Abstract
To better understand the role of dendritic cells (DCs) in human immunodeficiency virus (HIV) transmission at mucosal surfaces, we examined the expressions of the HIV adhesion molecule, dendritic-cell-specific ICAM-3 grabbing nonintegrin (DC-SIGN), its closely related homologue DC-SIGNR, and HIV coreceptors by distinct DC populations in the intestinal and genital tracts of humans and rhesus macaques. We also developed monoclonal antibodies (MAbs) specific for DC-SIGN or DC-SIGNR. In the Peyer's patches, DC-SIGN expression was detected in the interfollicular regions and in clusters of cells in the subepithelial dome regions. DC-SIGN expression was not found on plasmacytoid DCs. DC-SIGNR expression was restricted to endothelial cells in approximately one-third of the capillaries in the terminal ileum. In the vaginal epithelium, Langerhans' cells did not express DC-SIGN, whereas subepithelial DCs in the lamina propria expressed moderate levels of DC-SIGN. Finally, the rectum contained cells that expressed high levels of DC-SIGN throughout the entire thickness of the mucosa, while solitary lymphoid nodules within the rectum showed very little staining for DC-SIGN. Triple-color analysis of rectal tissue indicated that CCR5+ CD4+ DC-SIGN+ DCs were localized just beneath the luminal epithelium. These findings suggest that DC-SIGN+ DCs could play a role in the transmission of primate lentiviruses in the ileum and the rectum whereas accessibility to DC-SIGN+ cells is limited in an intact vaginal mucosa. Finally, we identified a MAb that blocked simian immunodeficiency virus interactions with rhesus macaque DC-SIGN. This and other specific MAbs may be used to assess the relevance of DC-SIGN in virus transmission in vivo.
Worldwide, human immunodeficiency virus (HIV) infection spreads primarily as a result of sexual exposure through mucosal surfaces. Identifying cellular factors that influence the efficiency of HIV transmission at mucosal surfaces is important not only in understanding the pathogenesis of HIV type 1 (HIV-1) infection, but also for the development of preventative measures such as topically applied microbicides. Dendritic cells (DCs) are professional antigen-presenting cells localized throughout the body that are ideally positioned to survey incoming microbial pathogens. DCs are capable of taking up microbial antigens at the sites of infection and migrating to draining lymph nodes to initiate antigen-specific T-lymphocyte activation. DCs may also serve as carriers of HIV-1 from mucosal tissues to secondary lymphoid organs (6, 13, 31). A C-type lectin that is highly expressed on DCs, termed dendritic cell-specific ICAM-3 grabbing nonintegrin (DC-SIGN), binds with high affinity to the envelope protein (Env) of HIV-1 and has been posited to play an important role in this process (11).
DC-SIGN is a type II integral membrane protein primarily expressed on DCs and on some types of tissue macrophages (3, 12, 33a). Human DC-SIGN has been shown to bind all HIV-1, HIV-2, and simian immunodeficiency virus (SIV) strains examined to date (3, 11, 26) and plays an important role in virus adhesion to DCs. Once bound to DC-SIGN, virus can be transmitted to susceptible target cells expressing CD4 and viral coreceptors (3, 11, 26), providing a molecular explanation for the well-known ability of DCs to enhance HIV infection of T cells in trans (7, 28, 41). A highly similar molecule termed DC-SIGNR (DC-SIGN related) is expressed on certain types of endothelial cells in vivo and also functions as an efficient virus attachment and transmission factor (4, 27).
While DC-SIGN can mediate virus attachment and transmission in vitro (3, 11, 26), its in vivo relevance is uncertain. To assess its in vivo role in viral transmission, the distribution of DC-SIGN, particularly in mucosal tissues, needs to be carefully assessed both in humans and in nonhuman primates that serve as important model systems for HIV transmission and pathogenesis. Previously, DC-SIGN expression was demonstrated in the human cervix, rectum, and uterus (11). In serial tissue sections, the majority of the mucosal DC-SIGN+ cells were shown to coincide with CD4 but not CCR5 staining (11). To more carefully document the expression of this virus attachment factor and to determine if viral coreceptors and DC-SIGN are expressed on the same cells, we utilized double and triple fluorescence labeling and confocal microscopy on sections of mucosal tissues of both humans and rhesus macaques. To unequivocally document DC-SIGN expression, we developed monoclonal antibodies (MAbs) that were specific for either DC-SIGN or DC-SIGNR. Several of these MAbs were able to block DC-SIGN-dependent virus transmission in vitro.
In this study, we demonstrate that in the Peyer's patches, DC-SIGN was mainly expressed on major histocompatibility complex (MHC) class II+, nonplasmacytoid DCs in the interfollicular regions and in clusters of cells within the subepithelial dome region. DC-SIGNR was expressed exclusively by the endothelial cells in the Peyer's patches and in villous lamina propria. In the rectum, DC-SIGN was expressed throughout the lamina propria, and DC-SIGN+ CCR5+ CD4+ DCs were present just beneath the luminal epithelium. In the vagina, subepithelial DCs but not Langerhans' cells expressed DC-SIGN. Taken together, our results for both humans and rhesus monkeys suggest that primate lentiviruses may gain access to the DC-SIGN+ DCs most readily in the intestinal tract, while the thick squamous epithelium serves as a barrier for viral access to DC-SIGN+ DCs in the vaginal mucosa. These data are consistent with the observation that the risk of HIV-1 transmission is greater in rectal intercourse than in vaginal exposure (40). Our results also revealed that tissue distribution of DC-SIGN and viral coreceptors is quite similar in humans and in rhesus macaques and further corroborate the validity of this animal model for mucosal HIV-1 transmission studies.
MATERIALS AND METHODS
Tissue samples.
Human vaginal, rectal, and ileum tissues were obtained from Yale Pathology Critical Technologies program under an institutionally approved HIC protocol. Tissues were obtained either from archived frozen blocks of tissues from areas with no pathological involvement or from autopsy from patients that died of causes unrelated to intestinal or genital diseases. All specimens used in this study were of adult origin, and all vaginal tissues were of premenopausal women. Rhesus macaque tissues were obtained from the New England Regional Primate Research Center, Southborough, Mass., supported by Division of Research Resources (National Institutes of Health) grant RR00168. The tissue usage has complied with all federal guidelines and institutional policies.
MAbs to DC-SIGN.
MAbs DC4, DC11, and DC28, which recognize the repeat region of DC-SIGN and cross-react with DC-SIGNR, were developed as described previously (3). To obtain antibodies to the lectin domain of DC-SIGN or DC-SIGNR, mice were immunized with 3T3 cells expressing human DC-SIGN or DC-SIGNR, and MAbs were produced as previously described 3; E. J. Soilleux et al., submitted). The characterization of these MAbs is described in Results. The isotypes of the antibodies are as follows: 120506 (immunoglobulin G2a [IgG2a]), 120507 (IgG2b), 120516 (IgG2a), 120518 (IgG2a), 120526 (IgG2a), 120531 (IgG1), 120604 (IgG2b), and 120612 (IgG2a).
Cell lines and flow cytometry analysis.
We engineered T-Rex cells to express either human DC-SIGN (hu DC-SIGN), human DC-SIGNR (hu DC-SIGNR), rhesus macaque DC-SIGN (rh DC-SIGN), pigtailed macaque DC-SIGN (pt DC-SIGN), murine DC-SIGN (mu DC-SIGN), a lectin domain-truncated hu DC-SIGN (ΔC), or a repeat region-truncated hu DC-SIGN (Δrepeat) following the manufacturer's instructions (Invitrogen, Carlsbad, Calif.). For fluorescence-activated cell sorter (FACS) analysis, each T-Rex cell line was induced with 0.01 μg of doxycycline per ml overnight, recovered, and washed once with FACS buffer (phosphate-buffered saline supplemented with 3% fetal bovine serum and 0.02% sodium azide). Half a million cells were then stained with each MAb at 10 μg/ml in FACS buffer for 30 min on ice. The samples were washed and incubated with phycoerythrin-conjugated goat anti-mouse Fab fragments (Caltag, Burlingame, Calif.) (1:100) for 30 min on ice and then washed and resuspended in FACS buffer containing 2% paraformaldehyde. The samples were analyzed with a FACScan (Becton Dickinson, San Jose, Calif.) cell analyzer using the CellQuest software for data evaluation. Dead cells were excluded on the basis of their forward and side scatter characteristics.
Multiple-color immunofluorescence staining.
To examine the distribution of DC subsets and their DC-SIGN expression patterns, frozen sections of Peyer's patches, vagina, and rectum from rhesus macaques and humans were stained with a variety of antibodies in a procedure similar to that described previously (18) with minor modifications. Briefly, 6- to 8-μm frozen sections were fixed in cold acetone and blocked with NEN blocking buffer (NEN Life Science Products Inc., Boston, Mass.) containing normal donkey serum. To block endogenous biotin, the sections were further treated with Avidin/Biotin block (Vector Laboratories, Inc., Burlingame, Calif.). Next, endogenous peroxidase activity was quenched with 1% H2O2 for 10 min and primary antibodies against CD11c (Novocastra Laboratories Ltd., Newcastle, United Kingdom), CD123, CD4, CXCR4, CCR5, or HLA-DR/DP/DQ (BD PharMingen, San Diego, Calif.), CD31 or platelet-endothelial cell adhesion molecule-1 (eBioscience, San Diego, Calif.), mouse MAbs that recognize the ectodomains of both DC-SIGN and DC-SIGNR (clones DC4, DC11, or DC28), or mouse MAbs specific to either DC-SIGN (clone 120507; R&D Systems, Minneapolis, Minn.) or DC-SIGNR (clone 120604; R&D Systems) were applied for 1.5 h at room temperature. All mouse MAbs were used at 5 to 10 μg/ml except for 120507, which was applied at the concentration of 0.25 μg/ml. Slides were washed and incubated with biotin-conjugated donkey F(ab")2 anti-mouse IgG (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) for 30 min, followed by incubation with streptavidin-horseradish peroxidase conjugate (Zymed Laboratories, San Francisco, Calif.). The antigens were detected with tetramethylrhodamine-Tyramide, FITC-Tyramide, or Cy5-Tyramide (NEN Life Science Products, Inc.), or Alexa 488-Tyramide or Alexa 594-Tyramide (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions. In the case of double or triple labeling on the same section, the exact same procedure was carried out in sequence, with the following blocking steps in between: 2% H2O2 for 10 min, followed by Avidin/Biotin block, incubation with mouse IgG (Sigma-Aldrich, St. Louis, Mo.), and finally incubation with Fab goat anti-mouse IgG (Jackson Immunoresearch). At the end of the staining, slides were washed, incubated with 4",6"-diamidino-2-phenylindole (DAPI) (Molecular Probes), and mounted with Fluoromount G (Southern Biotechnology Associates, Inc. Birmingham, Ala.). The stained slides were analyzed by fluorescence microscope (Leitz Orthoplan 2) or by confocal microscopy with a Zeiss LSM510 confocal laser microscope equipped with a 40× objective lens with water.
Blocking SIV transmission by antibodies against DC-SIGN.
Inhibition of DC-SIGN-mediated transmission of SIV was assessed using a T-Rex cell line that expresses rhesus macaque DC-SIGN upon induction with doxycycline. DC-SIGN T-Rex cells and parental T-Rex cells were seeded in 96-well plates, induced with 0.01 μg of doxycycline per ml for 12 h, incubated with DC-SIGN- and DC-SIGNR-specific antibodies or mannan for 30 min, and pulsed with replication-competent SIVmac239 reporter virus harboring the MER Env. After a 3-h incubation, the cells were vigorously washed and cocultivated with CEMx174 target cells. The cultures were lysed 3 days later, and luciferase activity in 20 μl of lysate was determined by using a commercially available kit (Promega, Madison, Wis.).
RESULTS
Generation of DC-SIGN- and DC-SIGNR-specific MAbs.
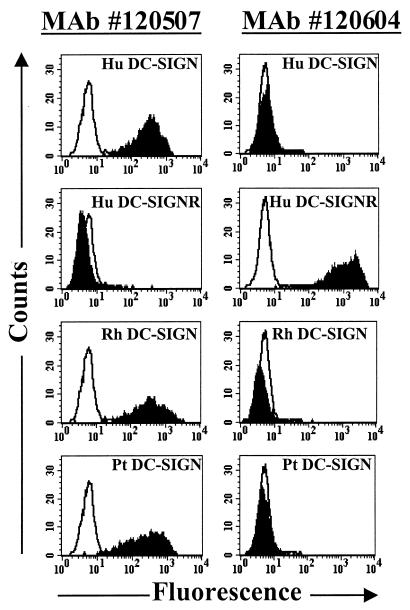
The fact that many MAbs to DC-SIGN cross-react with the closely related homologue DC-SIGNR (3, 4, 27) necessitates the development of antibodies that are specific for each of these molecules. To accomplish this, we immunized mice with murine 3T3 cells overexpressing either human DC-SIGN or DC-SIGNR. Hybridomas were generated, and the resulting MAbs were analyzed by staining cell lines expressing DC-SIGN or DC-SIGNR. By FACS analysis, we found that MAbs 120506, 120507, 120516, and 120531 bound to human, rhesus macaque, and pigtailed macaque DC-SIGN but not to DC-SIGNR; that MAb 120604 recognized human DC-SIGNR but not DC-SIGN; and that MAbs 120518, 120526, and 120612 recognized both DC-SIGN and DC-SIGNR (Fig. 1). All seven of the MAbs that bound to DC-SIGN recognized a construct lacking the repeat region of DC-SIGN (Δrepeat) (Table 1) (3). In contrast, binding to a construct lacking the lectin binding domain (ΔC) (Table 1) was not observed. Expression of this construct was confirmed by the use of a MAb to the repeat region (3). None of the MAbs recognized DC-SIGN efficiently by Western blotting (data not shown). Therefore, we conclude that these MAbs bind to conformation-dependent epitopes in the lectin binding domain of DC-SIGN. Finally, none of the MAbs recognized murine DC-SIGN (Table 1). We chose MAbs 120507 and 120604 as well as MAbs that recognize both DC-SIGN and DC-SIGNR (DC4, DC11, and DC28 [3]) to study the distribution of these molecules in human and rhesus macaque mucosal tissues.
FIG. 1.
Characterization of MAbs to DC-SIGN and DC-SIGNR. T-Rex cells stably expressing either human DC-SIGN (Hu DC-SIGN), human DC-SIGNR (Hu DC-SIGNR), rhesus macaque DC-SIGN (Rh DC-SIGN), or pigtailed macaque DC-SIGN (Pt DC-SIGN) were stimulated overnight with doxycycline and stained with DC-SIGN-specific MAb 120507 or DC-SIGNR-specific MAb 120604 as described in Materials and Methods. Overlay histograms show the staining obtained with an isotype-matched control (in white) and the specific staining (in black) obtained for each cell line and MAb. A representative experiment out of three is shown.
TABLE 1.
Monoclonal antibody reactivitiesa for stably transfected T-Rex cells
| T-Rex cell | MAb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 120604 | 120612 | 120506 | 120507 | 120516 | 120518 | 120526 | 120531 | |
| Hu DC-SIGN | − | + | + | + | + | + | + | + |
| Hu DC-SIGNR | + | + | − | − | − | + | + | − |
| Mu DC-SIGN | − | − | − | − | − | − | − | − |
| Pt DC-SIGN | − | + | + | + | + | + | + | + |
| Rh DC-SIGN | − | + | + | + | + | + | + | + |
| Hu ΔC | − | − | − | − | − | − | − | − |
| Hu Δrepeat | − | + | + | + | + | + | + | + |
Reactivities of DC-SIGN/DC-SIGNR MAbs were tested on various stably transfected T-rex cells. Data are representative of three experiments done in duplicate. +, reactivity; −, nonreactivity.
DC subset distribution in human Peyer's patch.
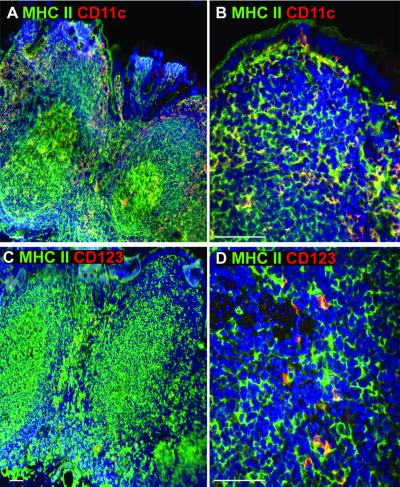
Peyer's patches harbor a significant fraction of gut-associated lymphoid tissue. While myeloid and lymphoid DCs are known to localize in the subepithelial dome and the interfollicular regions of mouse Peyer's patches, respectively (18), the tissue distribution of myeloid and plasmacytoid DCs in human Peyer's patches is not known. Prior to assessing DC-SIGN expression in the Peyer's patches, we studied the distribution of both myeloid DCs that express CD11c and plasmacytoid DCs that express interleukin 3R (IL-3R) (8, 14, 21, 29, 30) by using double immunofluorescence labeling of frozen sections of the terminal ileum (Fig. 2). We found that all CD11c+ cells expressed high levels of MHC class II and were distributed in the subepithelial dome region, the interfollicular regions, and the villous lamina propria. The germinal centers also contained large CD11c+/MHC class II+ DCs. In contrast, cells expressing IL-3R (CD123) were found exclusively in the interfollicular regions, and also expressed moderate levels of MHC class II molecule (Fig. 2C and D). IL-3R staining was also found on the high endothelial venules as reported previously (8, 14). Thus, in the Peyer's patches, CD11c+ DCs were found in the subepithelial dome region, germinal center, and interfollicular regions, whereas plasmacytoid DCs were localized exclusively in the interfollicular regions.
FIG. 2.
Immunofluorescence analysis of DC subset distribution in human Peyer's patches. Human terminal ileum tissues were embedded in OCT medium, and 6- to 8-μm frozen sections were stained with antibodies against MHC class II (HLA-DR/DP/DQ) (green) and CD11c (red) (A and B) or IL-3R (CD123; red) (C and D). The subepithelial dome region (B) and the interfollicular regions (D) containing CD11c+/MHC class II+ or IL-3R+/MHC class II+ DCs, respectively, are shown at higher magnifications. Images were captured using either 10× (A and C) or 40× (B and D) objective lenses. No background staining was detected when mouse IgG was used as a control (data not shown). The nucleus was stained with DAPI (blue). White bar, 100 μm in this and all subsequent figures. This figure gives results representative of three different donor tissues.
DC-SIGN is expressed in the dome and interfollicular regions of human Peyer's patches.
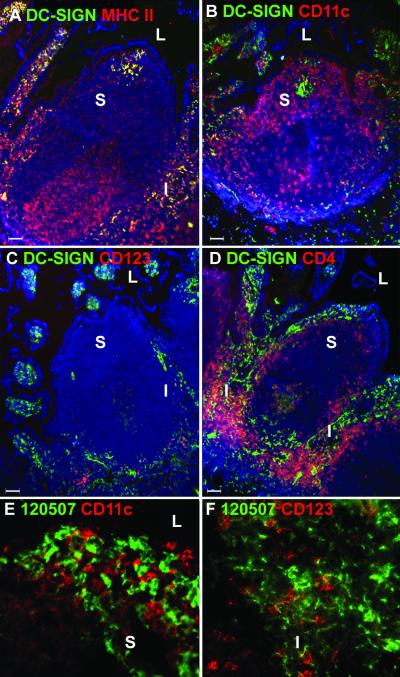
Having established the localization of myeloid and plasmacytoid DC populations in human Peyer's patches, we investigated whether these subsets of DCs express DC-SIGN. We used MAb DC4, which recognizes the repeat region of DC-SIGN and also cross-reacts with DC-SIGNR. Throughout this study we found that MAbs DC4, DC11, and DC28 gave very similar staining patterns but that the use of DC4 and DC11 resulted in the most intense staining with humans and rhesus macaques, respectively. We also used the DC-SIGN-specific MAb 120507 to demonstrate DC-SIGN expression by DC subsets in human Peyer's patches (Fig. 3E and F). As shown in Fig. 3A, all cells that were stained with MAb DC4, except for the high endothelial venules, were MHC class IIhi, confirming the earlier report that DC-SIGN is specifically expressed on DCs (12). However, not all MHC class II+ cells in the Peyer's patch were DC-SIGN+. MHC class II+ DC-SIGN− cells consisted of B cells and DCs in the germinal center, interfollicular region, and dome area (Fig. 3A). Most DC-SIGN+ cells in Peyer's patches were localized in the interfollicular regions (Fig. 3). We also observed numerous DC-SIGN+ cells within the villous lamina propria. In about half of the Peyer's patches, we detected distinct clusters of DC-SIGN+ cells in the subepithelial dome region. The DC-SIGN+ cell clusters were normally MHC class II+ (Fig. 3A) but did not express the CD11c molecule, as demonstrated by confocal microscopy analysis (Fig. 3E).
FIG. 3.
DC-SIGN expression in human Peyer's patches. Frozen sections of human Peyer's patches were stained with antibodies against DC-SIGN (DC4) (A to D) or DC-SIGN-specific MAb120507 (E and F) (green) and MHC class II (A) (red), CD11c (B and E) (red), IL-3R (C and F) (red), or CD4 (D) (red). Images were captured using a 10× objective lens (A to D). Notice the cluster of DC-SIGN+ cells in the subepithelial dome region (A and B). The nucleus was stained with DAPI (blue) (A to D). L, lumen; S, subepithelial dome region; I, interfollicular region. To examine DC-SIGN-specific staining on DC subsets, Peyer's patches stained with MAb 120507 were analyzed by confocal microscopy. (E) Subepithelial dome region stained with 120507 (green) and CD11c (red). (F) Interfollicular region stained with 120507 (green) and CD123 (red). Similar data were obtained on Peyer's patches from three different donors.
To determine if the interfollicular region DC-SIGN+ cells represented plasmacytoid DCs, we performed double labeling with antibodies to IL-3R and DC-SIGN (Fig. 3C) or with DC-SIGN-specific MAb 120507 (Fig. 3F). We did not observe a double-positive cell population, indicating that plasmacytoid DCs do not express DC-SIGN, at least in the tissues we have examined here. However, DC-SIGN+ cells and IL-3R+ cells were often found in close proximity to one another (Fig. 3C and F). Further, we analyzed the expression of the viral coreceptor CCR5 since the absence of this molecule is associated with a high degree of protection from HIV infection (20, 32). The human Peyer's patches contained very few CCR5+ cells, and most CCR5+ cells were found in the villous lamina propria and not within the Peyer's patches (data not shown). On the other hand, many CD4+ T cells were found in the interfollicular region and in the lamina propria of the villi (Fig. 3D). Within the interfollicular region, we detected only a few cells that coexpressed DC-SIGN and CD4. Thus, in human Peyer's patches, DC-SIGN+ DCs localize predominantly in the interfollicular region and in clusters within the subepithelial dome region but do not express appreciable levels of CD4 or the viral coreceptor CCR5.
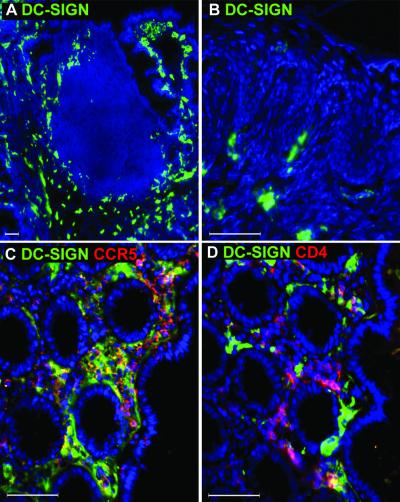
DC-SIGN+ cells are not found within the human vaginal epithelium.
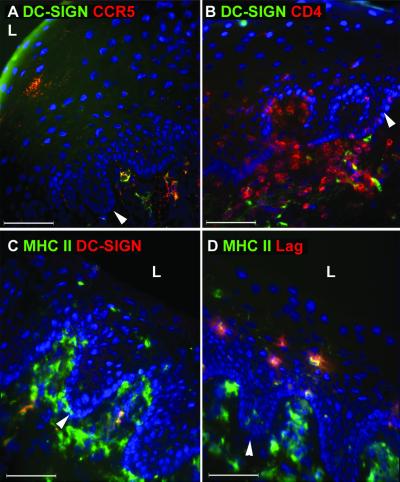
To determine whether Langerhans' cells within the vaginal epithelium express DC-SIGN, we analyzed human and macaque vaginal tissues. In none of the tissues examined did we find DC-SIGN+ cells within the epithelium, despite the abundance of Langerhans' cells expressing Birbeck granules (recognized by the Lag antibody) and MHC class II (Fig. 4D). Instead, DC-SIGN+ cells were detected in the subepithelial lamina propria, and a small portion of these cells coexpressed CCR5 (Fig. 4A). A similar pattern of DC-SIGN expression has been described on subepithelial cells in the cervix, although no CCR5 expression on these cells was detected (11). Some of the DC-SIGN+ cells were found to be in close contact with CD4+ T cells in the lamina propria (Fig. 4B). All DC-SIGN+ cells were MHC class II+ as shown in the Peyer's patches, but only a subset of DCs in the lamina propria expressed DC-SIGN (Fig. 4C). We did not observe any DC-SIGNR-specific staining in the vagina (data not shown), indicating that staining detected by MAb DC4 is DC-SIGN specific in this tissue. Overall, the expression levels of DC-SIGN in the vaginal mucosa appeared to be significantly lower than that found in the gastrointestinal mucosa since we had to use higher magnification and longer exposures to detect DC-SIGN. In summary, Langerhans' cells in the vaginal mucosa did not express DC-SIGN, and only a very small portion of the subepithelial DC-SIGN+ cells expressed CCR5.
FIG. 4.
Langerhans' cells in the vaginal epithelium do not express DC-SIGN. Frozen sections of human vaginal tissue were stained with antibodies against DC-SIGN (DC4) in green (A and B) or red (C) in combination with antibodies specific for CCR5 (red) (A), CD4 (red) (B), or MHC class II (green) (C). To demonstrate the presence of Langerhans' cells in the same tissue, a serial section was stained with antibodies to MHC class II (green) and Lag antibodies which recognize the Birbeck granules (red) (D). The nucleus was stained with DAPI (blue). The white arrow indicates the base of the vaginal epithelium. Images were captured using a 40× objective lens. L, lumen. This figure is representative of vaginal samples from three different donors examined.
Human rectal mucosa contains abundant DC-SIGN+ cells.
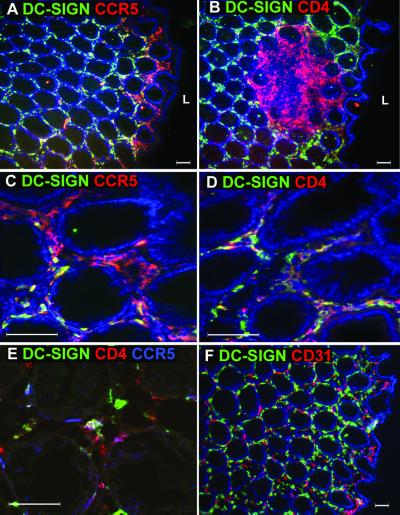
By far the most remarkable expression of DC-SIGN was found in the rectal mucosa (Fig. 5). DC-SIGN expression was found throughout the lamina propria of the rectum, though there were no intraepithelial DC-SIGN+ DCs. Moreover, CCR5+ cells were found to line the subepithelial region near the lumen of the rectum. DC-SIGN+ CCR5+ cells formed a narrow band of cells close to the lumen. We also noted that the solitary lymphoid nodules found along the length of the rectum contained abundant CD4+ T cells but that DC-SIGN expression was minimal except on rare cells in the perimeter within these follicles (Fig. 5D). By confocal microscopy analysis, cells which expressed all three molecules, CCR5, CD4, and DC-SIGN, were found near the lumen of the rectum (Fig. 5E). Since MAb DC4 used in these assays cross-reacted with DC-SIGNR, we examined whether some of the staining observed represented DC-SIGNR. We did not detect any DC-SIGNR-specific staining with MAb 120604 in the rectum (data not shown). Moreover, although numerous capillary endothelial cells were present in the rectal tissues, we did not detect any staining of endothelial cells with MAb DC4 (Fig. 5F). In addition, MAbs DC4 and 120507 gave identical staining patterns (data not shown), supporting the lack of DC-SIGNR expression by the rectal endothelial vessels. Thus, the rectum contains numerous DC-SIGN+ cells in the lamina propria, some of which coexpress CD4 and CCR5.
FIG. 5.
Rectal mucosa contains DC-SIGN+ cells that also express CCR5 and CD4 close to the lumen. Rectal sections were stained with antibodies against DC-SIGN (DC4) (green) and CCR5 (red) (A and C), CD4 (red) (B and D), or CD31 (red) (F). (C and D) Close-ups of the areas of rectum tissue near the lumen shown in panels A and B, respectively. Images were captured using either 10× (A, B, and F) or 40× (C and D) objective lenses. The nucleus was stained with DAPI (blue). In order to colocalize DCs that expressed D-SIGN, CCR5, and CD4, rectum sections were stained with three antibodies: anti-DC-SIGN (green), anti-CCR5 (blue), and anti-CD4 (red). Confocal microscopy was used to determine triple-positive cells (indicated by the white arrow) with a 40× objective lens (E). L, lumen. This figure is representative of rectal samples from six different donors examined.
DC-SIGN expression in Peyer's patches, vagina, and rectum of rhesus macaque.
Infection of rhesus macaques with SIV or simian-human immunodeficiency virus represents the most widely used animal model to study HIV pathogenesis in vivo. A number of studies have utilized this primate model of HIV to examine viral entry and infection via mucosal surfaces (16, 23, 33, 34, 35, 38). This model provides the best system to study the role of DC-SIGN in virus transmission and pathogenesis. We have previously shown that rhesus macaque DC-SIGN binds and transmits all HIV-1, HIV-2, and SIV strains tested (3). However, the pattern of DC-SIGN expression in rhesus macaque tissues is not known. To determine DC-SIGN expression in rhesus macaques, we performed immunofluorescence staining of tissue sections using MAbs that recognize rhesus macaque DC-SIGN. Overall, the distribution of DC-SIGN in rhesus tissues was very comparable to that found with humans. In rhesus macaque Peyer's patches, DC-SIGN+ cells were localized in the interfollicular regions and sometimes in a cluster within the subepithelial dome region (Fig. 6A). Although IL-3R expression on rhesus tissues was very similar to that found with humans (data not shown), the anti-human CD11c antibodies tested stained only a few cells within primate Peyer's patches, making it difficult to demonstrate CD11c+ DC distribution (data not shown). As in humans, vaginal epithelium did not contain DC-SIGN+ Langerhans' cells, whereas a subset of the subepithelial DCs expressed this molecule (Fig. 6B). Similar to what was observed for humans, the primate rectal tissue contained abundant DC-SIGN+ DCs, and the expression pattern of CCR5 was indistinguishable from that found in the human rectum, although somewhat fewer CD4+ DC-SIGN+ cells were found in the rhesus macaque rectum than in humans. Here again we observed DCs that were DC-SIGN+ CD4+ and DC-SIGN+ CCR5+ just beneath the luminal epithelium (Fig. 6C and D).
FIG. 6.
Expression of DC-SIGN on rhesus macaque tissues. Primate tissues containing the Peyer's patches (A) and vaginal (B) and rectal (C and D) tissues were stained with mouse MAb against DC-SIGN (DC11). The rectal sections were also doubly labeled with antibodies against CCR5 (red) (C) or CD4 (red) (D). The nucleus was stained with DAPI (blue). Images were captured using 10× (A) or 40× (B to D) objective lenses. L, lumen. This figure is representative of tissues from two monkeys examined.
Differential expression of DC-SIGN and DC-SIGNR in Peyer's patches.
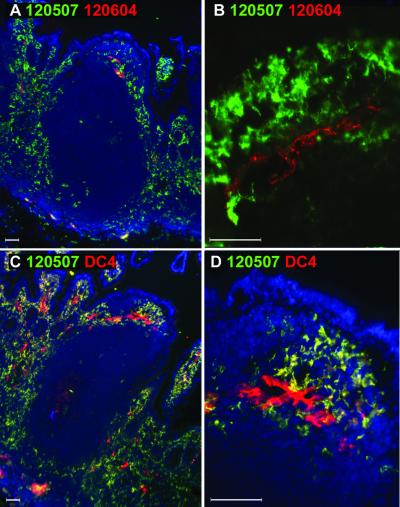
Because MAbs DC4, DC11, and DC28 recognized both DC-SIGN and DC-SIGNR, the staining pattern observed thus far could represent either of these molecules, though expression of DC-SIGNR thus far appears to be limited to certain types of endothelial cells (4, 27). In order to distinguish staining for DC-SIGN and DC-SIGNR, we performed double labeling of tissue sections using MAbs specific for DC-SIGN (120507) or DC-SIGNR (120604) (Fig. 7A and B). Although there were rare double-positive cells observed inside some of the endothelial vessels (Fig. 7A), confocal analysis of this region ruled out the existence of cells that express both DC-SIGN and DC-SIGNR (Fig. 7B). Similarly, we compared the staining by MAb DC4, which recognizes the ectodomains of both DC-SIGN and DC-SIGNR, to DC-SIGN-specific-MAb 120507 (Fig. 7C and D). Cells which stained specifically for DC-SIGN were dendritic in morphology and were identical to those stained with MAb DC4. Strikingly, the only cells that stained with DC4 but not with DC-SIGN-specific MAb 120507 were the endothelial cells found in the interfollicular regions and within villous lamina propria, confirming DC-SIGNR expression detected by MAb 120604 (Fig. 7C and D; red cells). By double labeling with a MAb specific for endothelial cells, we determined that about 30% of all endothelial cells in the Peyer's patches expressed DC-SIGNR (Fig. 8A and B). Although capillary vessels composed of endothelial cells were clearly present in the rectal and vaginal tissues, we did not detect staining specific for DC-SIGNR with MAb 120604 as described earlier (data not shown). Thus, DC-SIGNR expression in the human Peyer's patches is limited to endothelial cells, whereas DC-SIGN expression is restricted to DCs in the tissues examined.
FIG. 7.
Comparison of DC-SIGN and DC-SIGNR expression in human Peyer's patches. Human Peyer's patches were doubly labeled with mouse MAbs specific for DC-SIGN (120507; green) or DC-SIGNR (120604; red) (A and B). The staining by MAb DC4 (recognizing both DC-SIGN and DC-SIGNR; red) and DC-SIGN-specific staining by MAb 120507 (green) were compared (C and D). The nucleus was stained with DAPI (blue). To examine the presence of DC-SIGN+/DC-SIGNR+ cells found in the endothelium of the dome region in A, confocal microscopy analysis was carried out using a 40× objective lens (B). Images were captured using 10× (A and C) or 40× (B and D) objective lenses. Close-up of subepithelial dome region in panel C is shown in panel D.
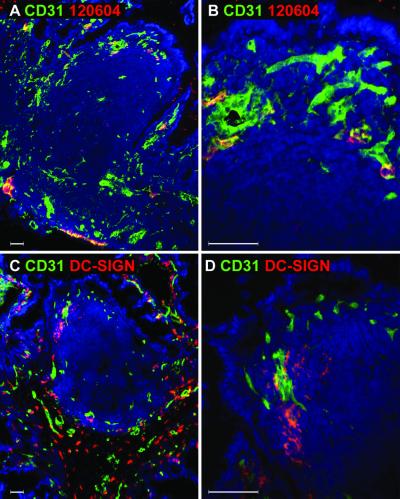
FIG. 8.
Endothelial expression of DC-SIGNR and DC-SIGN in human and rhesus macaque Peyer's patches. Human (A and B) and rhesus (C and D) Peyer's patches were doubly labeled with a MAb to endothelial cells (CD31; green) in conjunction with a MAb specific for DC-SIGNR (120604; red) (A and B) or MAb DC11, which recognizes both human DC-SIGN and DC-SIGNR (red) (C and D). No staining was detected with MAb 120604 on rhesus tissues (data not shown). The nucleus was stained with DAPI (blue). Images were captured using 10× (A and C) or 40× (B and D) objective lenses. Close-ups of the subepithelial dome regions shown in panels A and C are shown in panels B and D, respectively. This figure is representative of at least five Peyer's patches examined.
Lack of DC-SIGNR expression in rhesus macaque Peyer's patches.
To demonstrate DC-SIGNR expression in rhesus Peyer's patches, we performed immunofluorescence labeling with human DC-SIGNR-specific MAb 120604. In contrast to results obtained with human tissues, we were not able to detect DC-SIGNR expression when using MAb 120604 on rhesus macaque tissues (data not shown). We hypothesized that MAb 120604 may recognize an epitope in human DC-SIGNR that is not conserved in the rhesus DC-SIGNR homologue. Thus, we used MAb DC11, which recognizes rhesus DC-SIGN and cross-reacts with human DC-SIGNR, to determine if DC-SIGNR is expressed on endothelial cells in rhesus Peyer's patches. As shown in Fig. 8C and D, endothelial cells stained with anti-CD31 antibody (green) did not stain with MAb DC11 (red). Thus, in rhesus macaque Peyer's patches, no DC-SIGN or DC-SIGNR staining was detected on endothelial cells with MAb DC11 and no DC-SIGNR-specific staining was observed with MAb 120604. However, we have not been able to clone rhesus macaque DC-SIGNR, and as a consequence we cannot be certain that our MAbs, which recognize human DC-SIGN and DC-SIGNR and cross-react with rhesus DC-SIGN, actually bind to rhesus DC-SIGNR.
Rhesus DC-SIGN-dependent transmission of SIV is blocked by an anti-DC-SIGN MAb.
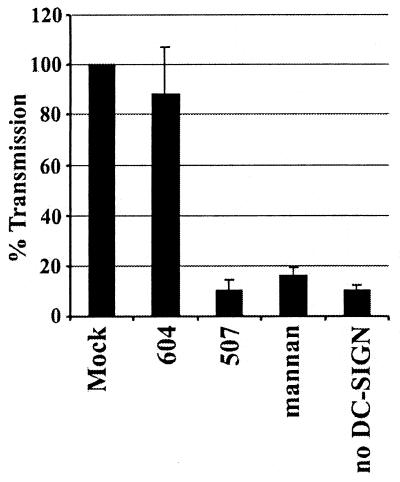
Our studies showed that DC-SIGN expression in rhesus macaques was similar to that observed with human mucosal tissues. To assess the importance of DC-SIGN for virus transmission and dissemination in vivo, specific antibodies or small molecules that block SIV or simian-human immunodeficiency virus interactions with rhesus DC-SIGN will be needed. To determine if the DC-SIGN-specific MAb 120507 could block transmission of SIV by rhesus DC-SIGN, we used an inducible T-Rex cell line that expresses rhesus DC-SIGN but does not express CD4 or CCR5. Thus, these cells cannot be infected by SIV. The DC-SIGN-positive cells were incubated with MAb 120507, the DC-SIGNR-specific MAb 120604, or the carbohydrate mannan, which has been shown to block HIV binding to human DC-SIGN. A luciferase reporter virus bearing the SIVmac239MER Env protein was then added for 3 h, the cells were washed vigorously to remove unbound virus, and CEMx174 target cells were added. Three days later the extent of virus infection was determined by measuring luciferase activity. As shown in Fig. 9, preincubation with MAb 120507 and mannan, but not with the DC-SIGNR-specific MAb 120604, reduced rhesus DC-SIGN-mediated virus transmission to background levels. Thus, MAb 120507 represents a specific immunological reagent that can be used to prevent SIV interactions with rhesus macaque DC-SIGN.
FIG. 9.
Blocking of rhesus macaque DC-SIGN-mediated SIV transmission by anti-DC-SIGN antibodies. T-Rex cells that express rhesus macaque DC-SIGN under a doxycycline-inducible promoter and control T-Rex cells were seeded in 96-well plates and induced with doxycycline. The cells were incubated with 20 μg of the indicated antibodies or mannan per ml and then pulsed with SIVmac239 MER Env replication-competent luciferase reporter virus. After a 3-h incubation, the cells were vigorously washed and cocultivated with CEMx174 target cells. After 72 h the cocultures were lysed and the luciferase activity was quantified. The results are presented as percent transmission ± standard deviation observed from untreated cells. A representative experiment carried out in triplicate is shown, and similar results were obtained in two independent experiments.
DISCUSSION
The precise role of DCs in mediating HIV-1 transmission and pathogenesis at mucosal surfaces is unclear. In vitro, infection of peripheral blood mononuclear cells by HIV-1 can be strongly enhanced by including cocultured DCs (7, 15, 17, 28, 41). While DCs are often not infected by HIV-1, virus binds to DCs efficiently and, once bound, can be retained in an infectious state for a prolonged period of time (11). Subsequent addition of susceptible cell types results in infection by virus in trans (7, 15, 17, 28, 41). The ability of DCs to bind HIV-1 and to transmit bound virus to receptor-positive cells has been linked to DC-SIGN (2, 3, 11, 26). These in vitro observations, coupled with the fact that mucosal DCs are among the first cell types encountered by HIV-1 at mucosal surfaces (10, 16, 34, 36), raise the possibility that HIV-1 interactions with DC-SIGN could impact sexual transmission.
To assess the role of DC-SIGN for virus transmission and pathogenesis, it will be important to develop reagents that specifically block virus binding to DC-SIGN. The abilities of such compounds to impact virus transmission could then be assessed in nonhuman primate models such as rhesus macaques, provided that rhesus DC-SIGN functions like human DC-SIGN and is expressed in a manner similar to that of its human homologue. We have recently shown that rhesus macaque DC-SIGN efficiently binds and transmits HIV-1, HIV-2, and SIV strains (3). In this study, we document the expression of DC-SIGN in mucosal tissues from rhesus macaques and identify a MAb that blocks SIV interactions with DC-SIGN in vitro. Our work extends previous studies by using MAbs specific for DC-SIGN and DC-SIGNR, by studying DC-SIGN expression in macaque tissues, and by using double and triple fluorescence labeling and confocal microscopy to correlate DC-SIGN expression with expression of CD4, CCR5, and specific DC subsets. The expressions of DC-SIGN in humans and in rhesus macaques were highly similar.
In the Peyer's patches, DC-SIGN+ cells were found predominantly in the interfollicular regions, with the exception of aggregates of DC-SIGN+ cells in the subepithelial dome region. It is now clear that there is considerable variation in the localization, phenotype, and function of different DC populations in various tissues. The human DC subsets in the peripheral blood have been categorized on the basis of differential expression of myeloid (CD11c) and plasmacytoid (IL-3R) markers (reviewed in reference 21). We show here that CD11c+ DCs are distributed in the dome and interfollicular regions and in the germinal center of the Peyer's patches, whereas the IL-3R+ plasmacytoid DCs are found exclusively in the interfollicular regions. Although all DC-SIGN+ cells expressed MHC class II molecules, we did not observe expression of DC-SIGN on IL-3R+ plasmacytoid DCs. This finding is consistent with a recent report of the lack of DC-SIGN mRNA expression by peripheral blood-derived plasmacytoid DCs (24). Thus, plasmacytoid DCs are unlikely to participate in HIV infection of CD4+ cells in trans, at least in a DC-SIGN-dependent manner. Further, CD11c+ DCs in the dome region were not found to express DC-SIGN. These data suggest that the DC-SIGN+ cells belong to another subset of DCs that express neither CD11c nor IL-3R within the Peyer's patches. Recently, a careful analysis of human tonsils revealed the presence of the CD11c− CD123− DCs, which expressed MHC class II and CD68 (37). When DC-SIGN expression on CD68+ cells was examined, we indeed observed that many of the dome region DC-SIGN+ cells coexpressed CD68 (unpublished observation). Yet, the majority of DC-SIGN+ cells in the interfollicular regions were CD68− CD11c− IL-3R− (unpublished observation). Future studies must address how these distinct DC subsets mediate virus transmission and immune induction during HIV-1 infection in vivo.
The epithelium covering the Peyer's patches over the dome contains M cells specialized in the uptake of luminal antigens and is known as the follicle-associated epithelium (19). HIV-1 has been shown to gain entry through M cells in rabbit and mouse Peyer's patches, although neither species supports efficient HIV infection (1). Thus, Peyer's patches are potentially the first sites of HIV entry during oral transmission in cases such as mother-to-child breastfeeding (9). Further, Peyer's patches may represent sites of HIV entry following rectal exposure since rectally injected materials have been shown to be processed and presented within Peyer's patches (5). The presence of clusters of DC-SIGN+ DC in the subepithelial dome region raises the possibility that these DCs may be the first to bind HIV-1 that enters the Peyer's patches via M cells or through tears in the mucosa. Since the dome region DC-SIGN+ DCs did not express the viral coreceptors CCR5 or CD4 at detectable levels, they are less likely to be directly infected by the virus but rather participate in infection of nearby CD4+ T cells. Aside from its HIV binding property, DC-SIGN has also been shown to interact with ICAM-3 expressed on resting T cells and mediate DC-T-cell conjugation (12). Thus, it is also possible that DC-SIGN+ DC aggregates in the dome region acts as the gatekeeper capable of capturing, processing, and presenting HIV antigenic peptides and intact virus to CD4+ T cells within this region. Indeed, CD4+ T cells were found in close proximity to the DC-SIGN+ cells of the subepithelial dome region (Fig. 3D).
In the vaginal mucosa, DC-SIGN expression was not detected within the squamous epithelial layer, but it was detected on rare DCs in the subepithelial lamina propria, comparable to what has been reported for the cervix (11). A small percentage of these cells expressed CCR5. In contrast, DC-SIGN+ DCs were abundantly distributed throughout the lamina propria immediately adjacent to the epithelium of the rectal mucosa. Moreover, DC-SIGN+ DCs that coexpressed the HIV-1 coreceptors CCR5 and CD4 formed a narrow band beneath the rectal luminal epithelium. These data are in discordance with previous studies showing that CCR5 is not expressed by mucosal DCs. Hladik and colleagues demonstrated that, unlike peripheral blood-derived DCs, cervicovaginal DCs express undetectable CCR5 but form stable conjugates with T cells which permit productive infection by HIV-1 (15). More recently, Geijtenbeek et al. reported that CCR5 is not expressed in the rectum or uterus (11). These apparent differences may be attributed to the sensitivities of the assays. For instance, we had to amplify the staining signal using the Tyramide amplification system in order to visualize CCR5 on these sections. Since only low levels of CCR5 are needed to support entry by many virus strains, especially if CD4 is expressed at high levels, the levels of CCR5 detected here could be relevant for virus infection (25). Further, as discussed above, we found CCR5 expression to be restricted to the lamina propria immediately beneath the luminal epithelium in the rectum. Thus, the ability to detect CCR5+ cell population in the rectum may also depend on the tissue orientation. By the same token, it is possible that CCR5 is expressed by Peyer's patch DCs below the limit of detection by our immunofluorescence protocol, since CCR5 expression in the gut-associated lymphoid tissue has been demonstrated by a much more sensitive flow cytometry analysis (39). Thus, future studies are needed to examine the virological relevance of the levels of the HIV coreceptors expressed by each DC subset during in vivo infection.
The examination of the relevance of mucosal DCs for the trans infection of T cells must also take into consideration the level of DC-SIGN expressed by these cells. Not only are the tissue distributions of DC-SIGN+ cells different in the vaginal and rectal mucosae, but also the levels of DC-SIGN expression by DC in these tissues appeared to differ considerably. We have recently demonstrated that the efficiency of HIV-1 binding and transmission is strongly dependent on the level of DC-SIGN expression (26). Although we were not able to estimate the level of DC-SIGN expressed on mucosal DCs due to the nonquantitative nature of the immunofluorescence technique, such measurements may prove useful in predicting the efficiency with which DCs in different mucosal sites can transmit HIV-1 to T cells in vivo.
Since most MAbs against DC-SIGN also detect DC-SIGNR, it was important to distinguish whether the staining was specific for DC-SIGN or DC-SIGNR. In previous studies, DC-SIGNR has been detected on endothelial cells in placenta, liver, and lymph nodes (4, 27). By producing a DC-SIGNR-specific MAb, we were able to show that DC-SIGNR is also expressed on about one-third of the capillary endothelial cells in the human ileum. In contrast, no DC-SIGNR-specific staining was observed in rhesus tissues. The MAb DC11, which recognizes the ectodomain of human and rhesus DC-SIGN and cross-reacts with human DC-SIGNR, did not stain rhesus endothelial cells. In fact, double labeling of rhesus Peyer's patches with MAb DC11 and DC-SIGN-specific MAb 120507 resulted in an identical staining pattern (data not shown), suggesting that MAb DC11 detects only DC-SIGN in rhesus tissues. Thus, either the rhesus DC-SIGNR homologue is distinct in the regions recognized by MAb 120604 and MAb DC11, it is not expressed in the tissues we examined, or no homologue for DC-SIGNR exists in rhesus macaques.
If DC-SIGN plays a role in virus transmission, then the accessibility of DC-SIGN+ DCs within different mucosal exposure sites would likely influence the efficiency of this process. During vaginal exposure, virus must somehow cross the epithelial layer to reach DC-SIGN+ DCs in the subepithelial lamina propria. It is interesting that progesterone treatment, which results in thinning of the vaginal epithelium, has been shown to contribute to a higher incidence of vaginal SIV transmission in rhesus macaques (22). Hormonal influences and microbial flora within the vaginal mucosa that result in thinning of the epithelium may potentially enhance the ability of HIV to gain access to the DC-SIGN+ cells in the lamina propria. In contrast, DC-SIGN+ CD4+ CCR5+ DCs in the rectal mucosa are separated from the lumen by only a single columnar epithelium, which should allow virus access to DC-SIGN+ DCs more easily. This hypothesis is corroborated by the fact that the HIV-1 transmission risk is greater in anal intercourse than in vaginal coitus in women (40).
In summary, DCs expressing DC-SIGN were distributed similarly in the mucosal surfaces of humans and rhesus macaques. The physical barriers that exist between the lumen and the closest DC-SIGN+ DCs were greatest in the vaginal mucosa and least in the rectum. An intriguing finding, however, is that the DC-SIGN+ DCs located near the lumen of the rectum were also positive for CD4 and CCR5. These DCs may not only ferry HIV to the draining lymph nodes but also become infected within the rectal mucosa and form a local viral factory in DC-T-cell conjugates. Further understanding of this viral adhesion molecule may provide insight into its potential use in novel preventative microbicidal agents against HIV transmission.
Acknowledgments
We thank Ken Price, Tracey Crisp, and Jim David for their assistance in the production and characterization of the monoclonal antibodies and Charles Dela Cruz for editorial assistance.
A.I. was supported by a Burroughs Wellcome Fund Career Award in Biomedical Sciences and by the American Foundation for AIDS Research. F.B. was supported by a fellowship from the Swiss National Science Foundation (grant 823A-611772). S.P. was supported by a fellowship from the Deutsche Forschungsgemeinshaft. R.W.D. was supported by NIH grants AI35383 and 40880, a Burroughs Wellcome Fund Transnational Research Award, and an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
REFERENCES
- 1.Amerongen, H. M., R. Weltzin, C. M. Farnet, P. Michetti, W. A. Haseltine, and M. R. Neutra. 1991. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 4:760-765. [PubMed] [Google Scholar]
- 2.Baribaud, F., S. Pohlmann, and R. W. Doms. 2001. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286:1-6. [DOI] [PubMed] [Google Scholar]
- 3.Baribaud, F., S. Pöhlmann, T. Sparwasser, M. T. Yu Kimata, Y.-K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. Edwards, G. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtail macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, P., M. Pope, A. Granelli-Piperno, and R. M. Steinman. 1996. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 59:158-171. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 8.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 9.Embree, J. E., S. Njenga, P. Datta, N. J. Nagelkerke, J. O. Ndinya-Achola, Z. Mohammed, S. Ramdahin, J. J. Bwayo, and F. A. Plummer. 2000. Risk factors for postnatal mother-child transmission of HIV-1. AIDS 14:2535-2541. [DOI] [PubMed] [Google Scholar]
- 10.Frankel, S. S., K. Tenner-Racz, P. Racz, B. M. Wenig, C. H. Hansen, D. Heffner, A. M. Nelson, M. Pope, and R. M. Steinman. 1997. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am. J. Pathol. 151:89-96. [PMC free article] [PubMed] [Google Scholar]
- 11.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 13.Grouard, G., and E. A. Clark. 1997. Role of dendritic and follicular dendritic cells in HIV infection and pathogenesis. Curr. Opin. Immunol. 9:563-567. [DOI] [PubMed] [Google Scholar]
- 14.Grouard, G., M. C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hladik, F., G. Lentz, R. E. Akridge, G. Peterson, H. Kelley, A. McElroy, and M. J. McElrath. 1999. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J. Virol. 73:5833-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ignatius, R., F. Isdell, U. O'Doherty, and M. Pope. 1998. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J. Med. Primatol. 27:121-128. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraehenbuhl, J. P., and M. R. Neutra. 2000. Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16:301-332. [DOI] [PubMed] [Google Scholar]
- 20.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Y. J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585-589. [DOI] [PubMed] [Google Scholar]
- 22.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 23.Miller, C. J., P. Vogel, N. J. Alexander, S. Sutjipto, A. G. Hendrickx, and P. A. Marx. 1992. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am. J. Pathol. 141:655-660. [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75:6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 29.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 30.Robinson, S. P., S. Patterson, N. English, D. Davies, S. C. Knight, and C. D. Reid. 1999. Human peripheral blood contains two distinct lineages of dendritic cells. Eur. J. Immunol. 29:2769-2778. [DOI] [PubMed] [Google Scholar]
- 31.Rowland-Jones, S. L. 1999. HIV: the deadly passenger in dendritic cells. Curr. Biol. 9:R248-R250. [DOI] [PubMed] [Google Scholar]
- 32.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 33.Sodora, D. L., A. Gettie, C. J. Miller, and P. A. Marx. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S119-S123. [PubMed] [Google Scholar]
- 33a.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. DC-SIGN is expressed on subsets of dendritic cells and specialized macrophages in tissue, and on a sub-population of plasmacytoid blood dendritic cells. J. Leukoc. Biol., in press.
- 34.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 36.Steinman, R., L. Hoffman, and M. Pope. 1995. Maturation and migration of cutaneous dendritic cells. J. Investig. Dermatol. 105:2S-7S. [DOI] [PubMed] [Google Scholar]
- 37.Summers, K. L., B. D. Hock, J. L. McKenzie, and D. N. Hart. 2001. Phenotypic characterization of five dendritic cell subsets in human tonsils. Am. J. Pathol. 159:285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 39.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voeller, B. 1991. AIDS and heterosexual anal intercourse. Arch. Sex. Behav. 20:233-276. [DOI] [PubMed] [Google Scholar]
- 41.Weissman, D., T. D. Barker, and A. S. Fauci. 1996. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J. Exp. Med. 183:687-692. [DOI] [PMC free article] [PubMed] [Google Scholar]