. K. Mahajan, and M. C. Schaad. 1996. Cell-to-cell and long distance transport of viruses in plants. Plant Cell 8:1669-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman, S., T. Kavanagh, and D. Baulcombe. 1992. Potato virus X as a vector for gene expression in plants. Plant J. 2:549-557. [DOI] [PubMed] [Google Scholar]
- 12.Citovsky, V., D. Knorr, G. Schuster, and P. Zambryski. 1990. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell 60:637-647. [DOI] [PubMed] [Google Scholar]
- 13.Citovsky, V., and P. Zambryski. 2000. Systemic transport of RNA in plants. Trends Plant Sci. 5:52-54. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, B., I. Schmitz, A. L. N. Rao, R. N. Beachy, and J. A. Dodds. 1996. Cell-to-cell transport of movement-defective cucumber mosaic and tobacco mosaic viruses in transgenic plants expressing heterologous movement protein genes. Virology 216:208-213. [DOI] [PubMed] [Google Scholar]
- 15.Covey, S. N., N. Al-Kaff, A. Lángara, and D. S. Turner. 1997. Plants combat infection by gene silencing. Nature 385:781-782. [Google Scholar]
- 16.Crescenzi, A., L. d’Aquino, S. Comes, M. Nuzzaci, P. Piazzolla, D. Boscia, and A. Hadidi. 1997. Characterization of the sweet cherry isolate of plum pox potyvirus. Plant Dis. 81:711-714. [DOI] [PubMed] [Google Scholar]
- 17.Cronin, S., J. Verchot, R. Haldeman-Cahill, M. C. Schaad, and J. C. Carrington. 1995. Long-distance movement factor: a transport function of the potyvirus helper component proteinase. Plant Cell 7:549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jong, W., and P. Ahlquist. 1992. A hybrid RNA virus made by transferring the noncapsid movement protein from a rod-shaped to an icosahedral virus is competent for systemic infection. Proc. Natl. Acad. Sci. USA 89:6808-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deom, C. M., M. Lapidot, and R. N. Beachy. 1992. Plant virus movement proteins. Cell 69:221-224. [DOI] [PubMed] [Google Scholar]
- 20.Ding, S.-W., W.-X. Li, and R. H. Symons. 1995. A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 14:5762-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorkin, O. N., A. G. Solovyev, N. E. Yelina, A. A. Zamyatnin, R. A. Zinovkin, K. Makinen, J. Schiemann, and S. Y. Morozov. 2001. Cell-to-cell movement of potato virus X involves distinct functions of the coat protein. J. Gen. Virol. 82:449-458. [DOI] [PubMed] [Google Scholar]
- 22.Fenczik, C. A., H. S. Padgett, C. A. Holt, S. J. Casper, and R. N. Beachy. 1995. Mutational analysis of the movement protein of odontoglossum ringspot virus to identify a host-range determinant. Mol. Plant-Microbe Interact. 8:666-673. [DOI] [PubMed] [Google Scholar]
- 23.Gal-On, A., I. B. Kaplan, and P. Palukaitis. 1996. Characterization of cucumber mosaic virus. II. Identification of movement protein sequences that influence its accumulation and systemic infection in tobacco. Virology 226:354-361. [DOI] [PubMed] [Google Scholar]
- 24.Gal-On, A., and B. Raccah. 2000. A point mutation in the FRNK motif of the potyvirus helper component-protease gene alters symptom expression in cucurbits and elicits protection against the severe homologous virus. Phytopathology 90:467-473. [DOI] [PubMed] [Google Scholar]
- 25.Giesman-Cookmeyer, D., S. Silver, A. A. Vaewhongs, S. A. Lommel, and C. M. Deom. 1995. Tobamovirus and dianthovirus movement proteins are functionally homologous. Virology 213:38-45. [DOI] [PubMed] [Google Scholar]
- 26.Gilbertson, R. L., and W. J. Lucas. 1996. How do viruses traffic on the ‘vascular highway’? Trends Plant Sci. 1:260-268. [Google Scholar]
- 27.Guerini, M. N., and J. F. Murphy. 1999. Resistance of Capsicum annuum ‘Avelar’ to pepper mottle potyvirus and alleviation of this resistance by co-infection with cucumber mosaic cucumovirus are associated with virus movement. J. Gen. Virol. 80:2785-2792. [DOI] [PubMed] [Google Scholar]
- 28.Hacker, D. L., and B. C. Fowler. 2000. Complementation of the host range restriction of southern cowpea mosaic virus in bean by southern bean mosaic virus. Virology 266:140-149. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton, R. I., and J. A. Dodds. 1970. Infection of barley by tobacco mosaic virus in single and mixed infection. Virology 42:266-268. [DOI] [PubMed] [Google Scholar]
- 30.Heinlein, M., B. L. Epel, H. S. Padgett, and R. N. Beachy. 1995. Interaction of tobamoviruses movement proteins with the plant cytoskeleton. Science 270:1983-1985. [DOI] [PubMed] [Google Scholar]
- 31.Kachroo, P., K. Yoshioka, J. Shah, H. K. Dooner, and D. F. Klessig. 2000. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12:677-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan, I. B., M. H. Shintaku, Q. Li, L. Zhang, L. E. Marsh, and P. Palukaitis. 1995. Complementation of virus movement in transgenic tobacco expressing the cucumber mosaic virus 3a gene. Virology 209:188-199. [DOI] [PubMed] [Google Scholar]
- 33.Kasschau, K. D., and J. C. Carrington. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95:461-470. [DOI] [PubMed] [Google Scholar]
- 34.Kasschau, K. D., S. Cronin, and J. C. Carrington. 1997. Genome amplification and long-distance movement functions associated with the central domain of tobacco etch potyvirus helper component-proteinase. Virology 228:251-262. [DOI] [PubMed] [Google Scholar]
- 35.Kerlan, C., and J. Dunez. 1979. Différenciation biologique et sérologique de souches du virus de la Sharka. Ann. Phytopathol. 11:241-250. [Google Scholar]
- 36.Klein, P. G., R. R. Klein, E. Rodr&ıacute;guez-Cerezo, A. Hunt, and J. G. Shaw. 1994. Mutational analysis of the tobacco vein mottling virus genome. Virology 204:759-769. [DOI] [PubMed] [Google Scholar]
- 37.Li, H. W., A. P. Lucy, H. S. Guo, W. X. Li, L. H. Ji, S. M. Wong, and S. W. Ding. 1999. Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J. 18:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindbo, J. A., L. Silva-Rosales, W. M. Proebsting, and W. G. Dougherty. 1993. Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell 5:1749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Moya, J. J., M. R. Fernández-Fernández, M. Cambra, and J. A. García. 2000. Biotechnological aspects of plum pox virus. J. Biotechnol. 76:121-136. [DOI] [PubMed] [Google Scholar]
- 40.Maia, I. G., and F. Bernardi. 1996. Nucleic acid-binding properties of a bacterially expressed potato virus Y helper component-proteinase. J. Gen. Virol. 77:869-877. [DOI] [PubMed] [Google Scholar]
- 41.Maia, I. G., A.-L. Haenni, and F. Bernardi. 1996. Potyviral HC-Pro: a multifunctional protein. J. Gen. Virol. 77:1335-1341. [DOI] [PubMed] [Google Scholar]
- 42.Malyshenko, S. I., L. G. Lapchic, O. A. Kondakova, M. E. Taliansky, and J. G. Atabekov. 1989. Plant virus transport function: complementation by helper viruses is non-specific. J. Gen. Virol. 70:2751-2757. [Google Scholar]
- 43.McLean, B. G., J. Zupan, and P. C. Zambryski. 1995. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell 7:2101-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mise, K., R. F. Allison, M. Janda, and P. Ahlquist. 1993. Bromovirus movement protein genes play a crucial role in host specificity. J. Virol. 67:2815-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno, M., B. F. Brandwagt, J. G. Shaw, and E. Rodríguez-Cerezo. 1999. Infectious virus in transgenic plants inoculated with a nonviable, P1-proteinase defective mutant of a potyvirus. Virology 257:322-329. [DOI] [PubMed] [Google Scholar]
- 46.Nicolas, O., S. W. Dunnington, L. F. Gotow, T. P. Pirone, and G. M. Hellmann. 1997. Variations in the VPg protein allow a potyvirus to overcome va gene resistance in tobacco. Virology 237:452-459. [DOI] [PubMed] [Google Scholar]
- 47.Pruss, G., X. Ge, X. M. Shi, J. C. Carrington, and V. B. Vance. 1997. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajamäki, M.-L., and J. P. T. Valkonen. 1999. The 6K2 protein and the VPg of potato virus A are determinants of systemic infection in Nicandra physaloides. Mol. Plant-Microbe Interact. 12:1074-1081. [DOI] [PubMed] [Google Scholar]
- 49.Ratcliff, F., B. D. Harrison, and D. C. Baulcombe. 1997. A similarity between viral defense and gene silencing in plants. Science 276:1558-1560. [DOI] [PubMed] [Google Scholar]
- 50.Ratcliff, F. G., S. A. MacFarlane, and D. C. Baulcombe. 1999. Gene silencing without DNA. RNA-mediated cross-protection between viruses. Plant Cell 11:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Revers, F., O. Le Gall, T. Candresse, and A. J. Maule. 1999. New advances in understanding the molecular biology of plant/potyvirus interactions. Mol. Plant-Microbe Interact. 12:367-376. [Google Scholar]
- 52.Riechmann, J. L., S. Laín, and J. A. García. 1992. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 73:1-16. [DOI] [PubMed] [Google Scholar]
- 53.Riechmann, J. L., S. Laín, and J. A. García. 1990. Infectious in vitro transcripts from a plum pox potyvirus cDNA clone. Virology 177:710-716. [DOI] [PubMed] [Google Scholar]
- 54.Rojas, M. R., F. M. Zerbini, R. F. Allison, R. L. Gilbertson, and W. J. Lucas. 1997. Capsid protein and helper component proteinase function as potyvirus cell-to-cell movement proteins. Virology 237:283-295. [DOI] [PubMed] [Google Scholar]
- 55.Ryabov, E. V., D. J. Robinson, and M. E. Taliansky. 1999. A plant virus-encoded protein facilitates long-distance movement of heterologous viral RNA. Proc. Natl. Acad. Sci. USA 96:1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sáenz, P., L. Quiot, J.-B. Quiot, T. Candresse, and J. A. García. 2001. Pathogenicity determinants in the complex virus population of a Plum pox virus isolate. Mol. Plant-Microbe Interact. 14:278-287. [DOI] [PubMed] [Google Scholar]
- 57.Schaad, M. C., A. D. Lellis, and J. C. Carrington. 1997. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 71:8624-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seron, K., and A.-L. Haenni. 1996. Vascular movement of plant viruses. Mol. Plant-Microbe Interact. 9:436-442. [DOI] [PubMed] [Google Scholar]
- 59.Shi, X. M., H. Miller, J. Verchot, J. C. Carrington, and V. B. Vance. 1997. Mutations in the region encoding the central domain of helper component-proteinase (HC-Pro) eliminate potato virus X/potyviral synergism. Virology 231:35-42. [DOI] [PubMed] [Google Scholar]
- 60.Sonoda, S., H. Koiwa, K. Kanda, H. Kato, M. Shimono, and M. Nishiguchi. 2000. The helper component-proteinase of Sweet potato feathery mottle virus facilitates systemic spread of Potato virus X in Ipomoea nil. Phytopathology 90:944-950. [DOI] [PubMed] [Google Scholar]
- 61.Spitsin, S., K. Steplewski, N. Fleysh, H. Belanger, T. Mikheeva, S. Shivprasad, W. Dawson, H. Koprowski, and V. Yusibov. 1999. Expression of alfalfa mosaic virus coat protein in tobacco mosaic virus (TMV) deficient in the production of its native coat protein supports long-distance movement of a chimeric TMV. Proc. Natl. Acad. Sci. USA 96:2549-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taliansky, M., and F. García-Arenal. 1995. Role of cucumovirus capsid protein in long-distance movement within the infected plant. J. Virol. 69:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomenius, K., D. Clapham, and T. Meshi. 1987. Localization by immunogold cytochemistry of the virus-coded 30K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology 160:363-371. [DOI] [PubMed] [Google Scholar]
- 64.Urcuqui-Inchima, S., J. Walter, G. Drugeon, S. German-Retana, A. L. Haenni, T. Candresse, F. Bernardi, and O. Le Gall. 1999. Potyvirus helper component-proteinase self-interaction in the yeast two-hybrid system and delineation of the interaction domain involved. Virology 258:95-99. [DOI] [PubMed] [Google Scholar]
- 65.van Oosten, H. J. 1970. Herbaceous host plants for the sharka (plum pox) virus. Neth. J. Plant Pathol. 76:253-260. [Google Scholar]
- 66.van Oosten, H. J. 1975. Susceptibility of some woody plant species, mainly Prunus spp., to sharka (plum pox) virus. Neth. J. Plant Pathol. 81:199-203. [Google Scholar]
- 67.Vance, V. B., P. H. Berger, J. C. Carrington, A. G. Hunt, and X. M. Shi. 1995. 5" proximal potyviral sequences mediate potato virus X/potyviral synergistic disease in transgenic tobacco. Virology 206:583-590. [DOI] [PubMed] [Google Scholar]
- 68.Verchot, J., and J. C. Carrington. 1995. Evidence that the potyvirus P1 proteinase functions in trans as an accessory factor for genome amplification. J. Virol. 69:3668-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]