Abstract
In the presence of complementing adeno-associated virus type 2 (AAV-2) Rep proteins, AAV-2 genomes can be pseudotyped with the AAV-5 capsid to assemble infectious virions. Using this pseudotyping strategy, the involvement of the ubiquitin-proteasome system in AAV-5 and AAV-2 capsid-mediated infections was compared. A recombinant AAV-2 (rAAV-2) proviral luciferase construct was packaged into both AAV-2 and AAV-5 capsid particles, and transduction efficiencies in a number of cell lines were compared. Using luciferase expression as the end point, we demonstrated that coadministration of the viruses with proteasome inhibitors not only increased the transduction efficiency of rAAV-2, as previously reported, but also augmented rAAV-5-mediated gene transfer. Increased transgene expression was independent of viral genome stability, since there was no significant difference in the amounts of internalized viral DNA in the presence or absence of proteasome inhibitors. Western blot assays of immunoprecipitated viral capsid proteins from infected HeLa cell lysates and in vitro reconstitution experiments revealed evidence for ubiquitin conjugation of both AAV-2 and AAV-5 capsids. Interestingly, heat-denatured virus particles were preferential substrates for in vitro ubiquitination, suggesting that endosomal processing of the viral capsid proteins is a prelude to ubiquitination. Furthermore, ubiquitination may be a signal for processing of the capsid at the time of virion disassembly. These studies suggest that the previously reported influences of the ubiquitin-proteasome system on rAAV-2 transduction are also active for rAAV-5 and provide a clearer mechanistic framework for understanding the functional significance of ubiquitination.
Adeno-associated viruses (AAV) are members of the dependent parvovirus family that requires helper viruses, such as adenovirus, to initiate productive infection and genome replication (27). Six distinct serotypes of primate AAV have been reported to date (2, 5, 6, 26, 31, 41). Cloning and sequence characterization of these serotypes indicate that they share a similar genomic organization, which consists of two large open reading frames (ORFs) flanked by two inverted terminal repeats (ITRs). The ITR structure is the minimal sequence required for AAV DNA replication, provirus integration, and packaging of progeny AAV DNA into virus particles. The left ORF encodes four nonstructural Rep proteins. These proteins not only are the regulators of AAV transcription (22) but also are involved in AAV replication (35) and virus assembly (21) and play a role in site-specific integration of the viral genome into the host chromosome during latent infection (1, 24). The sequences of the Rep ORFs of AAV-2, AAV-3, AAV-4, and AAV-6 are approximately 85% identical, but AAV-5 has only 54.5% homology with the other AAV serotypes (5). The right half of the AAV genome encodes three viral capsid proteins referred to as VP1, VP2, and VP3 and is less conserved than the Rep ORF. Although AAV-2, AAV-3, and AAV-6 share about 80% homology in the amino acid sequences of the capsid proteins, alignment of the capsid protein ORFs of all six serotypes results in a reduction of the overall amino acid identity to less than 45% (2). This diversity in the capsid protein sequences is likely the basis for differences in the serological characteristics and altered tissue tropism among the six AAV serotypes. However, the contribution of the packaged genome to cell tropism has yet to be determined.
AAV is currently considered an ideal vehicle for human gene therapy, as it is a small, defective, nonpathogenic, single-stranded DNA virus with the ability to infect nondividing cells and to establish long-term, latent infection in vivo in a wide variety of organs without cell-mediated immune responses (14). All six of the reported AAV serotypes have been cloned, and recombinant viral stocks have been created. AAV-2 was the first primate AAV to be cloned and has been under intensive development as a gene therapy vector. Additionally, promising results were recently obtained from a clinical trial with recombinant AAV-2 (rAAV-2)-based gene therapy for hemophilia B (20). Other recent advances based on the circularization and concatamerization of AAV-2 genomes have made it possible to overcome the inherent 4.7-kb packaging limitation of rAAV (9, 11, 28, 39, 44). These new approaches allow the delivery of large transgenes and/or large regulatory elements by using dual-vector heterodimerization approaches.
Compared to the other serotypes, AAV-5 is the most unique member of the AAV family, and it has recently attracted considerable interest for development as a gene delivery vector (2, 5). Although less is known about AAV-5 molecular biology than about that of AAV-2, this virus has been reported to have a higher transduction efficiency than AAV-2 in certain cell types, including cells in the human airway (45), ependymal cells in the cerebral ventricles and the cerebral hemispheres (7), and muscles (3, 10, 19). Detailed sequence comparisons of the AAV-2 and AAV-5 capsids indicate less than 45% homology, with the most divergent regions being predicted to reside on the exterior surface of the virion (5). A recent study demonstrated that 2,3-linked sialic acid is a necessary component of the AAV-5 receptor complex (40). In contrast, cell surface heparan sulfate proteoglycan is thought to be the primary receptor component involved in AAV-2 transduction (38). Although little is presently known about the coreceptors used for AAV-5 infection, both fibroblast growth factor receptor 1 (30) and αVβ5-integrin (37) have been demonstrated to be functional coreceptors for AAV-2 infection. The significant differences between the viral structural proteins (Cap proteins) and the cellular receptors for transduction result in differences in tissue tropism and host range characteristics of these two AAVs (5).
Previously, a dramatic enhancement in rAAV-2 transduction in polarized airway cells was demonstrated when proteasome inhibitors were coadministered with the virus (12). Modulation of the ubiquitin-proteasome system resulted in a significant enhancement of rAAV-2-mediated transgene expression and a concurrent augmentation in nuclear trafficking of the virus. These results led to the hypothesis that ubiquitination of the AAV-2 capsid proteins may play a role as a barrier for rAAV-2 by rerouting intracellular trafficking to a nonexpressible compartment or by promoting the degradation of incoming virions. Evidence that intracellular trafficking may be a barrier to AAV-2 transduction has also been described for fibroblasts (16, 17).
In the present study, we compared the effect of proteasome inhibitors on AAV-2 and AAV-5 capsid-mediated infections by using transgene expression as an end point for efficient transduction. However, since the AAV-5 ITR is only 58% homologous with the AAV-2 ITR (5), it is very possible that the mechanisms of DNA strand conversion between these two types of rAAV are different. To exclude possible effects of the AAV ITRs on transgene expression, the same AAV-2 proviral genome was packaged into either AAV-2 or AAV-5 capsids. Transgene expression assays for rAAV-2 and pseudotyped rAAV-5 (rAAV-2cap5) facilitated direct evaluation and comparison of the transduction efficiencies of these two different serotypes under the same infection conditions.
MATERIALS AND METHODS
Cloning of helper plasmids for pseudotyping.
Wild-type AAV-5 DNA was isolated as previously described (2) and annealed by heating at 95°C for 5 min, followed by overnight slow cooling to 60°C. A PCR approach permitted cloning of the full-length AAV-5 coding region by reassembly of two PCR products with a unique restriction enzyme site. The primers for AAV-5 Rep were as follows: forward, 5"-gctctagaGATGTAATGCTTATTGTCACGCGA-3", and reverse, 5"-cccaagcttGATTGGGTTTTGGTTTCGGTGGGC-3" (see below). For AAV-5 Cap, the primers were as follows: forward, 5"-tgcactgcagGCGAGTAGTCATGTCTTTTGTTGATCACCC-3", and reverse, 5"-cccaagcttcgtctagaGACCACAAGAGGCAGTATTTTACTGAC-3". Sequences homologous to AAV gene components are presented in uppercase bases; lowercase bases represent overhangs that include sequences complementary to cloning restriction sites (underlined). The AAV-5 2.1-kb Rep and 2.3-kb Cap coding regions were amplified separately, and each fragment was subcloned into pBluescript II SK(−). With the unique BclI site in the overlap region of each fragment, the two AAV-5 fragments were ligated to generate a 4.3-kb AAV-5 genome with no ITR structure at either end. The helper plasmid for AAV-5 packaging (pAV5-Trans) was generated by replacing the AAV-2 sequence in the AAV-2 packaging helper plasmid (pAAV-2/Ad) (32) with the 4.3-kb full-length AAV-5 coding fragment. A second helper plasmid with only the AAV-2 Rep sequence (pAV2-Rep) was generated by deleting the 1.1-kb ApaI fragment in the AAV-2 Cap coding region of pAAV-2/Ad. To confirm that no AAV-2 capsids were generated, Western blotting of an Ad5.CMVlacZ (13)-infected 293 cell lysate transfected with pAV2-Rep was performed.
Generation of rAAV stocks.
Stocks of rAAV-2 (rAAV-2RSVluc) and pseudotyped rAAV-5 (rAAV-2cap5RSVluc) were generated with the previously described plasmid pcisAV2RSVluc (11). This rAAV-2 proviral plasmid contains a Rous sarcoma virus (RSV) promoter-driven luciferase gene flanked by two AAV-2 ITRs from pSub201. A routine calcium phosphate cotransfection protocol was used to produce rAAV from Ad5.CMVlacZ-coinfected 293 cells. To produce rAAV-2, the cotransfection protocol included the proviral plasmid pcisAV2RSVluc and pAAV-2/Ad at a ratio of 1:3. Pseudotyped rAAV-2cap5 was generated by transfecting the same rAAV-2 construct, pcisAV2RSVluc, into adenovirus-infected 293 cells together with pAV2-Rep and pAV5-Trans at a ratio of 1:1:3. Cells were harvested 40 h after transfection, and virus particles were released by freezing-thawing, DNase I digestion, and deoxycholate treatment. Both viral stocks were purified by using the same CsCl ultracentrifugation procedure. Following three rounds of CsCl banding, fractions of 1.36 to 1.42 g/cm3 were collected. To inactivate any possible remaining adenovirus contamination, the AAV fractions were heated at 60°C for 1 h. After dialysis against HEPES-buffered saline at 4°C to remove the CsCl, the viral stocks were quantified by slot blot analysis and transgene expression was tested with cultured cells. Contamination with wild-type AAV-2 was determined as previously described (8) and was found to be less than one functional particle per 1010 rAAV-2 particles. Wild-type AAV-2/AAV-5 hybrid contamination was evaluated by nested PCR for the Rep and Cap genes. Less than one particle of the wild-type hybrid virus was detected in 1010 pseudotyped virus particles (10). Contamination with helper adenovirus Ad5.CMVlacZ was evaluated by histochemical staining for β-galactosidase activity as described previously (8). Typically, helper virus contamination was less than 1 in 1010 rAAV particles.
Transduction of cells in vitro.
HeLa, 293, and IB3 cells and primary fetal ferret fibroblasts were cultured as monolayers in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc.) and penicillin (100 U/ml)-streptomycin (100 μg/ml) and maintained in a 37°C incubator at 5% CO2. The C2C12 mouse muscle cell line was obtained from the American Type Culture Collection (CRL-1772). Undifferentiated C2C12 cells were cultured under similar conditions, but horse serum (HyClone Laboratories) rather than FBS was used to induce the differentiation of these cells. Typically, well-differentiated cultures of C2C12 cells developed into myotubes within 5 to 7 days following the addition of 10% horse serum, at which time they were used for experiments (10, 43). All other cell lines were seeded in 6-well (1 × 106/well) or 12-well (5 × 105/well) plates and allowed to adhere for 18 h. At 1 h prior to infection, cells were fed with fresh medium with or without proteasome inhibitors. The tripeptidyl aldehyde proteasome inhibitor N-acetyl-l-leucyl-l-leucyl-norleucine (LLnL or MG101) was purchased from Boston Biochem, Inc. (Boston, Mass.). Carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (Z-LLL or MG132) was obtained from Calbiochem-Novabiochem (La Jolla, Calif.). These inhibitors were dissolved in dimethyl sulfoxide as 1,000-fold stock solutions, with LLnL at 40 mM and Z-LLL at 4 mM, and stored at −20°C. Virus infection was performed with serum-free DMEM, and an equal amount of DMEM-20% FBS was added 2 h after infection to bring the final serum level to 10%. Maximal final concentrations of proteasome inhibitors were 40 μM LLnL and 4 μM Z-LLL. The chemicals were diluted in culture medium, added to the cells 1 h prior to infection, and present in the medium throughout the 24-h infection.
Transduction analysis.
For analysis of transgene expression, luciferase activity in infected cells was measured with an assay kit from Promega 24 h after infection. Cells were lysed with 200 μl of lysis buffer per well of the 12-well plates. Cell membranes and debris were pelleted by microcentrifugation at 10,000 × g for 1 min. The supernatant was incubated with the luciferase substrate in a luminometer (TD-20/20; Turner Designs Instruments, Sunnyvale, Calif.) according to the manufacturer's instructions, and luciferase activity was determined at a sensitivity of 70%. For the viral DNA assays, low-molecular-weight DNA was extracted according to a modified Hirt procedure as previously described (44). The Hirt DNA from ∼2 × 106 infected cells was dissolved in 50 μl of Tris-EDTA, and one-half was resolved on a 1% agarose gel. Southern blots of the Hirt DNA were hybridized with a luciferase fragment probe labeled with [α-32P]dCTP by random priming.
Immunoprecipitation of ubiquitinated AAV capsids.
Detection of AAV capsid ubiquitination in HeLa cells treated with a proteasome inhibitor was performed as previously described but with modifications (12). HeLa cells (2 × 106) were infected with 2 × 109 DNA-containing particles of rAAV-2RSVluc or rAAV-2cap5RSVluc in serum-free DMEM. Infections were performed in parallel, with or without 40 μM LLnL. At 4 h after infection, cells were lysed in 0.8 ml of radioimmunoprecipitation (RIPA) buffer. Cell lysates were precleared with 10 μl of protein G PLUS-agarose (Santa Cruz Biotechnology) and then were incubated with 10 μl of mouse anti-VP1, -VP2, and -VP3 monoclonal antibody (clone B1; American Research Products) at 4°C for 1 h, followed by the addition of 30 μl of protein G PLUS-agarose. After overnight incubation at 4°C, the beads were washed four times with 1 ml of ice-cold RIPA buffer and resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE). After transfer to a nitrocellulose membrane, the blot was probed with a 1:200 dilution of antiubiquitin monoclonal antibody (clone P4D1; Santa Cruz Biotechnology), followed by horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution). After the final washings, the ubiquitinated viral protein was visualized with an enhanced chemiluminescence system (Amersham Pharmacia).
In vitro ubiquitination of AAV particles.
All the reagents used for the in vitro ubiquitination assay were purchased from Boston Biochem. The ubiquitin-protein conjugation kit (catalog no. K960) consists of ATP-containing energy buffer, ubiquitin substrate solution, and purified conjugation enzymes (E1, E2s, and E3s) from HeLa cell cytoplasmic extract fraction II (15, 18). This supplied fraction does not contain 20S and 26S proteasomes but contains ubiquitin C-terminal hydrolases (UCHs). To improve the yield of the ubiquitinated protein product, ubiquitin aldehyde (catalog no. U-201) is used for the inhibition of UCH activity (25). Ubiquitin conjugation to purified AAV virions was performed according to standard protocols provided by the supplier but with modifications. In brief, 32 μg (40 μl) of fraction II conjugation enzyme components, 100 μg of ubiquitin, 3.5 μg of ubiquitin aldehyde, and 8 μl of 10× energy buffer were mixed and brought to a final 74-μl reaction volume with 50 mM HEPES buffer (pH 7.6). The mixture was incubated at 37°C for 5 min to allow for inhibition of the UCHs. Conjugation was initiated by the addition of 6 μl of virus, which contained 3 × 108 particles of rAAV-2 or rAAV-2cap5. After 2 h of incubation at 37°C, the reactions were quenched by the addition of EDTA (final concentration, 10 mM), and samples were concentrated to ∼15 μl in a Speed-Vac. The samples were mixed with 2× SDS loading buffer, heated for 5 min at 90°C, and resolved by SDS-10% PAGE. The AAV proteins were analyzed by Western blotting with anti-AAV capsid monoclonal antibody B1 at a 1:100 dilution. Ubiquitination was indicated by decreased mobility of immunoreactive capsid proteins.
RESULTS
Pseudotyping of the rAAV-2 genome with AAV-5 capsid proteins.
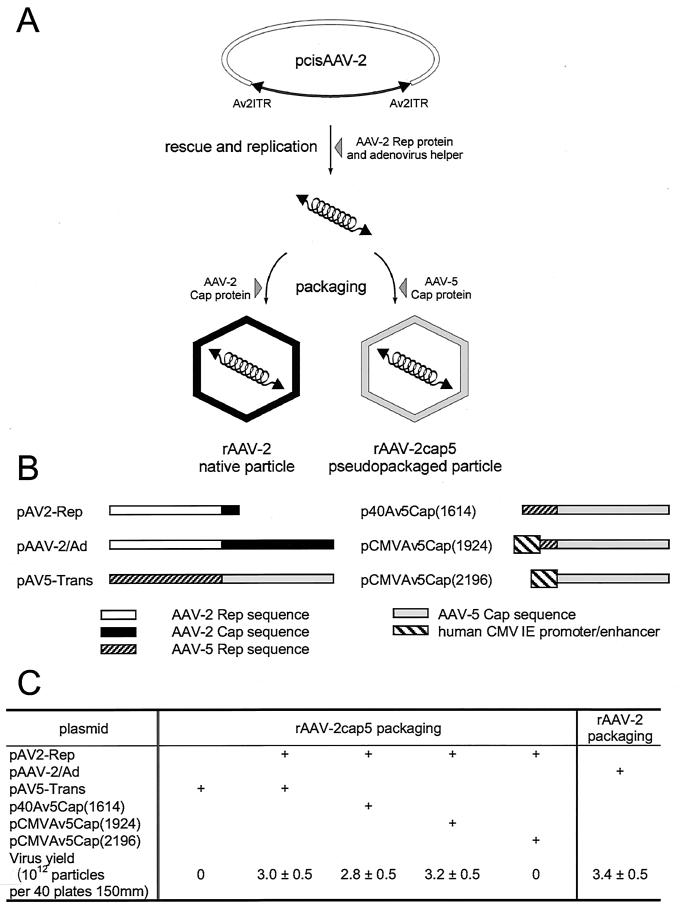
Unlike other serotypes of AAV that have shown cross-complementation of ITRs and Rep genes, AAV-5 is more distinct. The 58% homology between the ITRs of AAV-2 and AAV-5 and the low conservation of Rep protein binding and terminal resolution site recognition motifs suggest that AAV-5 Rep and AAV-2 Rep are not interchangeable. Therefore, the rAAV-2 construct was pseudopackaged with AAV-5 capsids in the presence of AAV-2 Rep proteins to assemble infectious particles (4). As shown in Fig. 1A, our initial goal was to create a pseudo-AAV-5 virion with an rAAV-2 genome containing an RSV-driven luciferase reporter gene in order to directly compare the efficiency of transduction with that of an intact rAAV-2 virion. When the rAAV-2 proviral plasmid pcisAV2RSVluc was transfected together with an AAV-2 Rep protein expression plasmid (pAV2-Rep) in adenovirus-infected 293 cells, the progeny viral DNA was efficiently packaged into either an AAV-2 capsid or an AAV-5 capsid, depending on which capsid expression plasmid was supplied. The viruses were purified by isopycnic density gradient centrifugation and quantified by DNA slot blot analysis. No significant difference in the efficiency of packaging of pcisAV2RSVluc into rAAV-2RSVluc or rAAV-2cap5RSVluc under our experimental conditions was observed (Fig. 1). The typical yield was 3 × 1012 particles/batch culture of 40 150-mm-diameter plates.
FIG. 1.
Production of rAAV-2 and rAAV-2cap5. (A) Principles underlying the pseudotyping of rAAV-2 genomes into AAV-5 particles. In the presence of AAV-2 Rep proteins and helper adenovirus, sequences flanked by the rAAV-2 ITRs are excised from the proviral plasmid (pcisAAV-2) and replicated. Depending on the serotype of capsid proteins provided by a second trans-plasmid, the rAAV-2 genome can be packaged in either rAAV-2 or pseudotyped rAAV-5 (rAAV-2cap5) particles. (B) Various helper plasmids that were tested for packaging of rAAV-2 proviral genomes into AAV-5 particles. AAV-2 Rep proteins are necessary for pseudopackaging of the rAAV-2 genome into AAV-5 particles and were provided by the helper plasmid pAV2-Rep. This plasmid was derived from pAAV-2/Ad, the routine helper plasmid for rAAV-2 production, by deletion of the AAV-2 capsid coding region. pAV5-Trans was generated by replacing the AAV-2 genome with the full-length AAV-5 Rep and Cap coding sequences. It can be used as the helper for generating authentic rAAV-5 vectors or for pseudotyping AAV-2 in an AAV-5 capsid. The AAV-5 capsid expression plasmid p40Av5Cap(1614) contains the native p40 promoter for Cap gene transcription. pCMVAv5Cap(1924) is similar, except that the human cytomegalovirus (hCMV) immediate-early (IE) promoter-enhancer replaces the p40 promoter. pCMVAv5Cap(2196) is derived from pCMVAv5Cap(1924) with the splicing signal deleted so that the CMV promoter is immediately upstream of the VP1 start codon. (C) Effects of the different AAV-5 helper plasmids on virus production. The yields of rAAV-2 and rAAV-2cap5 are the mean and standard error of the mean for three independent preparations.
Since AAV-2 has been under development as a gene transfer vector for a longer time than AAV-5, there is a greater understanding of the mechanisms for viral production in AAV-2 than in AAV-5. For example, it is known that reduced AAV-2 Rep 68/78 protein expression results in a much higher yield of rAAV-2 (23, 42). We attempted to increase the pseudotyped virus packaging efficiency by deleting the AAV-5 Rep gene coding region from the helper plasmid pAV5-Trans (Fig. 1B). As shown in Fig. 1C, use of the complementing plasmid p40Av5Cap(1614), with disabled AAV-5 Rep protein expression, resulted in no improvement in the yield of the pseudopackaged rAAV-2cap5. Similarly, substituting a strong heterologous promoter (the cytomegalovirus [CMV] immediate-early promoter-enhancer) for the AAV-5 p40 sequence [pCMVAv5Cap(1924)] resulted in only a slight increase in yield when this plasmid was used as the Cap5 complementing plasmid. Based on previous studies with rAAV-2 packaging (23, 42), these results suggest that AAV-5 and AAV-2 Rep proteins have distinct mechanistic influences on capsid expression and virus production.
To confirm that the native and pseudotyped AAV-2 vectors were packaged as expected, the immunologic characteristics of rAAV-2RSVluc and pseudotyped rAAV-2cap5RSVluc were evaluated. Mouse monoclonal antibody A20 (American Research Products), which only recognizes intact AAV-2 particles, did not demonstrate immunoreactivity to DNase-resistant particles of rAAV-2cap5, as assessed by either dot blot assays of intact virus followed by immunodetection or immunoprecipitation assays followed by Southern blotting for viral DNA with a transgene-specific probe (data not shown). In contrast, monoclonal antibody B1 reacted with the capsid proteins of both viruses with equal intensity in Western blots (data not shown). When equal numbers of purified DNase-resistant particles were evaluated by denaturing slot blot or Western blot analysis, indistinguishable levels of immunoreactivity were seen with antibody B1. Furthermore, the ratios of VP1, VP2, and VP3 capsid proteins were also indistinguishable for both purified rAAV-2 and rAAV-2cap5.
Encapsidation of rAAV-2 genomes into AAV-5 capsids alters the efficiency of transduction in several cell lines.
Given the various degrees of tropism of AAV-2 and AAV-5 for different cell lines, functional titer determination as a basis for comparison is problematic. For this reason, equivalent titers of DNase-resistant physical particles were used as the basis for comparison. However, since the primary goal of these experiments was to assess the effect of inhibiting the proteasome on transduction, overall differences in the extent of baseline transduction were of less concern. When equivalent numbers of physical particles of rAAV-2RSVluc or rAAV-2cap5RSVluc were used for infection (Fig. 2A), transgene expression was consistently lower (6- to 25-fold) for rAAV-2cap5 in nearly all cell lines evaluated (HeLa cells, primary fetal fibroblasts, IB3 cells, 293 cells, and undifferentiated C2C12 muscle cells) (Fig. 2B). The one exception was an approximately 30-fold-higher level of luciferase expression for rAAV-2cap5 than for rAAV-2 in differentiated C2C12 cells. A possible explanation for the differences in transduction between the two viruses in these cell types may be the levels of their respective cell surface receptors. For example, heparan sulfate proteoglycan is a primary receptor component for the binding of AAV-2 (38), while 2,3-linked sialic acid has been identified as a major receptor component for the binding of AAV-5 (40). In support of this notion, the induction of rAAV-2cap5 infection in differentiated C2C12 cells was recently shown to be due in part to an increased level of 2,3-linked sialic acid at the membrane (10).
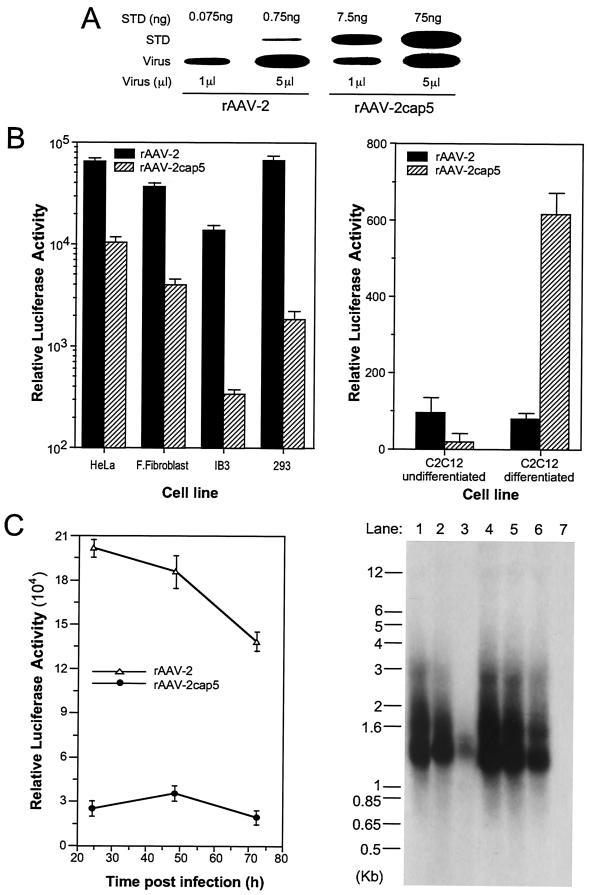
FIG. 2.
Evaluation of rAAV-2 and pseudotyped rAAV-2cap5 vectors. Both rAAV-2 and pseudotyped AAV-5 (rAAV-2cap5) contained the same luciferase reporter gene derived from the proviral plasmid pcisAV2RSVluc. The titers of both viral stocks were adjusted to equivalent physical particles per milliliter. (A) Titration of these two recombinant viral stocks by slot blot analysis against plasmid DNA standards (STD). The quantity of plasmid and the volume of virus evaluated in each well are marked above and below the blot, respectively. (B) Differences in transduction efficiencies following infection with either rAAV-2 or pseudotyped rAAV-2cap5 in a series of cell types (HeLa cells, primary fetal fibroblasts, IB3 cells, 293 cells, and undifferentiated or differentiated C2C12 muscle cells). Experiments were performed by infecting cells with 5 × 108 total particles/well in 12-well plates. Luciferase activity (relative light units/well) was determined 24 h after infection. Data represent the mean and standard error of the mean for four independent experiments. (C) Time course of transgene expression and viral genome persistence in HeLa cells following infection with rAAV-2 or rAAV-2cap5. A total of 109 particles of rAAV-2 or rAAV-2cap5 were used for infection of six-well plates. (Left panel) Luciferase activity was assayed at 24, 48, and 72 h postinfection. Data represent the mean and standard error of the mean for three independent experiments. (Right panel) Low-molecular-weight Hirt DNA was harvested from infected HeLa cells at 24 h (lanes 1 and 4), 48 h (lanes 2 and 5), and 72 h (lanes 3 and 6) and separated on a 1% agarose gel for Southern blotting and hybridization with a 32P-labeled luciferase probe. Each lane consists of Hirt DNA from one-half of a 35-mm dish of infected cells (note that microgram normalization of the DNA load did not provide an accurate comparison because cells were actively dividing over the time course of the study and contaminant genomic DNA in the Hirt DNA is dependent on cell number). Lanes 1 to 3, rAAV-2-infected cells; lanes 4 to 6, rAAV-2cap5-infected cells; 7, noninfected negative control.
To further investigate whether receptor binding or endocytosis accounted for the observed differences in transduction efficiency, low-molecular-weight Hirt DNA was purified from each cell type infected in parallel with the two viruses, and Southern blot analysis for intracellular viral DNA was performed. In fetal fibroblasts, IB3 cells, and 293 cells, a lower level of transduction with the pseudotyped virus appears to reflect a lower level of uptake of the virus, since only a limited amount of viral DNA could be retrieved from these cell lines after rAAV-2cap5RSVluc infection (data not shown). These observations would be consistent with differences in viral attachment or endocytosis being the major reason for various transduction efficiencies with these two recombinant viruses. However, in HeLa cells, the amount of internalized viral DNA was slightly higher for rAAV-2cap5 than for rAAV-2; thus, the decreased level of transduction seen with rAAV-2cap5 appears to be due to some aspect of intracellular processing.
Figure 2C demonstrates a kinetic analysis of the time course of transgene expression and uptake of viral DNA in HeLa cells. The level of transgene expression mediated by rAAV-2 was highest 24 h after infection and decreased progressively thereafter. Pseudotyped rAAV-2cap5 gave peak expression levels on day 2 following infection. Consistent with diminishing gene expression levels, the amount of internalized viral DNA following rAAV-2 infection decreased gradually over the course of 3 days. However, viral DNA following rAAV-2cap5 infection was both more abundant and more stable despite the low level of gene expression. These findings suggest that differences in infection of HeLa cells with rAAV-2 and rAAV-2cap5 are not likely to be solely due to receptor internalization but are apparently associated with differential intracellular processing.
Tripeptidyl aldehyde proteasome inhibitors enhance the transduction efficiencies of both rAAV-2 and rAAV-2cap5.
The sixfold-higher level of transgene expression with rAAV-2 than with rAAV-2cap5 in HeLa cells does not correlate with their levels of viral genome internalization (Fig. 2C). Since the viral genomes are identical in these viruses, viral DNA stability, strand conversion, and the efficiency of gene transcription should also remain the same with both serotypes. This notion prompted the hypothesis that differences in intracellular processing facilitated by either AAV-2 or AAV-5 capsid entry pathways may impart alternative fates that affect the efficiency of transduction with these two viruses. The proteasome system is known to modulate the intracellular processing of many proteins and viruses, such as human immunodeficiency virus (34). Previously, it was found that cell-permeable tripeptidyl aldehyde proteasome inhibitors, such as LLnL or Z-LLL, can substantially augment rAAV-2-mediated gene transfer to the apical surface of polarized cultures of human bronchial epithelial cells and mouse lung cells in vivo (12). Hence, we examined whether the proteasome pathway may also affect gene transfer with rAAV-2cap5.
The transduction efficiencies of rAAV-2RSVluc and rAAV-2cap5RSVluc were compared in the presence or absence of tripeptidyl proteasome inhibitors (40 μM LLnL or 4 μM Z-LLL). Results from these experiments with four different cell lines are summarized in Table 1. All four cell types tested demonstrated augmentation of both rAAV-2 and rAAV-2cap5 transduction (i.e., transgene expression) in the presence of LLnL or Z-LLL. No significant differences in the effects of these inhibitors on the transduction of rAAV-2 or rAAV-2cap5 were found for fetal fibroblasts and 293 cells. However, a significantly higher level of induction of transgene expression was seen with rAAV-2 infection of HeLa and IB3 cells than with rAAV-2cap5 infection (Table 1). These findings suggest that both serotypes of AAV may be susceptible to proteasome barriers for transduction; however, the extent of this susceptibility appears to be influenced by the cell type.
TABLE 1.
Fold induction of luciferase transgene expression with proteasome inhibitors
| Cell line | Mean ± SEM fold induction of luciferase activity in the presence ofa:
|
||||
|---|---|---|---|---|---|
| 40 μM LLnL
|
4 μM Z-LLL
|
||||
| rAAV-2 | rAAV-2cap5 | rAAV-2 | rAAV-2cap5 | ||
| HeLa | 16 ± 0.80 | 6.23 ± 0.32 | 20.34 ± 4.32 | 4.82 ± 1.03 | |
| Fetal fibroblasts | 30.09 ± 2.91 | 24.70 ± 3.33 | 12.05 ± 1.07 | 10.48 ± 0.85 | |
| 293 | 10.38 ± 2.92 | 7.20 ± 1.40 | 5.43 ± 1.29 | 6.25 ± 0.25 | |
| IB3 | 104.94 ± 10.87 | 24.07 ± 0.25 | 63.19 ± 1.23 | 24.58 ± 0.18 | |
Data are from four experiments. Fold induction was calculated with the following formula for each experimental point: relative luciferase activity with proteasome inhibitor/relative luciferase activity without proteasome inhibitor.
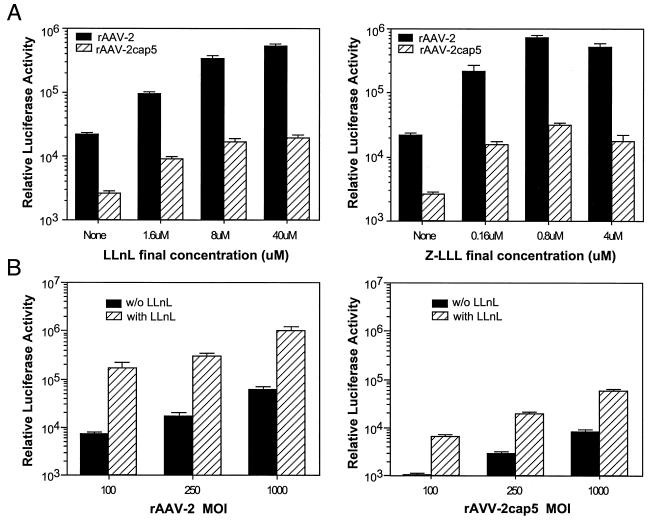
The effect of different viral multiplicities of infection (MOIs) and doses of both inhibitors were also evaluated with HeLa cells (Fig. 3). Cells were preincubated with increasing doses of LLnL (up to 100 μM) or Z-LLL (up to 10 μM) for 1 h prior to infection with rAAV-2 or rAAV-2cap5 (each at 250 particles/cell). The highest doses of the inhibitors were toxic to the cells and led to more than 20% cell attrition at 24 h after infection; hence, data are not presented for these conditions. However, concentrations as high as 40 μM LLnL or 4 μM Z-LLL showed no obvious toxicity to the cells. HeLa cells demonstrated a dose-dependent increase in transduction following LLnL treatment for both serotypes of virus. Subtle differences in the maximal effect of LLnL were seen between the two viruses, with peak induction at 40 μM for rAAV-2 and 8 μM for rAAV-2cap5. However, the maximal effect of Z-LLL was similar for both serotypes and peaked at 0.8 μM (Fig. 3A). The level of induction facilitated by 40 μM LLnL was independent of the MOI for both rAAV-2 and rAAV-2cap5 (Fig. 3B). In these experiments, the level of rAAV-2 transduction was approximately threefold higher than that seen following infection with pseudotyped rAAV-2cap5 (Fig. 3B).
FIG. 3.
Effects of proteasome inhibitors on rAAV-2 and rAAV-2cap5 transduction. (A) HeLa cells were infected with luciferase-expressing rAAV-2 or rAAV-2cap5 at an MOI of 250 particles/cell in the presence of different concentrations of the proteasome inhibitor LLnL or Z-LLL. (B) HeLa cells were infected with different doses of rAAV-2 or rAAV-2cap5 in the presence of 40 μM LLnL. In all panels, luciferase activity was measured 24 h after infection by using 12-well plates as described in Materials and Methods. Data represent the mean and standard error of the mean relative luciferase activity (per well) for four independent experiments.
Both AAV-2 and AAV-5 capsid proteins are substrates for ubiquitination.
Proteasome-dependent degradation of ubiquitinated molecules represents a major pathway for the disposal of both endogenous and foreign proteins (29, 33). A recent study also demonstrated that the ubiquitin-proteasome system can regulate receptor-mediated endocytosis (36). It was previously demonstrated that AAV-2 capsid proteins are ubiquitinated in primary fibroblasts and that LLnL treatment augments rAAV transgene expression 10-fold in this cell type (12). To test whether AAV-5 capsids are also ubiquitinated following infection, we performed immunoprecipitation experiments with anticapsid antibody B1, followed by Western blotting with antiubiquitin antibody. These experiments were performed with fetal fibroblasts and IB3, 293, and HeLa cells. However, due to the low level of infection of all cell types except HeLa with rAAV-2cap5, insufficient viral recovery prevented conclusive analysis with fetal fibroblasts, IB3 cells, and 293 cells. For example, the level of internalized viral genomes (as determined by Hirt DNA Southern blotting) was significantly lower following rAAV-2cap5 infection than following rAAV-2 infection for all cell lines except HeLa (data not shown). Hence, since viral uptake in HeLa cells was similar for both rAAV-2 and rAAV-2cap5 (Fig. 2C), we believed that comparative differences in the accumulation of ubiquitinated capsids would be easiest to interpret with this cell line. Furthermore, since LLnL similarly augmented both rAAV-2 and rAAV-2cap5 transduction (transgene expression was only 2.6-fold divergent), we hypothesized that if AAV capsid ubiquitination were linked to responsiveness to a proteasome inhibitor, then it would be evident in both AAV-2 and AAV-5 capsids.
Results from immunoprecipitation experiments demonstrated that capsid proteins from both rAAV-2 and rAAV-2cap5 were ubiquitinated in HeLa cells in the presence of LLnL (Fig. 4A, lanes 2 and 6). The presence of a proteasome inhibitor was required for an accumulation of ubiquitinated capsid, as might be expected if these molecules are quickly degraded by the proteasome following ubiquitination. Since two monoclonal antibodies were used for both capsid immunoprecipitation and ubiquitin detection, a cross-reactive immunoglobulin G band was seen in all lanes and was also seen in the absence of the addition of cell lysate to the immunoprecipitation reaction mixture (12). Interestingly, if indeed ubiquitinated virions are targeted to the proteasome for degradation, then one would expect that treatment with proteasome inhibitors might also increase the stability of viral genomes in cells. However, as shown in Fig. 4B, this was not the case for either rAAV-2 or rAAV-2cap5. No change in the abundance of intracellular viral DNA was detected at 24 h following infection, with or without LLnL. This result is consistent with a previous report that the presence of LLnL did not substantially prevent the enzymatic degradation of internalized AAV-2 DNA from the apical side of human airway epithelial cells, despite a significantly increased level of transduction (12).
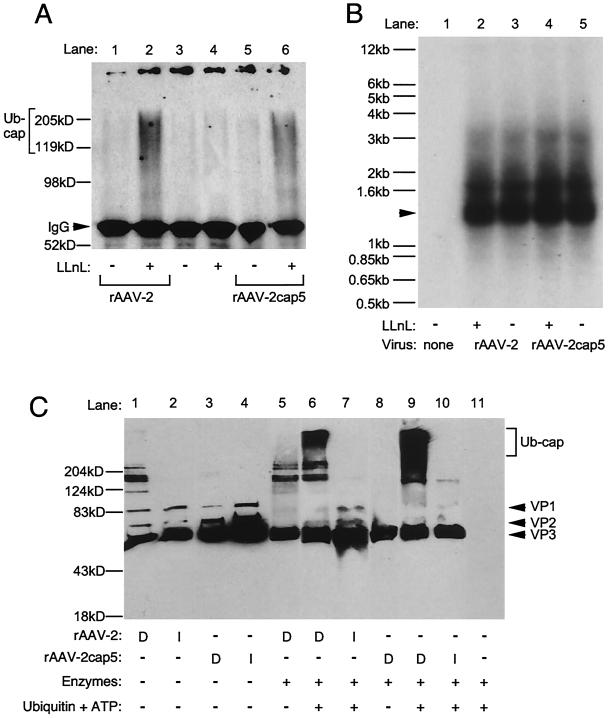
FIG. 4.
Ubiquitination of AAV-2 and AAV-5 capsid proteins. (A) Western blot analysis for ubiquitinated AAV-2 and AAV-5 capsid proteins in HeLa cells. HeLa cells were infected with luciferase-expressing rAAV-2 or rAAV-2cap5 with or without 40 μM LLnL. At 4 h after infection, cells were trypsinized, washed twice with phosphate-buffered saline, and lysed in 1 ml of RIPA buffer. As described in Materials and Methods, virus from HeLa cell lysates was immunoprecipitated with antibody B1 and subjected to Western blotting against antiubiquitin monoclonal antibody. Lane 1, rAAV-2 infection without LLnL; lane 2, rAAV-2 infection with LLnL; lane 3, mock-infected cells without LLnL; lane 4, mock-infected cells with LLnL; lane 5, rAAV-2cap5 infection without LLnL; lane 6, rAAV-2cap5 infection with LLnL. Ubiquitinated capsid proteins (Ub-cap; bracket) and cross-reactive immunoglobulin G (IgG) (arrowhead) are marked to the left of the blot. (B) Southern blot analysis of low-molecular-weight Hirt DNA from noninfected HeLa cells (lane 1) and HeLa cells infected with rAAV-2 (lanes 2 and 3) or rAAV-2cap5 (lanes 4 and 5) in the presence (lanes 2 and 4) or absence (lanes 3 and 5) of 40 μM LLnL. Single-stranded viral DNA is marked by an arrowhead to the left of the blot. (C) In vitro ubiquitin conjugation to rAAV-2 or rAAV-2cap5 particles. A total of 3 × 108 particles of rAAV-2 or rAAV-2cap5 were incubated with ubiquitin conjugation enzymes at 37°C for 120 min and then resolved by SDS-10% PAGE. Western blotting with anti-AAV capsid mouse monoclonal antibody B1 and enhanced chemiluminescence detection visualized increased apparent sizes of ubiquitinated AAV capsid proteins. The conditions for each conjugation reaction are marked below the blot. Lanes 1 to 4, virus without conjugation mixture; lanes 5 to 10, conjugation reactions with and without ubiquitin and ATP substrates as a control; lane 11, conjugation mixture without AAV. Both intact (I) and denatured (D) virus samples were evaluated to determine whether native and/or denatured capsid proteins are targets for ubiquitination. In reactions where virus was denatured, the virus was heated at 95°C for 10 min before addition to the conjugation reactions. Reactions in lanes 5 (rAAV-2) and 8 (rAAV-2cap5) were performed as the negative controls; the reaction conditions were the same as those for lane 6 (rAAV-2) and lane 9 (rAAV-2cap5), except that no ubiquitin or ATP was applied. VP1, VP2, and VP3 are marked by arrowheads to the right of the gel, and ubiquitinated capsids (Ub-cap) are bracketed.
Conjugation of ubiquitin side chains to the viral capsid proteins resulted in significant alterations of the migration patterns on SDS-PAGE. The high-molecular-weight smears seen for the AAV-2 or AAV-5 capsid proteins after ubiquitination in this HeLa cell experiment were consistent with previous results obtained with AAV-2-infected primary fibroblasts (12). However, in the current experiments, the high-molecular-weight smear of ubiquitinated AAV protein in HeLa cells had a more heterogeneous molecular mass than that found in the previous study. This finding could be related to a cell-type-specific difference. It also appears that ubiquitinated capsid proteins were slightly more abundant in rAAV-2- than in rAAV-2cap5-infected cells. Although this difference was small, it is interesting that rAAV-2 transduction was threefold more responsive to proteasome inhibitors and that intracellular rAAV-2 genomes were less stable than those derived from rAAV-2cap5 (Fig. 2C and 3A). Given the fact that nearly equivalent levels of viral DNA are taken up by HeLa cells following infection with rAAV-2 and rAAV-2cap5, we can assume that the abundances of capsid target molecules are also similar for both serotypes.
To further substantiate the finding of AAV-2 and AAV-5 capsid ubiquitination, we performed in vitro reconstitution experiments in an attempt to directly determine whether purified intact virions are substrates for ubiquitination. Purified active components of the ubiquitin conjugation system isolated from HeLa cell extracts (15, 18) were used with purified rAAV-2 or rAAV-2cap5 as a substrate. Western blot analysis with anticapsid antibody B1 was used to visualize a decrease in mobility for capsid proteins caused by the addition of multiple molecules of ubiquitin (7.6 kDa). As shown in Fig. 4C, lanes 7 and 10, intact virions of rAAV-2 and rAAV-2cap5 were poor substrates for in vitro ubiquitination. In contrast, after heat denaturation of both AAV-2 and AAV-5 at 95°C for 10 min, both AAV-2 and AAV5 capsid proteins were significantly ubiquitinated in the presence of ubiquitin, ATP, and purified ubiquitin ligases (Fig. 4C, lanes 6 and 9). Interestingly, AAV-5 capsids appeared to be slightly better substrates for ubiquitination, giving rise to a higher-molecular-mass smear (>200 kDa) of anticapsid immunoreactive bands. No appreciable increase in higher-molecular-mass capsid molecules was detected in the absence of ubiquitin and ATP (Fig. 4C, lane 5 and 8).
The results of this in vitro reconstitution experiment suggest that intact rAAV-2 or rAAV-2cap5 particles are not preferential substrates for conjugation with ubiquitin. Rather, these results suggest that ubiquitination of capsid proteins likely occurs after endosomal processing of the viruses (i.e., at the time of virion disassembly or following complete uncoating). An interesting complication of visualizing rAAV-2 capsid ubiquitination stemmed from the apparent aggregation of capsid proteins following heating (compare lanes 1 and 2 of Fig. 4C). The cause of this physical change in capsid proteins, which is resistant to denaturing (SDS) and reducing (β-mercaptoethanol) conditions of SDS-PAGE, is unknown. However, this change was not apparent with rAAV-2cap5.
DISCUSSION
The significant dissimilarities between the AAV-2 and AAV-5 genomes, Rep and Cap proteins, and ITRs forecast differences in tissue tropism, cellular receptors, host range, and possibly even replication mechanisms. Hence, one must consider several potential factors when looking for ways to increase the efficacy of gene transfer with these two types of AAV. Furthermore, differences in the reported efficiencies of recombinant AAV-2 and AAV-5 for gene transfer also provide an opportunity to learn about the biology responsible for the unique functional aspects of these two viruses as vectors (7, 10, 45). Such differences in biology could provide the foundation for improving vector delivery with many serotypes of AAV. Possible differences in biology include cell membrane receptor binding and endocytosis, intracellular trafficking, uncoating, initiation of secondary strand synthesis and conversion of single-stranded DNA to its active expressible form, the stability and long-term persistence of the viral genome, and more.
In the present study, we attempted to minimize differences in several of these aspects to concentrate on the biology of the ubiquitin-proteasome pathway in modulating the intracellular fate of rAAV. In these experiments, an rAAV-2 genome was pseudotyped with the AAV-5 capsid to minimize potential differences in viral genomes that might otherwise affect comparisons of gene expression with rAAV-2 vectors. We hypothesize that the presence of identical genomes within both capsid serotypes may enable direct comparison of intracellular processing events that control transduction. However, since the precise nature of the packaging signal has yet to be determined for AAV-5, we cannot rule out the possibility that subtle differences in transduction are due to nonnative interactions of the AAV-2 genome with the AAV-5 capsid. Furthermore, although a number of cell lines were screened for responsiveness of rAAV infection to proteasome inhibitors, the majority of mechanistic studies were performed with HeLa cells, which demonstrate equivalent levels of viral uptake for rAAV-2 and rAAV-2cap5 despite the reported divergent receptor entry pathways for other cell types (45). This consideration significantly simplified the comparative aspects of transduction between rAAV-2 and rAAV-2cap5.
In HeLa cells, rAAV-2 demonstrated a transduction efficiency six times higher than that for pseudotyped rAAV-2cap5. This result supports findings by Chiorini et al. comparing β-galactosidase expression in HeLa cells by using rAAV-2 and rAAV-5 vectors; they demonstrated a sevenfold-higher level of transduction with rAAV-2 (5). Interestingly, Southern blot analysis of low-molecular-weight Hirt DNA demonstrated that the levels of viral genomes taken up by cells within 24 h were very similar for rAAV-2 and rAAV-2cap5. This result demonstrates that the differences in AAV-2 and AAV-5 binding and internalization in HeLa cells may be minimal, even through they enter cells though different receptor-mediated mechanisms. Interestingly, viral DNA introduced into HeLa cells by pseudotyped rAAV-2cap5 tended to be more resistant to degradation than that introduced by rAAV-2. Together, these results could be explained by different endosomal processing and/or nuclear trafficking mechanisms for the two rAAV vector types.
A complete understanding of endocytic and nuclear trafficking mechanisms associated with AAV transduction has remained elusive. Various signaling pathways may play a role in these processes. It was previously reported (12) that the ubiquitin-proteasome pathway is involved in AAV-2 transduction. When proteasome function was inhibited, a substantial augmentation in rAAV-2-mediated transgene expression was observed. In the present study, we demonstrated that proteasome inhibitors enhanced not only rAAV-2- but also rAAV-2cap5-mediated gene transfer. The extent of this enhancement was significantly influenced by the cell type analyzed. In HeLa and IB3 cells, we saw much higher augmentation effects on rAAV-2 transduction than on rAAV-2cap5 transduction. This result also implies differences in processing after internalization of these viruses. Given the fact that proteasome inhibitors did not affect viral genome stability, it appears that these inhibitors do not augment transduction by altering the degradation of internalized virions or DNA.
The consequence of ubiquitin conjugation of the virus in cellular processing is currently undefined. From the present study, it is clear that both AAV-2 and AAV-5 capsids are ubiquitinated in HeLa cells. Furthermore, coadministration of the viruses with a proteasome inhibitor in HeLa cells resulted in a positive correlation of increased transgene expression with the amount of ubiquitinated AAV capsid protein. It is currently difficult to distinguish a causal relationship between viral ubiquitination and enhanced gene transfer in response to proteasome inhibitors. However, several possibilities may explain the functional involvement of the ubiquitin-proteasome pathway in both rAAV-2 and rAAV-2cap5 transduction. First, ubiquitination of the capsid may be a signal for intracellular rerouting of virus to a “dead-end” intracellular compartment in the absence of complete protease digestion of the relatively stable capsid. This hypothesis would invoke ubiquitination as a mechanism of intracellular innate immunity to incoming virus, as has been suggested for human immunodeficiency virus (34). In this case, ubiquitination of viral capsids would be detrimental to the capacity of rAAV to complete its latent life cycle. One would also anticipate that if ubiquitination were altering the intracellular routing of virus, then intact virions might be the substrate of ubiquitination. However, our in vitro ubiquitination assays appear to suggest that intact virions are not a preferential substrate for ubiquitination. Rather, denatured capsids of both AAV-2 and AAV-5 were more highly ubiquitinated in vitro. An alternative hypothesis consistent with this latter observation is that ubiquitination of AAV capsid proteins serves as a signal for viral particle disassembly. Since the treatment of cells with proteasome inhibitors augments the level of capsid ubiquitination, this alternative explanation may suggest that increased ubiquitination is a positive signal that enhances the completion of the rAAV latent life cycle. It is presently unclear whether in vivo immunoprecipitated ubiquitinated capsids are part of a partially denatured virion complex or represent fully disassembled capsid molecules. However, it is plausible that endosomal processing of the AAV virion renders it receptive to ubiquitination by altering the virion conformation or partially denaturing the capsid structure.
Despite the lack of a clear mechanism for enhanced transduction in the presence of proteasome inhibitors, these experiments suggest that ubiquitination of AAV capsids may be a common component of cellular interactions for both AAV-2 and AAV-5. Since AAV-2 and AAV-5 are the most divergent serotypes of AAV, these mechanisms may also apply to other serotypes as well. Further elucidation of the mechanisms and consequences of AAV ubiquitination may have significant therapeutic benefits in the application of multiple rAAV serotypes for gene therapy.
Acknowledgments
This work was supported by NIH grant HL58340 (to J.F.E.), the Center for Gene Therapy cofunded by the NIH and the Cystic Fibrosis Foundation (grant P30 DK54759) (to J.F.E.), and a research grant from Targeted Genetics Corp (to J.F.E.).
REFERENCES
- 1.Balague, C., M. Kalla, and W. W. Zhang. 1997. Adeno-associated virus Rep78 protein and terminal repeats enhance integration of DNA sequences into the cellular genome. J. Virol. 71:3299-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantel-Schaal, U., H. Delius, R. Schmidt, and H. zur Hausen. 1999. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J. Virol. 73:939-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. [DOI] [PubMed] [Google Scholar]
- 4.Chiorini, J. A., S. Afione, and R. M. Kotin. 1999. Adeno-associated virus (AAV) type 5 Rep protein cleaves a unique terminal resolution site compared with other AAV serotypes. J. Virol. 73:4293-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorini, J. A., L. Yang, Y. Liu, B. Safer, and R. M. Kotin. 1997. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J. Virol. 71:6823-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan, D., K. J. Fisher, J. F. Burda, and J. F. Engelhardt. 1997. Structural and functional heterogeneity of integrated recombinant AAV genomes. Virus Res. 48:41-56. [DOI] [PubMed] [Google Scholar]
- 9.Duan, D., P. Sharma, J. Yang, Y. Yue, L. Dudus, Y. Zhang, K. J. Fisher, and J. F. Engelhardt. 1998. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle. J. Virol. 72:8568-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan, D., Z. Yan, Y. Yue, W. Ding, and J. F. Engelhardt. 2001. Enhancement of muscle gene delivery with pseudotyped AAV-5 correlates with myoblast differentiation. J. Virol. 75:. [DOI] [PMC free article] [PubMed]
- 11.Duan, D., Y. Yue, Z. Yan, and J. F. Engelhardt. 2000. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat. Med. 6:595-598. [DOI] [PubMed] [Google Scholar]
- 12.Duan, D., Y. Yue, Z. Yan, J. Yang, and J. F. Engelhardt. 2000. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Investig. 105:1573-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, K. J., G. P. Gao, M. D. Weitzman, R. DeMatteo, J. F. Burda, and J. M. Wilson. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 70:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flotte, T. R., and B. J. Carter. 1995. Adeno-associated virus vectors for gene therapy. Gene Ther. 2:357-362. [PubMed] [Google Scholar]
- 15.Ganoth, D., E. Leshinsky, E. Eytan, and A. Hershko. 1988. A multicomponent system that degrades proteins conjugated to ubiquitin. Resolution of factors and evidence for ATP-dependent complex formation. J. Biol. Chem. 263:12412-12419. [PubMed] [Google Scholar]
- 16.Hansen, J., K. Qing, H. J. Kwon, C. Mah, and A. Srivastava. 2000. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 74:992-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen, J., K. Qing, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J. Virol. 75:4080-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershko, A., H. Heller, E. Eytan, and Y. Reiss. 1986. The protein substrate binding site of the ubiquitin-protein ligase system. J. Biol. Chem. 261:11992-11999. [PubMed] [Google Scholar]
- 19.Hildinger, M., A. Auricchio, G. Gao, L. Wang, N. Chirmule, and J. M. Wilson. 2001. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 75:6199-6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay, M. A., C. S. Manno, M. V. Ragni, P. J. Larson, L. B. Couto, A. McClelland, B. Glader, A. J. Chew, S. J. Tai, R. W. Herzog, V. Arruda, F. Johnson, C. Scallan, E. Skarsgard, A. W. Flake, and K. A. High. 2000. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 24:257-261. [DOI] [PubMed] [Google Scholar]
- 21.King, J. A., R. Dubielzig, D. Grimm, and J. A. Kleinschmidt. 2001. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 20:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labow, M. A., P. L. Hermonat, and K. I. Berns. 1986. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J. Virol. 60:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, J., R. J. Samulski, and X. Xiao. 1997. Role for highly regulated rep gene expression in adeno-associated virus vector production. J. Virol. 71:5236-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden, R. M., P. Ward, C. Giraud, E. Winocour, and K. I. Berns. 1996. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 93:11288-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melandri, F., L. Grenier, L. Plamondon, W. P. Huskey, and R. L. Stein. 1996. Kinetic studies on the inhibition of isopeptidase T by ubiquitin aldehyde. Biochemistry 35:12893-12900. [DOI] [PubMed] [Google Scholar]
- 26.Muramatsu, S., H. Mizukami, N. S. Young, and K. E. Brown. 1996. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 221:208-217. [DOI] [PubMed] [Google Scholar]
- 27.Muzyczka, N. 1992. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158:97-129. [DOI] [PubMed] [Google Scholar]
- 28.Nakai, H., T. A. Storm, and M. A. Kay. 2000. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 18:527-532. [DOI] [PubMed] [Google Scholar]
- 29.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 30.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samulski, R. J., L. S. Chang, and T. Shenk. 1989. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J. Virol. 63:3822-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz, A. L., and A. Ciechanover. 1999. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 50:57-74. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz, O., V. Marechal, B. Friguet, F. Arenzana-Seisdedos, and J. M. Heard. 1998. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J. Virol. 72:3845-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder, R. O., D. S. Im, and N. Muzyczka. 1990. Evidence for covalent attachment of the adeno-associated virus (AAV) rep protein to the ends of the AAV genome. J. Virol. 64:6204-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strous, G. J., and R. Govers. 1999. The ubiquitin-proteasome system and endocytosis. J. Cell Sci. 112:1417-1423. [DOI] [PubMed] [Google Scholar]
- 37.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 38.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun, L., J. Li, and X. Xiao. 2000. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat. Med. 6:599-602. [DOI] [PubMed] [Google Scholar]
- 40.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 21:21.. [DOI] [PubMed] [Google Scholar]
- 41.Xiao, W., N. Chirmule, S. C. Berta, B. McCullough, G. Gao, and J. M. Wilson. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 73:3994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaffe, D., and O. Saxel. 1977. A myogenic cell line with altered serum requirements for differentiation. Differentiation 7:159-166. [DOI] [PubMed] [Google Scholar]
- 44.Yan, Z., Y. Zhang, D. Duan, and J. F. Engelhardt. 2000. From the cover: trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc. Natl. Acad. Sci. USA 97:6716-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]