Abstract
Alfalfa mosaic virus (AlMV) coat protein is involved in systemic infection of host plants, and a specific mutation in this gene prevents the virus from moving into the upper uninoculated leaves. The coat protein also is required for different viral functions during early and late infection. To study the role of the coat protein in long-distance movement of AlMV independent of other vital functions during virus infection, we cloned the gene encoding the coat protein of AlMV into a tobacco mosaic virus (TMV)-based vector Av. This vector is deficient in long-distance movement and is limited to locally inoculated leaves because of the lack of native TMV coat protein. Expression of AlMV coat protein, directed by the subgenomic promoter of TMV coat protein in Av, supported systemic infection with the chimeric virus in Nicotiana benthamiana, Nicotiana tabacum MD609, and Spinacia oleracea. The host range of TMV was extended to include spinach as a permissive host. Here we report the alteration of a host range by incorporating genetic determinants from another virus.
The interaction between virus and plant proteins determines the capability of the virus to multiply and systemically infect the host plant (1). Systemic infection with plant viruses requires cell-to-cell and long-distance movement of viral genomic RNA (2, 3). Many plant viruses find access into cells through wounds. Upon initial entry into a plant cell, the virus multiplies and moves locally from cell to cell (local infection). In most cases, the transfer of viral RNA between cells is supported by a virus-encoded movement protein(s) (4–7), whereas in some viruses the capsid protein is a primary determinant of cell-to-cell movement (8–10). The movement protein interacts with the plasmodesmata and transfers the viral RNA into a neighboring uninfected cell (11–13). During systemic infection, the virus moves through the vascular system and multiplies in upper uninoculated leaves. Movement into the upper uninoculated tissue is a critical step in the infection by many plant viruses, and prevention of this step can result in significant protection of the host(s) from virus invasion (14). Coat proteins (CPs) of several plant viruses are essential for long-distance movement (15–23). Moreover, some RNA plant viruses, such as tobacco mosaic virus (TMV) (16, 17, 24), cowpea mosaic virus (25), alfalfa mosaic virus (AlMV) (18), tobacco etch potyvirus (26), and red clover necrotic mosaic virus (27) require functional CP for long-distance movement.
Here, to study the role of AlMV CP in long distance movement, we designed a hybrid virus consisting of TMV, the type member of the tobamovirus group, and the CP of AlMV. The TMV genome consists of a single plus-sense RNA (6,395 nt) encapsidated with a 17.5-kDa CP, which results in rod-shaped particles (300 nm in length). In addition to CP, TMV has three nonstructural proteins. Proteins (183 and 126 kDa) are translated from genomic RNA and are required for virus replication (28). The 30-kDa protein is a movement protein and provides the transfer of viral RNA from cell to cell (29). The only structural protein of TMV is a CP (17.5 kDa), which is required for the encapsidation and long-distance movement of the virus in an infected host (16, 17, 24). Movement and coat proteins are translated from subgenomic mRNAs (30–32).
AIMV is a member of the Bromoviridae family. The genome of this virus consists of three plus-sense RNAs (RNAs 1, 2, and 3), which are encapsidated by a single CP (24 kDa) that results in bacilliform or spherical particles depending on the size of RNA encapsidated. A fourth RNA (subgenomic RNA4) of AlMV is the messenger for the CP and is synthesized from genomic RNA3. The CP plays a key role in early and late AlMV infection, functioning in genome activation (33–36), RNA replication (37, 38), virus assembly (39, 40), stability of viral RNA (41, 42), and long-distance movement of viral RNA (18). Moreover, the AlMV CP is involved in symptom formation (43).
The multiple functions of the AlMV CP has made it difficult to analyze any single function without interfering with others. Thus, to address the role of AlMV CP in long-distance movement, we used the TMV-based expression vector Av, which is deficient in TMV CP production and, therefore, limited to inoculated leaves. Expression of the AlMV CP supported the long-distance movement of Av in Nicotiana benthamiana, Nicotiana tabacum MD609, and Spinacia oleracea.
MATERIALS AND METHODS
DNA Constructs.
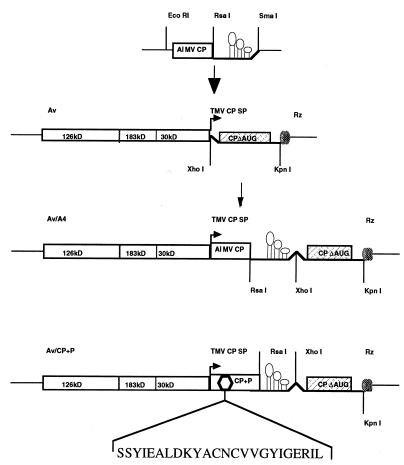
All cloning and cell transformations were performed according to Sambrook et al. (44). Escherichia coli DH5α-competent cells (Life Technologies, Gaithersburg, MD) were used for transformation. The TMV-derived vector was constructed such that the translation start codon ATG of TMV CP was replaced with AGA, and multiple cloning sites PacI, PmeI, AgeI, and XhoI were introduced 42 nt downstream of the mutated ATG codon. Av (Fig. 1) contains the full-length TMV but is defective in CP production. In addition, Av contains a ribozyme at the 3′ end for self-cleavage of in vitro RNA transcripts. A chimeric Av containing the AlMV CP was constructed using pSP65A4 (41), which contains the full-length cDNA of AlMV RNA4 (Fig. 1). The EcoRI–SmaI fragment of pSP65A4 containing the 5′ and 3′ noncoding regions in addition to the ORF of AlMV CP was cloned into Av (Fig. 1), which was linearized by XhoI using blunt-end ligation to create Av/A4 (Fig. 1). An additional construct, Av/CP+P (Fig. 1), containing a mutated AlMV CP was engineered to test whether particle formation is required for long-distance movement. The mutation was introduced by replacing coding sequences between amino acids 66 and 86 of AlMV CP with a synthetic peptide of equal size (Fig. 1), by using PCR cloning. Although the mutant protein CP+P is functional in genome activation, binds viral RNA, and forms dimers in vitro, no particle assembly was observed in infected protoplasts (V.Y., et al., unpublished results). Thus, mutant CP+P was digested with EcoRI and SmaI and cloned into Av linearized with XhoI to create Av/CP+P (Fig. 1).
Figure 1.
Schematic representation of the genome of TMV-derived Av and construction of Av/A4. The 126- and 183-kDa proteins are required for TMV replication, the 30-kDa protein is the viral movement protein, and CPΔAUG is the translation-deficient gene of the viral CP. Right arrow under TMV CP SP indicates the subgenomic promoter of TMV CP. Rz indicates ribozyme for self-cleavage. Av/A4 and Av/CP+P are the hybrid viruses engineered to express AIMV CP using Av. Amino acids used to replace the AlMV CP sequences to create CP+P are shown under Av/CP+P.
In Vitro Transcription.
In vitro transcripts of recombinant TMV were synthesized by using T7 RNA polymerase (Promega) and CsCl-purified plasmid DNA, according to the manufacturer’s guidelines. Transcripts were capped by using the RNA cap-structure analog m7G(5)ppp(5)G (New England Biolabs).
Plant Inoculation.
Three upper leaves of each plant were inoculated with in vitro transcription products of recombinant Av constructs after adding 1 vol (vol/vol) of FES buffer [1% sodium pyrophosphate (wt/vol)/1% macaloid (wt/vol)/1% celite (wt/vol)/0.5 M glycine/0.3 M K2HPO4, pH 8.5, with phosphoric acid]. Inoculum was applied by gentle rubbing on leaves after abrading the leaf surface with carborundum (320 grit; Fisher). In experiments to confirm the stability of chimeric Av/A4, new plants were inoculated with sap from leaves systemically infected with this chimera.
Northern Blot Hybridization and Reverse Transcription–PCR (RT-PCR).
Total RNA was extracted from locally and systemically infected leaf tissue. Briefly, 100–200 mg of leaf tissue was homogenized in 10 vol of TRIzol reagent (GIBCO) and processed according to the manufacturer’s guidelines. Northern blot analysis of RNA was performed as described (45) using 5 μg of total RNA. The RT reaction was performed using Moloney murine leukemia virus reverse transcriptase (Promega) and 5′-TTTTCCGGAACCTTTTCG-3′ as primer, which also was used in PCR with 5′-GGGCCCATGGAACTT ACAGAAGAA-3′ Taq DNA polymerase (Promega).
Western Blot Analysis.
AIMV CP produced in virus-infected plants was analyzed by Western immunoblotting (46) using antibodies from Agdia (Elkhart, IN). Proteins from crude plant extracts or from purified virus particles were separated electrophoretically on SDS-polyacrylamide gels and electroblotted onto a nylon membrane overnight at 33 mA. After blocking with milk (Kirkegaard & Perry Laboratories), proteins were allowed to react with appropriate antibodies and detected by using the Vectastain ABC kit (Vector Laboratories).
RESULTS
Infection of Plants with AlMV, Av/A4, and TMV.
Leaves of Nicotiana benthamiana, Nicotiana tabacum MD609, and Spinacia oleracea were inoculated with AlMV, TMV, or in vitro synthesized transcripts of Av/A4. N. benthamiana and N. tabacum MD609 are systemic hosts for both AlMV and TMV, whereas S. oleracea is systemically infected only with AlMV but not with TMV (47).
N. benthamiana.
Inoculation of N. benthamiana with either TMV, Av/A4, or AlMV resulted in systemic infection with symptoms developing in the upper uninoculated leaves within 7–10 days. Systemic infection of N. benthamiana with either AlMV, Av/A4, or TMV resulted in mild curling and yellowing of leaves, with no significant differences between the viruses in the symptoms induced early in infection. Although very similar amounts (0.6–0.8 mg/g fresh tissue) of AlMV, Av/A4, and TMV were recovered from systemically infected N. benthamiana leaves, the TMV-infected plants developed stem necrosis and died within 12–15 days postinoculation, whereas AlMV- or Av/A4-infected plants remained nonnecrotic and alive. Even after 30 days of infection, AlMV- or Av/A4-infected plants never developed the necrotic reactions observed with TMV.
N. tabacum MD609.
Movement of TMV into the upper uninoculated leaves of N. tabacum MD609 (9–12 days postinoculation; dpi) was indicated clearly by mild mosaic symptoms and yellowing along leaf veins, leaving occasional green islands of uninfected tissue. Whereas TMV caused no symptoms on a locally inoculated leaf, the infection with either AlMV or Av/A4 resulted in the formation of necrotic lesions (5–6 dpi), which led to the death of the inoculated leaf 15–17 dpi. This necrotic death of the inoculated leaf, however, did not prevent the systemic infection with AlMV or Av/A4 from proceeding. Systemic invasion (8–10 dpi) of N. tabacum MD609 with AlMV or Av/A4 resulted in distinct mosaic symptoms with occasional necrotic spots. Systemic infection of TMV began at the apex, whereas AlMV or Av/A4 never reached the apex, leaving the uppermost two to three leaves symptomless. These results indicate that AlMV and TMV differ in the mechanisms of systemic movement.
S. oleracea.
Inoculation of S. oleracea plants with either AlMV or Av/A4 resulted in mild mosaic yellowing of systemically infected leaves, whereas inoculation with TMV induced no symptoms and did not result in infection of uninoculated leaves as determined by Western and Northern blot analyses (not shown). In addition, we inoculated N. tabacum cv. Xanthi-nc plants with the sap from inoculated and the upper uninoculated leaves of S. oleracea exposed either to TMV, AlMV, or Av/A4. Inoculation of N. tabacum cv. Xanthi-nc with the sap from both inoculated and the upper uninoculated leaves of S. oleracea infected with AlMV resulted in infection. Similarly, sap from both inoculated and the upper uninoculated leaves of S. oleracea infected with Av/A4 resulted in infection, inducing local lesions on N. tabacum cv. Xanthi-nc. However, when we inoculated N. tabacum cv. Xanthi-nc with the sap from both the lower inoculated and the upper uninoculated leaves of S. oleracea exposed to TMV, only sap from inoculated leaves resulted in lesion formation. As it was reported by Holmes (47), S. oleracea is a local infection host for TMV. These results indicate that incorporation of AlMV CP into the TMV genome extended the host range of TMV, enabling it to systemically infect the upper uninoculated leaves of S. oleracea.
Western Analysis of Systemic Infection of Plants with Av/A4.
Leaves of N. benthamiana, N. tabacum MD609, and S. oleracea were inoculated with in vitro synthesized transcripts of Av or Av/A4. Western immunoblot analysis of tissue samples taken 10 dpi demonstrated the presence of AlMV CP in both locally and systemically infected leaves (Fig. 2A). N. benthamiana and N. tabacum MD609 plants inoculated with in vitro transcripts of Av developed symptoms only on locally inoculated leaves, and the virus did not move into the upper uninoculated leaves. S. oleracea infected with Av transcripts showed no signs of infection, and no viral RNA was detected by Northern blot analysis in the upper uninoculated leaves (Fig. 3A). TMV CP also was undetected by Western analysis (data not shown) in tissue samples from plants infected with Av or Av/A4. Plants inoculated with Av/CP+P did not develop systemic symptoms, and virus could not be purified from locally infected leaves, although the 24.0-kDa protein was detected in inoculated leaves using AlMV CP-specific antibodies (Fig. 2B).
Figure 2.
Western immunoblot analysis of AlMV CP expression in plants infected with Av/A4. Proteins were separated electrophoretically on a 13% SDS-polyacrylamide gel, transferred to a membrane, and reacted with mAbs specific for AIMV CP. (A) Antibodies recognized a 24-kDa protein in samples from locally (L) and systemically (S) infected tissue collected from N. benthamiana, N. tabacum MD609, and S. oleracea. (B) Antibodies also reacted with the 24-kDa protein in extracts from tissue locally infected (lane L) with Av/CP+P. Av/CP+P was not detected in upper uninoculated leaves (lane S). Left lanes show AlMV CP from wild-type virus (positive control) and the absence of CP in an Av-infected plant sample (negative control), respectively.
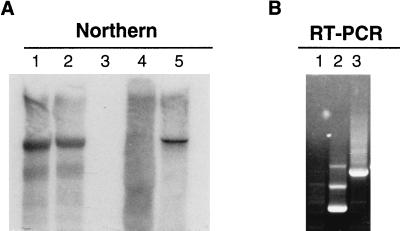
Figure 3.
Northern analysis (A) and RT-PCR (B) of viral RNA isolated from leaves of plants systemically infected with Av/A4. RNA molecules were separated by electrophoresis in denaturing conditions, transferred to a membrane, and probed with minus-strand RNA resembling 250 5′ nt of the TMV genome. In vitro synthesized transcripts of Av/A4 were used as a positive control (lane 5). Lanes 1 and 2 show RNA purified from systemically infected leaves of N. benthamiana and N. tabacum MD609, respectively. Total RNA purified from the upper uninoculated leaves of N. benthamiana (lane 3) and N. tabacum MD609 (lane 4) infected with Av/CP+P was used as a negative control. (B) RT-PCR of total RNA confirmed the presence of the full-size AlMV RNA4 insert in recombinant Av/A4 (lane 3). In vitro synthesized transcripts of Av (lane 2) and total RNA purified from upper uninoculated leaves of N. benthamiana infected with Av/CP+P were used as negative controls (lane 1).
Northern Blot Analysis and RT-PCR of Viral RNA from Systemically Infected Leaves.
N. benthamiana and N. tabacum MD609 plants were inoculated with sap from leaves systemically infected with chimeric Av/A4. RNA was isolated from leaves that were systemically infected 7–10 dpi. Northern blot analysis of the viral RNA using a minus-strand RNA probe (corresponding to the 250 5′ nt of TMV) revealed no difference between the migration of RNA from in vitro synthesized Av/A4 transcripts (Fig. 3A, lane 5) used as a positive control and RNA purified from systemically infected leaves of N. benthamiana (Fig. 3A, lane 1) or N. tabacum MD609 (Fig. 3A, lane 2), indicating the stability of construct during systemic infection. Total RNA purified from upper leaves of N. benthamiana (Fig. 3A, lane 3) and N. tabacum MD609 (Fig. 3A, lane 4) infected with Av/CP+P served as a control. RT-PCR of total RNA from systemically infected tissue confirmed the presence of the correct-sized insert of AlMV RNA4 in recombinant Av/A4 (Fig. 3B, lane 3). In vitro synthesized transcripts of Av (Fig. 3B, lane 2) and total RNA from upper uninoculated leaves of N. benthamiana infected with Av/CP+P were used as controls (Fig. 3B, lane 1). These results demonstrate that the AIMV CP supported long-distance movement of Av/A4, the chimeric TMV.
Infection of N. tabacum cv. Xanthi-nc with Av/A4.
N. tabacum cv. Xanthi-nc is a systemic host for AlMV, whereas TMV infection results only in local lesion formation. However, TMV can systemically invade N. tabacum cv. Xanthi-nc at 30°C, a temperature at which the plant cannot trigger a hypersensitive reaction (HR) to virus infection. Infection of N. tabacum cv. Xanthi-nc with Av/A4 at 27°C resulted in local lesions (Fig. 4A, Av/A4), and the virus did not move into upper uninoculated leaves. Local lesions in Av/A4-infected leaves appeared within 5–6 days after inoculation compared with 2–3 days in TMV-inoculated plants (Fig. 4A, TMV). Moreover, the local lesions in Av/A4-infected leaves were smaller (1–2 mm) than those of TMV-inoculated leaves (4–5 mm), perhaps reflecting the slower cell-to-cell movement in the Av/A4 infection. Inoculation of N. tabacum cv. Xanthi-nc with Av/A4, AlMV, or TMV at 30°C resulted in systemic spread of virus, which was phloem-mediated for TMV but not for Av/A4 or AlMV (Fig. 4B, Av/A4, AlMV, and TMV). Because the HR does not take place at 30°C, both TMV and Av/A4 moved into the upper uninoculated leaves (Fig. 4B, TMV and Av/A4). However, during the systemic infection, TMV reached the apex of the plant via the phloem and then spread to upper uninoculated leaves (Fig. 4B, TMV), whereas Av/A4 did not reach the apex and systemic infection started from the continuous movement of virus into the upper uninoculated leaves (Fig. 4B, Av/A4). Thus, inoculation of N. tabacum cv. Xanthi-nc with Av/A4 and incubation at 30°C resulted in systemic infection with symptoms similar to those of AlMV (Fig. 4B, AlMV). This similarity in the pattern of systemic infection of Av/A4 and AlMV suggests the key role of the AlMV CP in determining the systemic movement of virus. When N. tabacum cv. Xanthi-nc plants infected with Av/A4 were transferred into a 27°C room and maintained 3 days after inoculation at 30°C, local lesions and necrotic death of whole leaves, where the virus had moved, were observed, indicating the HR of the plant to Av/A4 infection.
Figure 4.
Comparison of symptoms induced by infection with TMV, AlMV, and Av/A4 on N. tabacum cv. Xanthi-nc at 27°C (A) and at 30°C (B). (A) TMV and Av/A4 caused necrotic local lesion formation, limiting the infection to the inoculated leaf, while AlMV moved into the upper uninoculated leaves. (B) Inoculation of N. tabacum cv. Xanthi-nc with Av/A4, AlMV, or TMV at 30°C resulted in the systemic spread of the virus. Local lesions and systemically infected leaves are indicated by circles and arrows, respectively.
DISCUSSION
Using a TMV-based expression vector, Av, we have addressed the role of AlMV CP in the long-distance movement of viral RNA without involving genome activation and replication, because TMV does not require the CP to initiate the infection or, unlike AlMV, to replicate. This study demonstrates that the AlMV CP is capable of encapsidating the TMV genomic RNA in vivo. Thus, Av provided an excellent system with which to study the role of AlMV CP in long-distance movement and systemic infection. Inoculation of N. benthamiana, N. tabacum MD609, and S. oleracea with Av/A4 resulted in systemic infection of plants that displayed symptoms similar to those caused by AlMV. Whereas N. benthamiana and N. tabacum MD609 are systemic hosts for both TMV and AlMV, S. oleracea is systemically infected only by AlMV. Expression of AlMV CP in Av supported the systemic infection of S. oleracea with chimeric TMV, thereby extending the host range of TMV. Another study, using p30BRzCPg24 (48), which produces AlMV CP and TM GMV U5 CP, suggested the key role of the AlMV CP in virus spread in this plant as well as the importance of the specificity of the interaction between AlMV CP and host molecules to enable virus spread. This report concerns the extension of the host range of a virus by the expression of heterologous coat protein. A number of hybrid viruses were designed by replacing the coat proteins (1, 49–51). None, however, resulted in the extension of the host range.
N. benthamiana plants inoculated with in vitro transcripts of Av/CP+P evidenced no virus in uninoculated upper leaves, and infection was limited to inoculated leaves. No virus, however, could be recovered from locally infected leaves of N. benthamiana expressing CP+P (Fig. 2B). Because no virus expressing CP+P could be recovered, it was evident that just the binding of CP+P to viral RNA may not be sufficient for systemic infection of the virus. From earlier experiments, it is expected that CP+P might bind viral RNA in vivo during infection.
The systemic invasion of the host plant with Av/A4 and the similarity of long-distance movement and symptoms in the Av/A4 and AlMV infections suggest a key role of the AlMV CP in systemic infection and symptom development. The experiments with Av/CP+P suggest that particle formation is essential for the long-distance movement of AlMV in the host plant. Moreover, expression of AlMV CP in Av extends the host range of TMV to include spinach. The latter may have an advantage in the development of plant virus-based vectors for functional studies of genes as well as the production of biomedicals in targeted host species.
Acknowledgments
We thank Dr. Alexander Karasev for the critical reading of this manuscript, Dr. Sue Loesch-Fries for the infectious cDNA clone of AlMV RNA4, and Shannon Cox and Mike Bertovich for technical help. Research at Biotechnology Foundation Laboratories is supported by a grant from the Commonwealth of Pennsylvania.
ABBREVIATIONS
- AlMV
alfalfa mosaic virus
- TMV
tobacco mosaic virus
- CP
coat protein
- RT-PCR
reverse transcription–PCR
References
- 1.Dawson W O. Virology. 1992;186:359–367. doi: 10.1016/0042-6822(92)90001-6. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Z, Kim K H, Giesman-Cookmeyer D, Lommel S A. Virology. 1993;192:27–32. doi: 10.1006/viro.1993.1004. [DOI] [PubMed] [Google Scholar]
- 3.Wang H L, Wang Y, Giesman-Cookmeyer D, Lommel S A, Lucas W J. Virology. 1998;245:75–89. doi: 10.1006/viro.1998.9154. [DOI] [PubMed] [Google Scholar]
- 4.Atabekov J G, Taliansky M E. Adv Virus Res. 1990;38:201–248. doi: 10.1016/s0065-3527(08)60863-5. [DOI] [PubMed] [Google Scholar]
- 5.Deom C M, Lapidot M, Beachy R N. Cell. 1992;69:221–224. doi: 10.1016/0092-8674(92)90403-y. [DOI] [PubMed] [Google Scholar]
- 6.Waigmann E, Lucas W J, Citovsky V, Zambryski P. Proc Natl Acad Sci USA. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding B, Li Q, Nguyen L, Palukaitis P, Lucas W J. Virology. 1995;207:345–353. doi: 10.1006/viro.1995.1093. [DOI] [PubMed] [Google Scholar]
- 8.van Lent J, Storms M, van der Meer F, Wellink J, Goldbach R. J Gen Virol. 1991;72:2615–2623. doi: 10.1099/0022-1317-72-11-2615. [DOI] [PubMed] [Google Scholar]
- 9.Dolja V V, Hadelman-Cahill R, Montgomery A E, Vandenbosch K A, Carrington J C. Virology. 1995;206:1007–1016. doi: 10.1006/viro.1995.1023. [DOI] [PubMed] [Google Scholar]
- 10.Rojas M R, Zerbini F M, Allison R F, Gilbertson R L, Lucas W J. Virology. 1997;237:283–295. doi: 10.1006/viro.1997.8777. [DOI] [PubMed] [Google Scholar]
- 11.Lucas W J, Gilbertson R L. Annu Rev Phytopathol. 1994;32:387–422. doi: 10.1146/annurev.phyto.43.040204.135939. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson R L, Lucas W J. Trends Plant Sci. 1996;1:260–267. [Google Scholar]
- 13.Mezitt L A, Lucas W J. Plant Mol Biol. 1996;32:251–273. doi: 10.1007/BF00039385. [DOI] [PubMed] [Google Scholar]
- 14.Beck D L, van Dolleweerd C J, Lough T J, Balmori E, Voot D M, Andersen M T, O’Brien I E, Forster R L. Proc Natl Acad Sci USA. 1994;91:10310–10314. doi: 10.1073/pnas.91.22.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamatsu N, Ishikawa M, Meshi T, Okada Y. EMBO J. 1987;6:307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson W O, Bubrick P, Grantham G L. Phytopathology. 1988;78:783–789. [Google Scholar]
- 17.Saito T, Yamanaka K, Okada Y. Virology. 1990;176:329–336. doi: 10.1016/0042-6822(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 18.van der Kuyl A C, Neeleman L, Bol J F. Virology. 1991;183:731–738. doi: 10.1016/0042-6822(91)91002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton L A, Lee T C, Wei N, Morris T J. Virology. 1991;183:143–150. doi: 10.1016/0042-6822(91)90127-w. [DOI] [PubMed] [Google Scholar]
- 20.Chapman S, Hills G, Watts J, Baulcambe D C. Virology. 1992;191:223–230. doi: 10.1016/0042-6822(92)90183-p. [DOI] [PubMed] [Google Scholar]
- 21.Hilf M E, Dawson W O. Virology. 1993;193:106–114. doi: 10.1006/viro.1993.1107. [DOI] [PubMed] [Google Scholar]
- 22.Taliansky M E, Garcia-Arenal F. J Virol. 1995;69:916–922. doi: 10.1128/jvi.69.2.916-922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flasinski S, Dzianott A, Pratt S, Bugarski J J. Mol Plant–Microbe Interact. 1995;8:23–31. doi: 10.1094/mpmi-8-0023. [DOI] [PubMed] [Google Scholar]
- 24.Siegel A, Zaitlin M, Seghal O P. Proc Natl Acad Sci USA. 1962;48:1845–1851. doi: 10.1073/pnas.48.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellink J, van Kammen A. J Gen Virol. 1989;70:2279–2286. [Google Scholar]
- 26.Dolja V V, Hadelman R, Robertson N L, Dougherty W G, Carrington J C. EMBO J. 1994;13:1482–1491. doi: 10.1002/j.1460-2075.1994.tb06403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaewhongs A A, Lommel S A. Virology. 1995;212:607–613. doi: 10.1006/viro.1995.1518. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa M, Meshi T, Motoyoshi F, Takamatsu N, Okada Y. Nucleic Acids Res. 1986;14:8291–8308. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meshi T, Watanabe Y, Saito T, Sugimoto A, Maede T, Okada Y. EMBO J. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruening G, Beachy R N, Scalla R, Zaitlin M. Virology. 1976;71:498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- 31.Beachy R N, Zaitlin M, Bruening G, Israel H W. Virology. 1976;73:498–507. doi: 10.1016/0042-6822(76)90411-6. [DOI] [PubMed] [Google Scholar]
- 32.Hunter T R, Hunt T, Knowland J, Zimmern D. Nature (London) 1976;260:759–760. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- 33.Bol J F, van Vloten-Doting L, Jaspars E M. Virology. 1971;46:73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- 34.van der Vossen E A, Neeleman L, Bol J F. Virology. 1994;202:891–903. doi: 10.1006/viro.1994.1411. [DOI] [PubMed] [Google Scholar]
- 35.Yusibov V, Loesch-Fries L S. Virology. 1995;208:405–407. doi: 10.1006/viro.1995.1168. [DOI] [PubMed] [Google Scholar]
- 36.Yusibov V, Loesch-Fries L S. Virology. 1998;242:1–5. doi: 10.1006/viro.1997.8973. [DOI] [PubMed] [Google Scholar]
- 37.van der Kuyl A C, Neeleman L, Bol J F. Virology. 1991;185:496–499. doi: 10.1016/0042-6822(91)90807-n. [DOI] [PubMed] [Google Scholar]
- 38.de Graaff M, Man in’t Veld M R, Jaspars E M. Virology. 1995;208:583–589. doi: 10.1006/viro.1995.1189. [DOI] [PubMed] [Google Scholar]
- 39.Jaspars E M. Adv Virus Res. 1974;19:37–149. doi: 10.1016/s0065-3527(08)60659-4. [DOI] [PubMed] [Google Scholar]
- 40.Reusken C B, Neeleman L, Brederode F T, Bol J F. J Virol. 1997;71:8385–8391. doi: 10.1128/jvi.71.11.8385-8391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loesh-Fries L S, Jarvis N P, Krahn K J, Nelson S E, Hall T C. Virology. 1985;146:177–187. doi: 10.1016/0042-6822(85)90002-9. [DOI] [PubMed] [Google Scholar]
- 42.Neeleman L, Van der Vossen E A, Bol J F. Virology. 1993;196:883–887. doi: 10.1006/viro.1993.1551. [DOI] [PubMed] [Google Scholar]
- 43.Neeleman L, van der Kuyl A C, Bol J F. Virology. 1991;181:687–693. doi: 10.1016/0042-6822(91)90902-n. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook S, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 45.Yusibov V, Loesch-Fries L S. Proc Natl Acad Sci USA. 1995;92:8980–8984. doi: 10.1073/pnas.92.19.8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusibov V, Modelska A, Steplewski K, Agadjanyan M, Weiner D, Hooper C, Koprowski H. Proc Natl Acad Sci USA. 1997;94:5784–5788. doi: 10.1073/pnas.94.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes F O. Phytopathology. 1946;36:643–649. [Google Scholar]
- 48.Modelska A, Dietzschold B, Fleysh N, Fu Z F, Steplewski K, Hooper D C, Koprowski H, Yusibov V. Proc Natl Acad Sci USA. 1998;95:2481–2485. doi: 10.1073/pnas.95.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaher R, French R, Ahlquist P. Virology. 1988;167:15–24. doi: 10.1016/0042-6822(88)90049-9. [DOI] [PubMed] [Google Scholar]
- 50.Osman F, Grantham G L, Rao A L N. Virology. 1997;238:452–459. doi: 10.1006/viro.1997.8849. [DOI] [PubMed] [Google Scholar]
- 51.Osman F, Choi Y G, Grantham G L, Rao A L N. Virology. 1998;251:438–448. doi: 10.1006/viro.1998.9421. [DOI] [PubMed] [Google Scholar]